1. The Types and Principles of Enzyme Electrode Electrochemical Gas Sensor
1.1. Three-Electrode Enzyme Electrochemical Gas Sensor
The three-electrode system is a classic biochemical detection technology, which has been applied for the transduction of electric signals of enzyme electrochemical gas sensors. The structure of a miniaturized planar electrochemical gas sensor is shown in
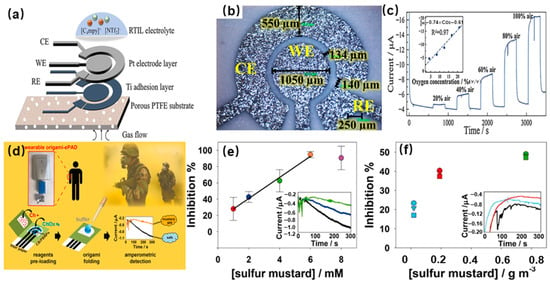
Figure 1a. This system consists of a working electrode (WE) to identify the target gas molecules, a counter electrode (CE) that acts as a current source, a reference electrode (RE) that applies a stabilizing potential, and liquid electrolytes
[1]. The current signal is generated by an electrochemical reaction on the WE and is used for the quantification of target gas molecules. The structure image of the electrodes by optical microscope is shown in
Figure 1b. The diameter of the WE is 1050 μm, and the width of the CE and RE is 550 μm. The gap between the WE and CE is about 130 μm, and the gap between the RE and CE is 140 μm. The detection principle of the enzyme electrode electrochemical sensor for gas molecules is described thoughtfully. Firstly, the enzyme is immobilized on the surface of the WE. Secondly, gas molecule measurements are performed by immersing the three-electrode system in an electrolyte. The enzyme catalyzes the oxidation or reduction of target gas molecules, resulting in the transfer of electrons. These electrons are then transferred to the WE, leading to the production of a current that is directly related to the concentration of target gas molecules
[2]. The miniaturized planar electrochemical gas sensor was applied for the detection of oxygen. The results in
Figure 1c show the sensor can rapidly respond to a series of oxygen concentrations. The inset of
Figure 1c shows the plot of current vs. oxygen concentration. The sensitivity of the sensor is 0.74 μA/[% oxygen], and the linearity is 0.97.
Three electrode electrochemical enzyme gas sensors are extensively utilized for gas detection, such as the formaldehyde dehydrogenase for the detection of formaldehyde
[3]. The alcohol oxidase was utilized for the monitoring of ethanol
[4]. The enzyme electrode of hydrogenase was designed for the detection of hydrogen
[5]. An electrochemical biosensor has been applied for the detection of sulfur mustard, which is one of the most dangerous and extensively used chemical warfare agents
[6]. A novel wearable electrochemical biosensor was prepared using the following steps (
Figure 1d). First, the filter paper-based chemical electrodes were produced by screen-printing. Second, both choline chloride (Ch) and choline oxidase enzyme (ChOx) solutions were separately preloaded. Finally, the enzymatic reaction was activated by adding phosphate buffer solution (PBS). Sulfur mustard (SM) agent detection was carried out by monitoring their inhibitory effects toward the choline oxidase enzyme, through the amperometric measurement of the enzymatic byproduct hydrogen peroxide. The origami-like devices used to create a wearable, ready, and easy-to-use electrochemical PAD (origami-ePAD) were applied for the detection of the standard solution of SM in a liquid phase. As shown in
Figure 1e, a linear between 1 and 6 mM was obtained. The inset shows the corresponding current (E = 0.0 V and t = 300 s) obtained from the detection of H
2O
2 enzymatic byproduct in the absence (black) and the presence of SM concentrations equal to 2 mM and 4 mM. This result demonstrated that the newly developed origami-ePAD is suitable for SM detection in the liquid phase. Meanwhile, the suitability of developed origami-ePAD for the detection of aerosolized SM was verified. As shown in
Figure 1f, current values were sampled three times to evaluate the extent of inhibition at different exposure times, indicating the sensor can alert for the presence of airborne SM in only 60 s. This analytical approach provides a strategy applicable to the real-time monitoring of a variety of chemical weapon threats.
