Heightened suspicion for pulmonary hypertension (PH) arises when the advancement of dyspnoea in interstitial lung disease (ILD) patients diverges from the expected pattern of decline in pulmonary function parameters. The complexity of PH associated with ILD (PH-ILD) diagnostics is emphasized by the limitations of transthoracic echocardiography in the ILD population, necessitating the exploration of alternative diagnostic approaches. Cardiac magnetic resonance imaging (MRI) emerges as a promising tool, offering insights into hemodynamic parameters and providing valuable prognostic information. The potential of biomarkers, alongside pulmonary function and cardiopulmonary exercise tests, is explored for enhanced diagnostic and prognostic precision.
- pulmonary hypertension
- interstitial lung disease
- cardiopulmonary exercise testing
- lung function tests
- echocardiography
- cardiac magnetic resonance imaging
1. Introduction
2. PH-ILD Pathophysiology
3. PH-ILD Disease Spectrum
4. PH-ILD Phenotypes
5. Challenges in Clinical Diagnosis of PH-ILD
6. PH-ILD Diagnostic Strategy
|
Imaging Modality |
PH Suggesting Features |
|---|---|
|
Chest CT |
|
|
Echocardiography |
|
|
Cardiac MRI |
|
Abbreviations: CT—computed tomography, LV—left ventricle, MRI—magnetic resonance imaging, PA—pulmonary artery, PH—pulmonary hypertension, RV—right ventricle, TRV—tricuspid regurgitation velocity.
7. Artificial Intelligence Applications
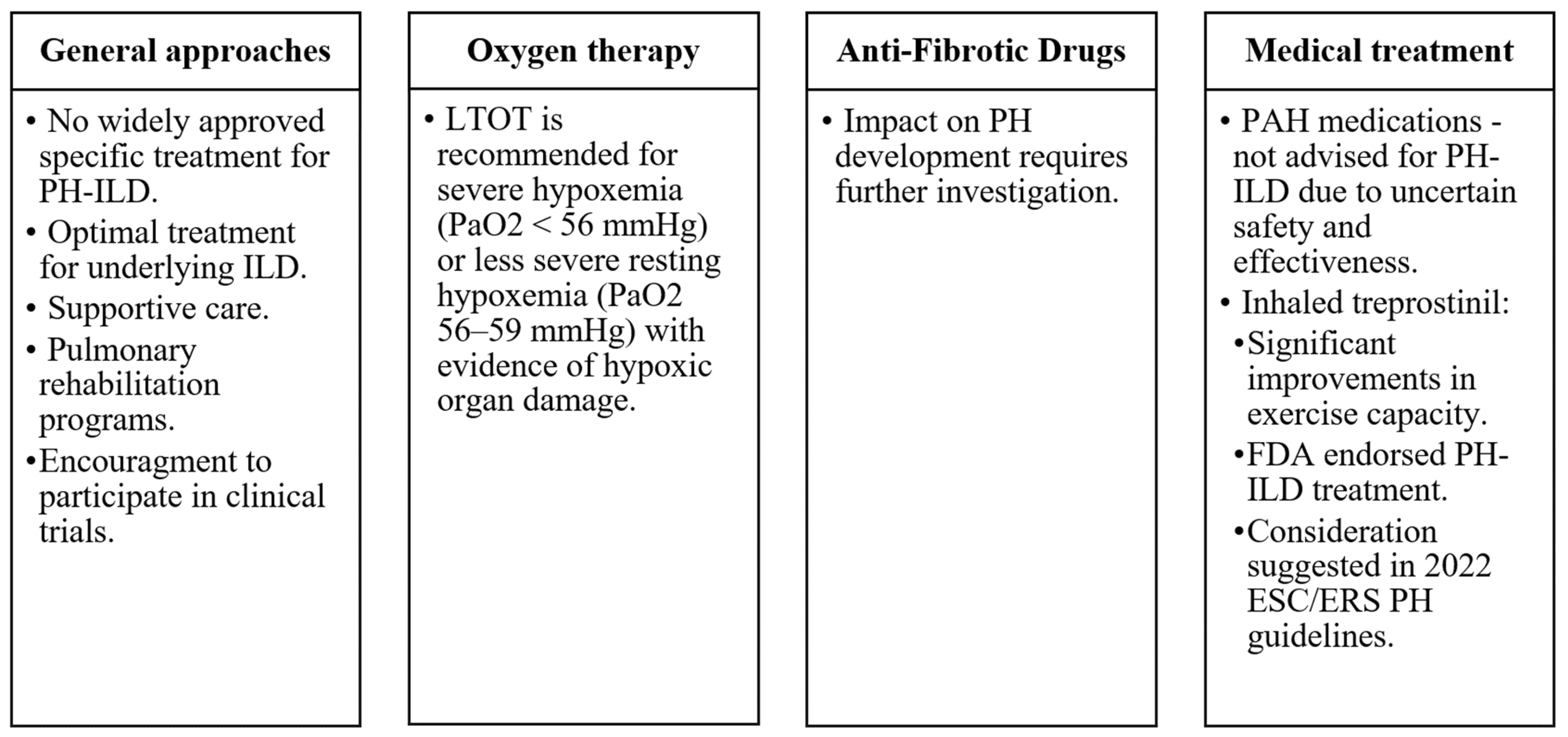
8. Management and Treatment
9. Prognosis and Outcomes
This entry is adapted from the peer-reviewed paper 10.3390/medicina60010058
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023, 61, 2200879.
