Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Toxoplasma gondii, a protozoan parasite with the ability to infect various warm-blooded vertebrates, including humans, is the causative agent of toxoplasmosis. This infection poses significant risks, leading to severe complications in immunocompromised individuals and potentially affecting the fetus through congenital transmission. A comprehensive understanding of the intricate molecular interactions between T. gondii and its host is pivotal for the development of effective therapeutic strategies.
- Toxoplasma gondii
- proteomics
- protozoan
1. Introduction
T. gondii is the causative agent of toxoplasmosis, one of the most common parasitic infections worldwide. The World Health Organization (WHO) estimates that about one-third of the world’s population has been infected with T. gondii. Among them, over 800,000 persons have been exposed and an estimated 750 deaths are caused by toxoplasmosis in the United States each year [1].
T. gondii engages in both sexual and asexual reproduction, utilizing felines as definitive hosts and other animals as intermediate hosts. Intermediate hosts become infected through consuming oocysts or tissues containing T. gondii cysts. Within the intermediate host, the parasite progresses through tachyzoites and bradyzoites development stages. Whereas in the definitive feline host, the sexual stages develop, leading to the excretion of unsporulated oocysts into the environment where they undergo sporulation to become infective, facilitating survival and dissemination.
T. gondii employs a range of mechanisms to invade, replicate, manipulate host metabolism, and establish virulence. It invades host cells through a process called gliding motility, which involves the coordinated action of proteins like myosin A, actin filaments, gliding-associated proteins (GAP), microneme proteins, rhoptry neck proteins (RONs), apical membrane antigen 1 (AMA1), and rhoptry proteins (ROPs). These proteins work together to facilitate attachment to host cells, form a moving junction, and, ultimately, the invasion and egress of host cells [2]. T. gondii also exhibits varying levels of virulence, with different strains having distinct characteristics. One of the virulence factors is ROP18, which is critical for the parasite’s ability to evade the host’s immune response [3][4]. Virulent strains can cause severe disease in immunocompromised individuals, while less virulent strains may result in milder symptoms or even asymptomatic infections in healthy hosts. Once inside host cells, T. gondii replicates quickly and divides within a host cell to form daughter parasites, which are then released to infect new cells. By manipulating the host’s metabolic pathways, T. gondii can downregulate the host’s metabolism of fatty acids, lipids, and energy, often through hijacking host-signaling pathways such as peroxisome proliferator-activated receptors (PPAR) [5].
Clinical outcomes of T. gondii infection vary based on the host’s immunity. Immunocompetent individuals typically experience mild symptoms or remain asymptomatic, while immunocompromised cases, like those with HIV/AIDS or organ transplants, face severe complications—encephalitis, retinochoroiditis, and organ damage. Congenital infection can affect fetuses. Existing clinical treatments are limited in scope so far.
Mass spectrometry (MS)-based proteomics, which involves the large-scale study of proteins and their functions, has become a cornerstone of biological and medical research [6][7][8][9][10][11]. Through investigating the dynamic changes in protein expression, PTMs, and protein-protein interactions (PPIs) that occur during T. gondii infection, host immune responses, and disease progression, scientists have acquired valuable insights into its biology, host interactions, virulence factors, and potential therapeutic targets [12][13][14][15][16][17][18].