Figure 1. (
a) The structure schematic of the electrochemical gas sensor; (
b) The image of the electrodes by optical microscope; (
c) The current response of miniaturized planar electrochemical gas sensor to oxygen
[1]; (
d) The preparation process and working principle of a wearable origami-like paper-based electrochemical biosensor for sulfur mustard detection; (
e) The detection results of origami-ePAD for the standard solution of SM in liquid phase (absence (black) and in presence of SM concentrations equal to 2 mM and 4 mM); (
f) The detection results of origami-ePAD for the aerosolized SM(absence (black line) and in presence of SM concentrations equal to 0.019 g/m
3 and 0.76 g/m
3)
[6].
1.2. Field-Effect Transistor (FET) Enzyme Electrochemical Gas Sensor
The field effect transistor serves as a platform for chemical detection. This is built upon an electrical device, utilizing the electric field effect of the control input circuit to regulate the output circuit’s current. This concept has garnered significant interest in the realm of research
[7]. It has three electrodes: drain (D), source (S), and gate (G)
[8]. Among them, a conductive channel composed of semiconductors is formed between the drain and the source, and the carriers in the channel can be controlled by adjusting the electric field of the gate, thus controlling the current between the drain and the source. Due to the advantages, including higher sensitivity and high integration real-time detection, FET has been used in many fields to detect various types of analytes such as proteins, gas, small molecules, etc. However, the design of the gas-sensing interface disturbs the development of the FET enzyme electrochemical gas sensor. To the best of our knowledge, prior research into the electrochemical gas sensor based on the FET enzyme remains limited.
Vianello et al.
[9] designed an ion-sensitive field-effect transistor (ISFET) in conjunction with an aldehyde dehydrogenase specific for the detection of formaldehyde. The silicon nitride is applied for the gate dielectric of silicon-integrated ISFET. The formaldehyde dehydrogenase enzyme is immobilized on the surface of silicon nitride through covalent binding. In the sensing process, the nicotinamide adenine dinucleotide (NAD) was applied as a cofactor, catalyzing the oxidation of the formaldehyde molecule. The detection limit of this sensor is about 0.1 ppm. Zhao et al.
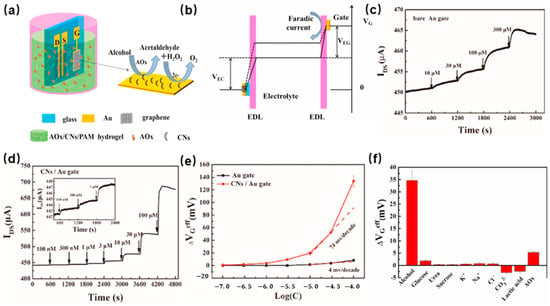
[10] developed a breath alcohol biosensor based on a hydrogel-gated graphed field-effect transistor. The carbon nanosheets (CNs) were synthesized through fast pyrolysis of chlorella. Then, alcohol oxidase (AOx) was introduced into the substrate to detect alcohol in real time. The structure of the hydrogel-gated graphed field-effect transistor is shown in
Figure 2a. There are three electrodes, namely gate, source, and drain, which contact the hydrogel. Due to the high carrier mobility feature of graphene enabling a significant field-effect amplification of the sensor, that was applied to construct the channel of the source and drain electrodes, the carbon nanosheets were employed to modify the gate electrode. The alcohol-sensing mechanism of the sensor depends on the oxidation reaction of alcohol by AOx and the electrocatalytic oxidation reaction of the generated H
2O
2. In detail, the enzymatically produced H
2O
2 will be oxidized on the surface of the Au gate electrode under a bias voltage. In this reaction, the electrons will be transferred to the gate electrode, and the Faradaic current will be generated by the redox reaction of H
2O
2 on the gate surface, which will cause the change of effective gate voltage, as shown in
Figure 2b. The channel current response to H
2O
2 for the bare Au gate and CN-modified Au gate, as shown in
Figure 2c,d. The CN-modified Au gate field-effect transistor alcohol oxidase electrochemical gas sensor exhibits a better sensitivity and lower limit of detection to 100 nM, 100 times lower than that of the device with a bare Au gate. As shown in
Figure 2e, the effective gate voltage change vs. H
2O
2 concentration in logarithm. The CN-modified gate SGGT shows a response of 74 mV per decade, which is higher than that of the bare Au gate SGGT (4 mV per decade). Meanwhile, the CN-modified Au gate exhibits a dramatic enhancement in the current response. In addition, to verify the sensor’s specificity, interfering components were used in the detection of alcohol. As
Figure 2f shows, the AOx/CN-modified gate sensor demonstrates excellent selectivity to alcohol. This sensor has been successfully applied to breath tests after alcohol drinking.