- Teramachi, R.; Taniguchi, H.; Kondoh, Y.; Ando, M.; Kimura, T.; Kataoka, K.; Suzuki, A.; Furukawa, T.; Sakamoto, K.; Hasegawa, Y. Progression of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis with mild to moderate restriction. Respirology 2017, 22, 986–990.
- Olsson, K.M.; Hoeper, M.M.; Pausch, C.; Grünig, E.; Huscher, D.; Pittrow, D.; Rosenkranz, S.; Gall, H. Pulmonary vascular resistance predicts mortality in patients with pulmonary hypertension associated with interstitial lung disease: Results from the COMPERA registry. Eur. Respir. J. 2021, 58, 2101483.
- Shorr, A.F.; Wainright, J.L.; Cors, C.S.; Lettieri, C.J.; Nathan, S.D. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur. Respir. J. 2007, 30, 715–721.
- Andersen, C.U.; Mellemkjær, S.; Hilberg, O.; Nielsen-Kudsk, J.E.; Simonsen, U.; Bendstrup, E. Pulmonary hypertension in interstitial lung disease: Prevalence, prognosis and 6 min walk test. Respir. Med. 2012, 106, 875–882.
- Pulito-Cueto, V.; Genre, F.; López-Mejías, R.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Portilla, V.; Sebastián Mora-Gil, M.; Ocejo-Vinyals, J.G.; Gualillo, O.; Blanco, R.; et al. Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 1275.
- Yanagihara, T.; Tsubouchi, K.; Zhou, Q.; Chong, M.; Otsubo, K.; Isshiki, T.; Schupp, J.C.; Sato, S.; Scallan, C.; Upagupta, C.; et al. Vascular–Parenchymal Cross-Talk Promotes Lung Fibrosis through BMPR2 Signaling. Am. J. Respir. Crit. Care Med. 2023, 207, 1498–1514.
- Samarelli, A.V.; Tonelli, R.; Marchioni, A.; Bruzzi, G.; Gozzi, F.; Andrisani, D.; Castaniere, I.; Manicardi, L.; Moretti, A.; Tabbì, L.; et al. Fibrotic Idiopathic Interstitial Lung Disease: The Molecular and Cellular Key Players. Int. J. Mol. Sci. 2021, 22, 8952.