2. Proteomics Approaches in T. gondii Research
Proteomics has evolved significantly over the past few decades (Figure 1) with the advancements in technology and methodology, playing a crucial role in our understanding of the parasite’s biology, host–parasite interactions, and potential drug targets.
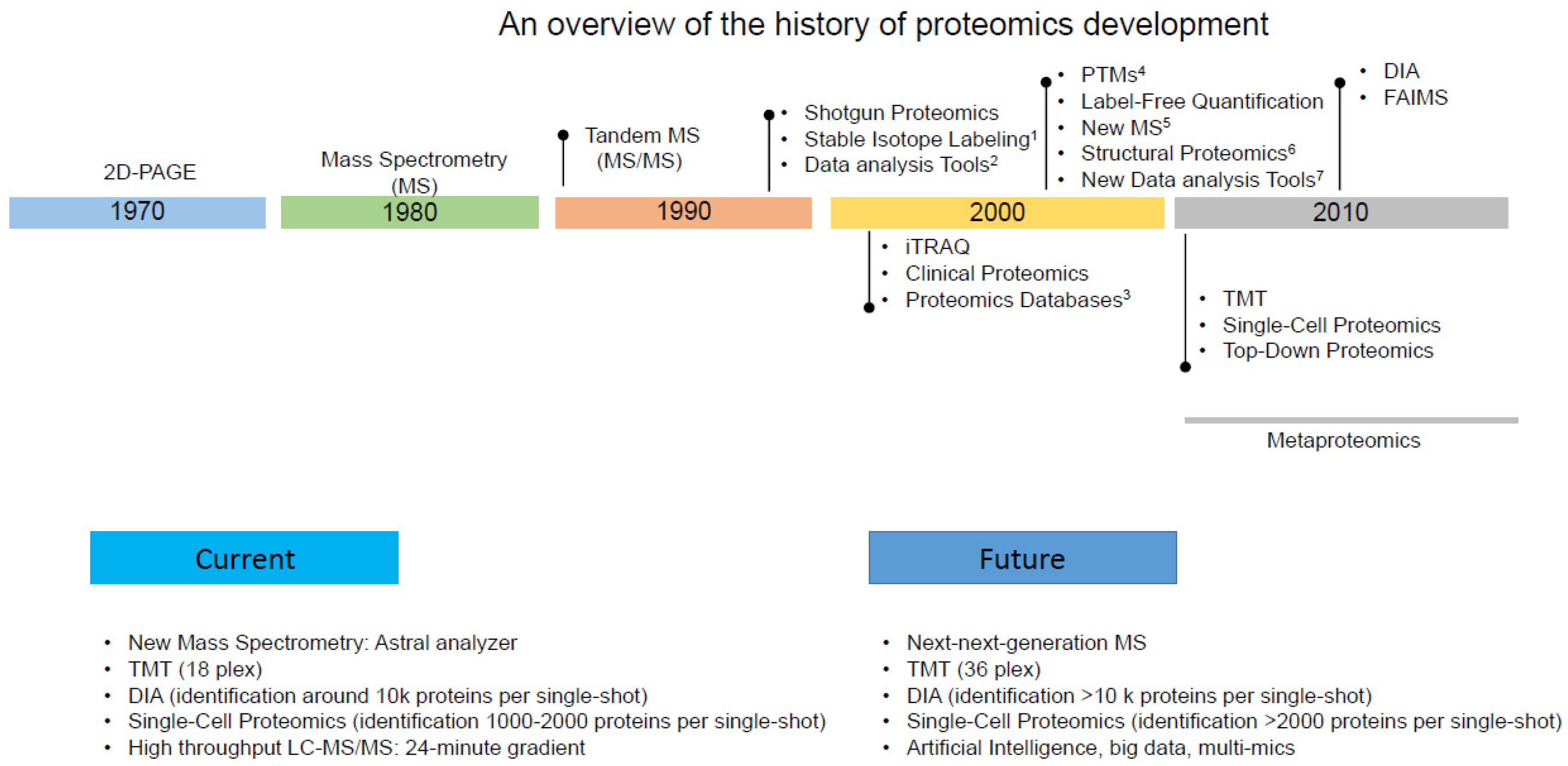
Figure 1. Timeline of the main events associated with proteomic studies. The vertical lines indicate early, middle, or late events depending on their position. Examples of some events: (1) stable isotope labeling by amino acids (SILAC), Isotope-coded Affinity Tag (ICAT); (2) SEQUEST, Mascot, and Scaffold; (3) UniProt, National Institutes of Health database; (4) Phosphoproteome, Acetylome, Ubiqutinome, etc.; (5) Orbitrap, Q-TOF; (6) Hydrogen Deuterium Exchange (HDX)-MS; (7) Skyline, MaxQuant. iTRAQ: Isobaric Tags for Relative and Absolute Quantitation. TMT: Tandem Mass Tags. FAIMS: Field Asymmetric Ion Mobility Spectrometry. DIA: Data-independent acquisition. The decades are not in scale.
2.1. Early Studies (Late 1990s to Early 2000s)
Proteomics studies on T. gondii began in the late 1990s and early 2000s, primarily using two-dimensional gel electrophoresis (2DGE) combined with matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) to separate and analyze proteins. These early studies helped identify and characterize some of the parasite’s proteins [19][20], enabling researchers to analyze thousands of proteins from a biological sample. However, they had limitations concerning sensitivity and the ability to analyze low-abundance proteins.
Shotgun proteomics, also known as bottom-up proteomics, gained popularity in the early 2000s. It involved digesting proteins into peptides and then analyzing those peptides using MS. Several databases, such as UniProt, and searching algorithms, like SEQUEST and Mascot, were established to annotate and search protein sequences and information. These resources have become essential for proteomics research. Liquid chromatography-tandem MS (LC-MS/MS) techniques began to gain prominence in T. gondii research due to their improved accuracy in protein identification and quantification [21]. During this stage, researchers could identify a few hundred to a few thousand proteins in a single experiment using techniques like LC-MS/MS coupled with 2DGE. However, this process often required multiple injections of LC-MS/MS, contributing to the overall cost and time investment associated with the research endeavor.
2.2. Mid-2000s to the Present
2.2.1. Comparative Proteomics
Comparative proteomics involves comparing the proteomes of T. gondii and its infected host cells/tissues under different conditions to identify differentially expressed proteins and reveal molecular changes associated with infection. This approach allows researchers to identify and quantify parasite-specific proteins, host factors targeted by the parasite, and alterations in host protein expression induced by T. gondii infection [22][23][24][25][26][27][28].
2.2.2. Subcellular Proteomics
Subcellular proteomics focuses on studying the proteome of specific cellular compartments or organelles involved in host–parasite interactions. By isolating and analyzing proteins from particular subcellular fractions, researchers can identify organelle-specific proteins and elucidate the role of specific compartments in T. gondii infection and host response [29][30][31]. Extensive proteomic studies on T. gondii have explored subcellular protein localization through diverse analyses, including membrane-soluble fraction comparisons, organelle exploration (e.g., mitochondria), secretory pathway investigation, microvesicle formation, and comprehensive analyses.