Figure 2. (
a) Schematic of the solution-gated graphene transistors (SGGT) modified with AOx/CNs in a polyacrylamide hydrogel-based system; (
b) The change in potential between the gate and channel before (solid line) and after (dotted line) the addition of alcohol in PBS solution; (
c) the detection of increasing H
2O
2 concentration in PBS solution by a bare Au gate; (
d) the detection of increasing concentration of H
2O
2 in PBS solution by CN-modified Au gate; (
e) Change in effective gate voltage of the SGGT with unmodified Au gate and CN-modified Au gate vs. the logarithm of H
2O
2 concentration; (
f) The selectivity of the SGGT device with the AOx/CN-modified gate
[10].
2. The Immobilization Method of Enzyme
The sensing interface plays a critical role in the electron transfer for enzyme catalysts. The process of enzyme sensing can be divided into two parts: gas molecular recognition, which correlates with the enzymes catalyzing gaseous chemical reactions; and signal transduction, which is related to the mechanism of enzymatic reaction. In consideration of electron transfer between an electrode and enzyme biocatalyst, it is important to rationally design an electrode surface to optimize the interaction between the enzyme and the electrode surface. Since enzymes that are used in gas biosensors are not in their natural surroundings, they tend to be less stable due to the changes in environmental conditions. If the bare enzyme (the enzyme that has not been immobilized in the substances) is modified on the electrode surface, the load amount of the enzyme becomes smaller, resulting in reduced enzyme activity. Furthermore, the electron transfer efficiency between the enzyme and the electrode will decrease. In the condition of enzyme gas sensor manufacturing, immobilization of the enzyme can enable close contact between the enzyme and the electrode surface while preserving the catalytic competence and avoiding the leakage of the enzyme into the sample. Meanwhile, in the process of enzyme immobilization, the introduction of substances should be avoided to block the specific sites of gas adsorption.
The most common method currently used to address this is by immobilizing the enzyme onto the electrode surface. One approach to solving this problem is immobilizing enzymes in polymers. For example, polystyrene sulfonate can be dropped onto the electrode surface to immobilize oxidases
[11], polyethyleneimine was applied to immobilize enzymes on agarose gels
[12], bilirubin oxidase was introduced into a Nafion and crosslinked with glutaraldehyde to form a stable electrochemical interface
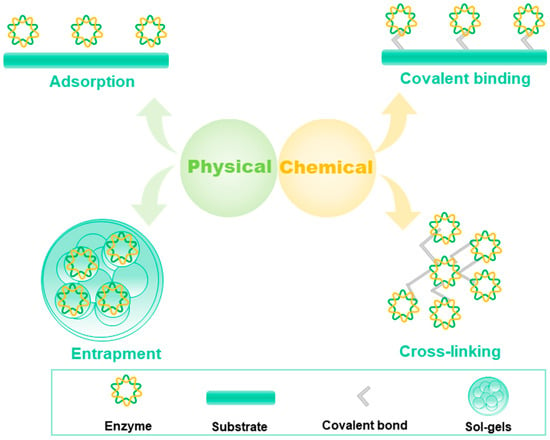
[13]. The immobilization of enzymes can be classified as physical and chemical methods
[14]. Adsorption
[15], entrapment, and encapsulation are physical immobilization methods
[16], whereas covalent bonding
[17], crosslinking, and electrostatic attraction are classified as chemical immobilization methods (
Figure 3).
Figure 3. Physical and chemical methods for immobilization of enzymes.
2.1. Physical Adsorption
Physical adsorption is based on van der Waals attractive forces between enzymes and electrodes, which is one of the first used and the simplest methods for enzyme immobilization
[18]. It is normally sufficient for short-term studies but makes the enzyme electrode easily polluted. Jiang et al.