- Miądlikowska, E.; Rzepka-Wrona, P.; Miłkowska-Dymanowska, J.; Białas, A.J.; Piotrowski, W.J. Review: Serum Biomarkers of Lung Fibrosis in Interstitial Pneumonia with Autoimmune Features—What Do We Already Know? J. Clin. Med. 2021, 11, 79.
- Borek, I.; Birnhuber, A.; Voelkel, N.F.; Marsh, L.M.; Kwapiszewska, G. The vascular perspective on acute and chronic lung disease. J. Clin. Investig. 2023, 133, e170502.
- Darwiche, T.; Collum, S.D.; Bi, W.; Reynolds, J.O.; Wilson, C.; Wareing, N.; Hernandez, A.M.; Mertens, T.C.J.; Zhou, Z.; Pandit, L.M.; et al. Alterations in cardiovascular function in an experimental model of lung fibrosis and pulmonary hypertension. Exp. Physiol. 2019, 104, 568–579.
- Dotan, Y.; Stewart, J.; Gangemi, A.; Wang, H.; Aneja, A.; Chakraborty, B.; Dass, C.; Zhao, H.; Marchetti, N.; D’Alonzo, G.; et al. Pulmonary vasculopathy in explanted lungs from patients with interstitial lung disease undergoing lung transplantation. BMJ Open Respir. Res. 2020, 7, e000532.
- Colombat, M.; Mal, H.; Groussard, O.; Capron, F.; Thabut, G.; Jebrak, G.; Brugière, O.; Dauriat, G.; Castier, Y.; Lesèche, G.; et al. Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: Histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum. Pathol. 2007, 38, 60–65.
- Kylhammar, D.; Rådegran, G. The principal pathways involved in the in vivo modulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol. 2017, 219, 728–756.
- Maimon, N.; Salz, L.; Shershevsky, Y.; Matveychuk, A.; Guber, A.; Shitrit, D. Sarcoidosis-associated pulmonary hypertension in patients with near-normal lung function. Int. J. Tuberc. Lung Dis. 2013, 17, 406–411.
- Duong, H.T.; Bonham, C.A. Sarcoidosis-associated Pulmonary Hypertension: Pathophysiology, Diagnosis, and Treatment. Clin. Pulm. Med. 2018, 25, 52–60.
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913.
- Collum, S.D.; Amione-Guerra, J.; Cruz-Solbes, A.S.; DiFrancesco, A.; Hernandez, A.M.; Hanmandlu, A.; Youker, K.; Guha, A.; Karmouty-Quintana, H. Pulmonary Hypertension Associated with Idiopathic Pulmonary Fibrosis: Current and Future Perspectives. Can. Respir. J. 2017, 2017, 1430350.
- Klinger, J.R. Group III Pulmonary Hypertension. Cardiol. Clin. 2016, 34, 413–433.
- Karampitsakos, T.; Tzouvelekis, A.; Chrysikos, S.; Bouros, D.; Tsangaris, I.; Fares, W.H. Pulmonary hypertension in patients with interstitial lung disease. Pulm. Pharmacol. Ther. 2018, 50, 38–46.
- Hachulla, E.; Gressin, V.; Guillevin, L.; Carpentier, P.; Diot, E.; Sibilia, J.; Kahan, A.; Cabane, J.; Francès, C.; Launay, D.; et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: A French nationwide prospective multicenter study. Arthritis Rheum. 2005, 52, 3792–3800.
- Vonk, M.C.; Broers, B.; Heijdra, Y.F.; Ton, E.; Snijder, R.; van Dijk, A.P.J.; van Laar, J.M.; Bootsma, H.; van Hal, P.T.W.; van den Hoogen, F.H.J.; et al. Systemic sclerosis and its pulmonary complications in The Netherlands: An epidemiological study. Ann. Rheum. Dis. 2009, 68, 961–965.
- Phung, S.; Strange, G.; Chung, L.P.; Leong, J.; Dalton, B.; Roddy, J.; Deague, J.; Playford, D.; Musk, M.; Gabbay, E. Prevalence of pulmonary arterial hypertension in an Australian scleroderma population: Screening allows for earlier diagnosis. Intern. Med. J. 2009, 39, 682–691.
- Avouac, J.; Airò, P.; Meune, C.; Beretta, L.; Dieude, P.; Caramaschi, P.; Tiev, K.; Cappelli, S.; Diot, E.; Vacca, A.; et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J. Rheumatol. 2010, 37, 2290–2298.
- Morrisroe, K.; Stevens, W.; Sahhar, J.; Rabusa, C.; Nikpour, M.; Proudman, S.; Australian Scleroderma Interest Group (ASIG). Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: Results from a real-life screening programme. Arthritis Res. Ther. 2017, 19, 42.
- Coghlan, J.G.; Denton, C.P.; Grünig, E.; Bonderman, D.; Distler, O.; Khanna, D.; Müller-Ladner, U.; Pope, J.E.; Vonk, M.C.; Doelberg, M.; et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: The DETECT study. Ann. Rheum. Dis. 2014, 73, 1340–1349.
- Chauvelot, L.; Gamondes, D.; Berthiller, J.; Nieves, A.; Renard, S.; Catella-Chatron, J.; Ahmad, K.; Bertoletti, L.; Camara, B.; Gomez, E.; et al. Hemodynamic Response to Treatment and Outcomes in Pulmonary Hypertension Associated With Interstitial Lung Disease Versus Pulmonary Arterial Hypertension in Systemic Sclerosis: Data From a Study Identifying Prognostic Factors in Pulmonary Hypertension Associated With Interstitial Lung Disease. Arthritis Rheumatol. 2021, 73, 295–304.
- Piccari, L.; Allwood, B.; Antoniou, K.; Chung, J.H.; Hassoun, P.M.; Nikkho, S.M.; Saggar, R.; Shlobin, O.A.; Vitulo, P.; Nathan, S.D.; et al. Pathogenesis, clinical features, and phenotypes of pulmonary hypertension associated with interstitial lung disease: A consensus statement from the Pulmonary Vascular Research Institute’s Innovative Drug Development Initiative—Group 3 Pulmonary Hypertension. Pulm. Circ. 2023, 13, e12213.
- Hage, R.; Gautschi, F.; Steinack, C.; Schuurmans, M.M. Combined Pulmonary Fibrosis and Emphysema (CPFE) Clinical Features and Management. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 167–177.
- Ryerson, C.J.; Hartman, T.; Elicker, B.M.; Ley, B.; Lee, J.S.; Abbritti, M.; Jones, K.D.; King, T.E.; Ryu, J.; Collard, H.R. Clinical Features and Outcomes in Combined Pulmonary Fibrosis and Emphysema in Idiopathic Pulmonary Fibrosis. Chest 2013, 144, 234–240.
- Tomioka, H.; Mamesaya, N.; Yamashita, S.; Kida, Y.; Kaneko, M.; Sakai, H. Combined pulmonary fibrosis and emphysema: Effect of pulmonary rehabilitation in comparison with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2016, 3, e000099.
- Sato, T.; Tsujino, I.; Tanino, M.; Ohira, H.; Nishimura, M. Broad and heterogeneous vasculopathy in pulmonary fibrosis and emphysema with pulmonary hypertension. Respirol. Case Rep. 2013, 1, 10–13.
- Cottin, V.; Le Pavec, J.; Prevot, G.; Mal, H.; Humbert, M.; Simonneau, G.; Cordier, J.-F. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur. Respir. J. 2010, 35, 105–111.
- Oliveira, R.P.; Ribeiro, R.; Melo, L.; Grima, B.; Oliveira, S.; Alves, J.D. Connective tissue disease-associated interstitial lung disease. Pulmonology 2022, 28, 113–118.