[19] prepared an amperometric ethanol biosensor by integrating alcohol dehydrogenase with mediator meldola’s blue (MB). Based on the strong π–π stacking interaction between the aromatic group of MB and the graphene, MB was adsorbed on the surface of carbon nanotubes, and the loading, electron transfer kinetics of MB, and stability were improved. By adsorbing MB onto the carbon nanotube surface, the loading, electron transfer kinetics of MB, and stability were enhanced. The alcohol dehydrogenase-based sensor demonstrated a lower detection limit of 19.1
± 0.58 μM and excellent selectivity. Unfortunately, the activity of the dehydrogenase sensor was only one week. In a separate study, Kundu et al [20]. developed an electrochemical formaldehyde dehydrogenase (FDH) enzyme sensor based on a screen-printed electrode for formaldehyde detection in corn. The formaldehyde dehydrogenase enzyme was immobilized on the α-Fe2O3/ITO electrode surface through physical adsorption. The fabrication process of the screen-printed electrode-based enzyme sensor involved preparing 𝛼-Fe2O3/ITO through electrophoretic deposition, followed by the addition of the enzyme onto the electrode surface and overnight incubation. Additionally, a cofactor (NAD+) was used in conjunction with FDH to enhance stability during the enzyme-catalyzed reaction. Subsequently, the enzyme electrode was treated with bovine serum albumin solution (BSA) to block nontargeted sites on the electrode surface. The enzyme sensor exhibited high sensitivity and low detection limits (0.03 mg/L). This immobilization method is simple and does not require chemical reactions, but the enzyme is easily inactivated.
2.2. Entrapment in Sol-Gels
Entrapment in sol–gels is as mild as adsorption, which is one of the major approaches for the immobilization of enzymes. This method involves caging the enzyme within a polymeric network through the formation of covalent or noncovalent bonds, which allow the passage of substrate and products but retain the enzyme. However, the two major issues obstruct the development of entrapment-based electrochemical enzyme sensors. One is the leaking of enzymes, and the other is the sluggish substrate–enzyme active site mass transfer
[21].
To address these challenges, Adhikari et al.
[22] proposed a new facile enzyme entrapment, a special cationic polymer poly(2-(dimethylamino)ethyl methacrylate) (MADQUAT) on single-wall carbon nanotube and reduced the graphene oxide (SWCNT–rGO) thin film to form an entrapment platform. Subsequently, alcohol dehydrogenase (ADH) is immobilized into the entrapment platform by the strong electrostatic affinity for the detection of ethanol. The entrapped alcohol dehydrogenase enzyme exhibits a high ability to transfer electrons and significantly enhances the enzyme catalytic activity. The developed ethanol sensor exhibits high sensitivity (26.27 μA mM
−1 cm
−2), and a low limit of detection (0.16 μM). Istrate et al.
[23] designed an alcohol sensor by entrapping alcohol dehydrogenase into sol–gel matrix that was immobilized on the surface of the screen-printed electrode (SPE) modified with poly(allylamine hydrochloride). Das et al.
[24] developed a direct electrochemistry enzyme sensor for the detection of alcohol. An alcohol oxidase (AOx) was immobilized on a multiwalled carbon nanotubes-Nafion (MWCNT-Nf) matrix and encapsulated with polyethylenimine (PEI) on the gold electrode (AuE). The surface morphology of bare Au shows a homogenous surface. MWCNTs with porous morphology uniform distribution on the electrode surface. When AOx was added to this film, the porosity disappeared, indicating the AOx was immobilized on the MWCNT-Nf film. Then, the alcohol oxidase (AOx) was immobilized on multiwalled carbon nanotubes-Nafion and encapsulated with polyethyleneimine (PEI) on the surface of the electrode. The electron was transferred directly between the AOx and the electrode. Meanwhile, the entrapped AOx presented good bioactivity and electrocatalytic activity. The AOx enzyme sensor has a rapid response time of 55 s, a low detection limit of 5 μM, and exhibits potential applications for detecting alcohol in real samples. Hiroyuki et al.
[25] designed a choline oxidase-based choline vapor sensor, which was fabricated by entrapping the choline oxidase in sol–gels on a Clark-type dissolved oxygen electrode, this sensor shows excellent choline gas-sensing performances. These findings provide a new paradigm for the design of enzyme electrodes. This method can protect the enzyme from the external environment but may reduce the reaction rate of the enzyme.