- Saggar, R.; Giri, P.C.; Deng, C.; Johnson, D.; McCloy, M.K.; Liang, L.; Shaikh, F.; Hong, J.; Channick, R.N.; Shapiro, S.S.; et al. Significance of autoimmune disease in severe pulmonary hypertension complicating extensive pulmonary fibrosis: A prospective cohort study. Pulm. Circ. 2021, 11, 1–12.
- Cottin, V.; Harari, S.; Humbert, M.; Mal, H.; Dorfmüller, P.; Jaïs, X.; Reynaud-Gaubert, M.; Prevot, G.; Lazor, R.; Taillé, C.; et al. Pulmonary hypertension in lymphangioleiomyomatosis: Characteristics in 20 patients. Eur. Respir. J. 2012, 40, 630–640.
- Freitas, C.S.G.; Baldi, B.G.; Jardim, C.; Araujo, M.S.; Sobral, J.B.; Heiden, G.I.; Kairalla, R.A.; Souza, R.; Carvalho, C.R.R. Pulmonary hypertension in lymphangioleiomyomatosis: Prevalence, severity and the role of carbon monoxide diffusion capacity as a screening method. Orphanet J. Rare Dis. 2017, 12, 74.
- Wu, X.; Xu, W.; Wang, J.; Tian, X.; Tian, Z.; Xu, K. Clinical characteristics in lymphangioleiomyomatosis-related pulmonary hypertension: An observation on 50 patients. Front. Med. 2019, 13, 259–266.
- Bhattacharyya, P.; Saha, D.; Bhattacherjee, P.; Das, S.; Bhattacharyya, P.; Dey, R. Tuberculosis associated pulmonary hypertension: The revelation of a clinical observation. Lung India 2016, 33, 135.
- Park, S.Y.; Lee, S.M.; Shin, J.W.; Choi, B.W.; Kim, H.; Lee, J.S.; Lee, S.D.; Park, S.S.; Moon, H.S.; Park, Y.B. Epidemiology of chronic thromboembolic pulmonary hypertension in Korea: Results from the Korean registry. Korean J. Intern. Med. 2016, 31, 305–312.
- Walsh, K.F.; Lui, J.K. Post-tuberculosis pulmonary hypertension: A case of global disparity in health care. Lancet Glob. Health 2022, 10, e476.
- DuBrock, H.M.; Nathan, S.D.; Reeve, B.B.; Kolaitis, N.A.; Mathai, S.C.; Classi, P.M.; Nelsen, A.C.; Olayinka-Amao, B.; Norcross, L.N.; Martin, S.A. Pulmonary hypertension due to interstitial lung disease or chronic obstructive pulmonary disease: A patient experience study of symptoms and their impact on quality of life. Pulm. Circ. 2021, 11, 1–9.
- Nikkho, S.M.; Richter, M.J.; Shen, E.; Abman, S.H.; Antoniou, K.; Chung, J.; Fernandes, P.; Hassoun, P.; Lazarus, H.M.; Olschewski, H.; et al. Clinical significance of pulmonary hypertension in interstitial lung disease: A consensus statement from the Pulmonary Vascular Research Institute’s innovative drug development initiative—Group 3 pulmonary hypertension. Pulm. Circ. 2022, 12, e12127.
- Hoeper, M.M.; Lee, S.H.; Voswinckel, R.; Palazzini, M.; Jais, X.; Marinelli, A.; Barst, R.J.; Ghofrani, H.A.; Jing, Z.-C.; Opitz, C.; et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J. Am. Coll. Cardiol. 2006, 48, 2546–2552.
- Kogan, E.; Didden, E.-M.; Lee, E.; Nnewihe, A.; Stamatiadis, D.; Mataraso, S.; Quinn, D.; Rosenberg, D.; Chehoud, C.; Bridges, C. A machine learning approach to identifying patients with pulmonary hypertension using real-world electronic health records. Int. J. Cardiol. 2023, 374, 95–99.