2.3. Covalent Coupling
Covalent coupling is one of the most promising methods in the process of enzyme immobilization for the design and construction of enzyme-based electrochemical sensors. Covalent coupling provides a potential way to preserve enzyme activity for a long period. This method mainly depends on the formation of a covalent bond between the enzyme and the support material. Soylemez et al.
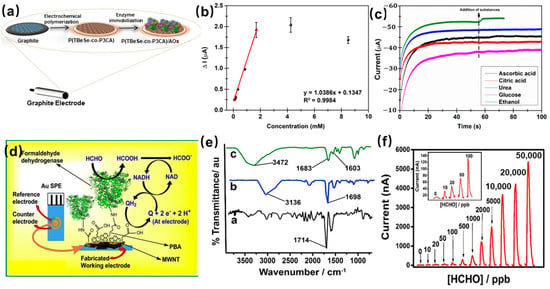
[26] designed a newly synthesized copolymer with enhanced enzyme-sensing properties as a novel sensor for the detection of ethanol. The conjugated copolymer (TBeSe-co-P3CA) was prepared on the surface of the electrode by electrochemical polymerization. After the alcohol oxidase (AOx) was immobilized through the covalent linkage between the enzyme’s amino group and the carboxyl group derived from P3CA, the schematic of the sensing device and the preparation process is shown in
Figure 4a. The prepared sensor exhibits excellent ethanol sensing performance, as
Figure 4b shows; the amperometric response increases and then reaches the steady-state value, the limit of detection is 0.37 mM. Meanwhile, the sensor specificity was measured against interfering compounds, including ascorbic acid, citric acid, urea, glucose, and ethanol. It can be seen from
Figure 4c that the sensor exhibits a distinct change of current to ethanol, indicating excellent specificity. Soylemez et al.
[27] constructed the enzyme electrode by immobilizing the AOx onto the carbon nanotubes modified conducting polymer with the help of EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide)/NHS (N-hydroxysuccinimide) crosslinking, the preparation of biosensor. EDC and NHS were used to activate the free carboxylic acid groups of the conducting nanotube, and the amide bond formed between enzyme molecules and carbon nanotubes for the covalent attachment of enzymes. The XPS result confirmed the formation of the covalent bond. This alcohol oxidase-based sensor has been successfully used for the detection of alcohol, with a lower limit of detection is 0.17 μM.
Figure 4. (
a) Schematic of sensing device containing graphite electrode, a network of polymer and AOx; (
b) Amperometric response of the alcohol sensors to increasing ethanol concentrations; (
c) Effect of interfering substances on biosensor performance
[26]; (
d) the immobilization of the formaldehyde dehydrogenase and the mechanism for catalyzing formaldehyde; (
e) The FTIR spectra of formaldehyde dehydrogenase immobilized on pyrenyl carbon nanostructures; (
f) Amperometric responses for various concentration of formaldehyde
[28].
The other example of an enzyme immobilized by covalent coupling is as follows: the first pyrenyl carbon nanostructure-based enzyme gas sensor for urine formaldehyde quantitation was fabricated by Gayan et al.
[28]. The NAD+-dependent formaldehyde dehydrogenase (FDH) was immobilized by covalent coupling on the surface of the carboxylated multiwalled carbon nanotubes stacked with π–π 1-pyrenebutyric acid units, the fabrication steps, and the mechanism for catalyzing formaldehyde as
Figure 4d shows. EDC and NHS were used to activate the free carboxylic acid groups of the conducting nanotube, and the amide bond formed between enzyme molecules and carboxylated multi-walled carbon nanotubes for the covalent attachment of enzymes. The FTIR spectra results confirmed the formation of the covalent bond (
Figure 4e). The appearance of a new board peak at 3472 cm
−1, indicates the covalent immobilization via the formation of an amide bond between the carboxylated multiwalled carbon nanotubes and formaldehyde dehydrogenase. This sensor shows an excellent formaldehyde-sensing performance, as
Figure 4f shows. The biosensor provides an ideal immobilization matrix for the formaldehyde dehydrogenase and has been successfully used for the detection of formaldehyde samples in urine with satisfactory results. However, the fabricated formaldehyde dehydrogenase-based sensor, with a short lifetime of 30 h, is under continuous testing. In conclusion, this method can immobilize the enzyme stably but may affect the activity and stability of the enzyme.
2.4. Crosslinking Method
Crosslinking is a method of immobilizing enzymes on a carrier surface. In this method, chemical crosslinking agents are used to bind the enzyme to the functional groups on the carrier surface, forming a chemical bond to immobilize the enzyme. Glutaraldehyde is one of the commonly used crosslinking agents. Enzyme–chemical crosslinking has advantages such as high efficiency of enzyme immobilization, strong binding force between the enzyme and the carrier, and good stability. Additionally, this method can control the density and position of the immobilized enzyme and adjust the chemical properties of the carrier surface to further improve the catalytic efficiency and stability of the enzyme.
Razmshoar et al.
[29] created a conductometric enzymatic methanol sensor by grafting alcohol oxidase (AOx) onto electrospun polystyrene-poly(amidoamine) dendritic polymer nanofibers. To form a strong link between AOx and the surface of nanofibers, glutaraldehyde coupling was used to form a bond between the amine groups of the AOx enzyme and the nanofiber dendritic polymer groups (
Figure 5a). The FTIR spectra, which were used to evaluate the chemical structure, showed a peak at 1674 cm
−1. This peak is assigned to the
C=Ngroup and confirms the covalent link between the polystyrene-poly(amidoamine) and the glutaraldehyde. Notably, this peak is due to the binding between the AOx and glutaraldehyde, as depicted in
Figure 5b. This sensor was applied for the detection of gaseous methanol concentrations above different methanol/water solutions (
Figure 5c). The sensor showed rapid response/recovery performance: the response time was from 13 s to 35 s, the recovery time from 6 s to 10 s was from a lower concentration of methanol to its higher concentrations, and the detection limit was 100 ppm. Niculescu et al.
[30] have developed an ethanol biosensor based on dehydrogenases, Poly(vinyl imidazole) complexed with Os(4,40-dimethyl bipyridine)
2Cl employed as the electrochemical mediator, and poly(ethylene glycol)-diglycidyl ether as the crosslinking agent. The developed enzyme sensor has been successfully employed for the detection of ethanol in wines. Cinti et al.
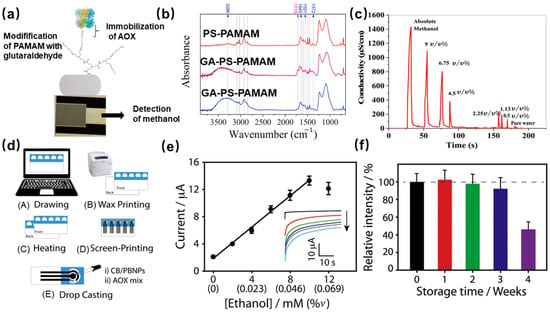
[4] reported a paper-based electrochemical ethanol biosensor. The electrode was modified by carbon black (CB) and Prussian blue nanoparticles (PBNPs), and then the AOx enzyme was immobilized via crosslinking, the fabrication route is shown in
Figure 5d. The ethanol-sensing performance as
Figure 5e shows, the chronoamperometric records of ethanol concentration up to 10 mM in 100 μL of 0.05 M phosphate-buffered solution containing 0.1 M KCl at pH 7.4, displaying a very quick and sensitive response after just 40 s. Meanwhile, the shelf-life of the biosensor was evaluated, as
Figure 5f shows no significant decrease in initial activity after storage of 3 weeks, but the response decreased by 50% after the fourth week. This method can improve the stability and reaction rate of the enzyme but may affect the activity of the enzyme.
Figure 5. (
a) The schematic of preparation alcohol oxidase-based methanol sensor; (
b) FTIR spectra of PS-PAMAM, GA-PS-PAMAM, and AOX/PS-PAMAM ESNFs samples; (
c) the response of sensor to a series concentration of gas-phase methanol
[29]; (
d) The schematic diagram of the process for preparing paper-based ethanol biosensor; (
e) The calibration plot of ethanol concentration vs. current (The inset shows the chronoamperometric records with the concentration increase from 0–12 mM); (
f) the evaluation of shelf-life at 4 °C of the ethanol sensor, fresh biosensor (black bar), 1 week (red bar), 2 weeks (green bar), 3 weeks (blue bar) and 4 weeks (violet bar)
[4].
This entry is adapted from the peer-reviewed paper 10.3390/molecules29010005