- Kwon, J.; Kim, K.-H.; Medina-Inojosa, J.; Jeon, K.-H.; Park, J.; Oh, B.-H. Artificial intelligence for early prediction of pulmonary hypertension using electrocardiography. J. Heart Lung Transplant. 2020, 39, 805–814.
- Kusunose, K.; Hirata, Y.; Tsuji, T.; Kotoku, J.; Sata, M. Deep learning to predict elevated pulmonary artery pressure in patients with suspected pulmonary hypertension using standard chest X ray. Sci. Rep. 2020, 10, 19311.
- Sprecher, V.P.; Didden, E.; Swerdel, J.N.; Muller, A. Evaluation of code-based algorithms to identify pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension patients in large administrative databases. Pulm. Circ. 2020, 10, 2045894020961713.
- Waxman, A.B.; Elia, D.; Adir, Y.; Humbert, M.; Harari, S. Recent advances in the management of pulmonary hypertension with interstitial lung disease. Eur. Respir. Rev. 2022, 31, 210220.
- Cordeiro, R.; Nunes, A.; Smith, O.; Renzoni, E.A. Oxygen in interstitial lung diseases. Breathe 2023, 19, 220271.
- Dowman, L.; Hill, C.J.; May, A.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2021, 2, CD006322.
- Kapnadak, S.G.; Raghu, G. Lung transplantation for interstitial lung disease. Eur. Respir. Rev. 2021, 30, 210017.
- Zhao, N.; Chen, J.; Zhang, M.; Zhou, L.; Liu, L.; Yuan, J.; Pang, X.; Hu, D.; Ren, X.; Jin, Z. PAH-specific therapy for pulmonary hypertension and interstitial lung disease: A systemic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 992879.
- King, C.S.; Shlobin, O.A. The Trouble With Group 3 Pulmonary Hypertension in Interstitial Lung Disease. Chest 2020, 158, 1651–1664.
- Idiopathic Pulmonary Fibrosis Clinical Research Network; Zisman, D.A.; Schwarz, M.; Anstrom, K.J.; Collard, H.R.; Flaherty, K.R.; Hunninghake, G.W. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N. Engl. J. Med. 2010, 363, 620–628.
- Raghu, G. Treatment of Idiopathic Pulmonary Fibrosis With Ambrisentan: A Parallel, Randomized Trial. Ann. Intern. Med. 2013, 158, 641.
- Nathan, S.; Behr, J.; Collard, H.R.; Cottin, V.; Hoeper, M.M.; Martinez, F.; Corte, T.; Keogh, A.; Leuchte, H.; Mogulkoc, N.; et al. RISE-IIP: Riociguat for the treatment of pulmonary hypertension associated with idiopathic interstitial pneumonia. Eur. Respir. Soc. 2017, 50 (Suppl. 61), OA1985.
- Kimura, M.; Taniguchi, H.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Nishiyama, O.; Aso, H.; Sakamoto, K.; Hasegawa, Y. Pulmonary Hypertension as a Prognostic Indicator at the Initial Evaluation in Idiopathic Pulmonary Fibrosis. Respiration 2013, 85, 456–463.
- Hayes, D.; Black, S.M.; Tobias, J.D.; Kirkby, S.; Mansour, H.M.; Whitson, B.A. Influence of Pulmonary Hypertension on Patients With Idiopathic Pulmonary Fibrosis Awaiting Lung Transplantation. Ann. Thorac. Surg. 2016, 101, 246–252.
- Suzuki, A.; Taniguchi, H.; Watanabe, N.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Matsuda, T.; Yokoyama, T.; Sakamoto, K.; Nishiyama, O.; et al. Significance of pulmonary arterial pressure as a prognostic indicator in lung-dominant connective tissue disease. PLoS ONE 2014, 9, e108339.
- Kessler, R.; Faller, M.; Weitzenblum, E.; Chaouat, A.; Aykut, A.; Ducoloné, A.; Ehrhart, M.; Oswald-Mammosser, M. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 2001, 164, 219–224.