2.2.3. Time-Resolved Proteomics
Time-resolved proteomics approach involves collecting samples at different time points during a biological process, infection, or treatment, and then analyzing the proteomic changes that occur over those time intervals. It is a powerful tool for deciphering temporal changes in disease progression [32], cellular responses [33], biological processes [34], and drug action [35]. These applications in T. gondii provide valuable knowledge about the dynamics of complex systems and molecular mechanisms.
2.2.4. Post-Translational Modifications (PTMs)
PTMs such as phosphorylation [36], glycosylation [37], and acetylation [38][39] play crucial roles in regulating various cellular processes in apicomplexan parasites, including T. gondii. These modifications affect protein activity, localization, and interactions, and contribute to the complexity of the proteome, thereby likely influencing developmental transitions, biology, and pathogenesis of these parasites.
2.3. Recent Advancements in Proteomics Techniques
2.3.1. Single-Cell Proteomics
With the advance of MS, single-cell proteomics is experiencing rapid technological advancements and taking center stage [40]. These improvements include increased proteome coverage, which has grown from analyzing around 200 protein groups to over 1000 protein groups within individual mammalian cells [41]. Tandem Mass Tag (TMT) is typically ideal for studies requiring high multiplexing, compatibility with various sample types, and higher throughput. Junho et al. optimized the real-time search MS3-based TMT quantification method for the 126 single cells study; about 1000 out of the 1200 identified proteins were quantifiable [42].
While single-cell proteomics publications in T. gondii applications are currently limited, scholars anticipate an upcoming surge in both applications and publications. This is expected to significantly enhance research on T. gondii. One of the main benefits of single-cell proteomics in T. gondii research is to strengthen our understanding of the heterogeneity of parasite–host interactions and provide insights into how different cells respond to the infection. It also reveals variations in parasite protein expression within the host environment. On the other hand, integrating single-cell proteomics with spatial information can help researchers understand the localized effects of T. gondii infection within infected tissues.
2.3.2. Data-Independent Acquisition (DIA) and High-Throughput Proteomics
DIA represents an emerging MS-based approach that effectively addresses the limitations associated with traditional data-dependent acquisition (DDA) methods. Through systematically fragmenting all discernible ions within a defined mass range, DIA offers several notable advantages, including expanded coverage of the proteome, enhanced precision in quantification, and the ability to detect proteins and PTMs at low abundance levels. Srinivasan et al. compared DIA with DDA methods for phosphoproteomics from nocodazole-treated and untreated U2OS cells. They found 15,548 unique site-localized phosphopeptides using DDA and 6817 using DIA. While DDA excels in identifying more unique analytes, DIA demonstrated better reproducibility, with approximately 66% of localized peptides consistently identified in at least 5 out of 10 replicates compared to 32% in DDA [43]. It anticipates that novel data analysis methods, such as machine learning, will leverage advanced MS instrumentation to enhance the capabilities of DIA-MS, enabling more comprehensive and accurate measurements of PTMs [44].
The use of DIA in T. gondii research is limited, with only a few applications. For instance, Alex et al. employed DIA to quantitatively analyze immunoprecipitated proteins from T. gondii cWT, cMut, and TIR1 parasites, leading to the measurement of over 2.5 thousand proteins [45]. DIA offers robust and reproducible quantifications across multiple samples, which are crucial for clinical implications. Scholars anticipate that DIA will aid researchers in discovering novel proteins, protein isoforms, and PTMs in T. gondii studies. DIA is also well-suited for investigating the temporal dynamics of T. gondii proteome during different life cycle stages or in response to drug treatments. This is particularly valuable for identifying parasite proteins with rapid turnover rates that may play crucial roles in dynamic cellular processes or stress responses.
2.3.3. Targeted Proteomics
Targeted proteomics, which includes techniques like selected reaction monitoring (SRM) and parallel reaction monitoring (PRM), is an active and rapidly advancing field in proteomics research. With advances in high-resolution mass spectrometers, targeted proteomics is gaining prominence in clinical and translational research [46]. It plays a crucial role in biomarker discovery and validation for various diseases. Researchers are developing targeted assays for specific proteins and PTMs relevant to disease.
Targeted proteomics, recognized for its sensitivity, specificity, and reproducibility in quantitatively assessing and validating parasite protein expression levels, has been extensively employed in biomedical research and the pharmaceutical industry. Targeted proteomics often requires well-defined and characterized samples. Obtaining such samples from T. gondii, especially across different life stages (tachyzoite, bradyzoite, and oocyst), or during specific host interactions, each expressing a unique set of proteins, presents significant challenges. Despite its widespread use, the applications of targeted proteomics in T. gondii have been notably restricted, and there are scarce publications employing these techniques in the context of T. gondii.
2.3.4. Plasma Proteome
While fractionation and depletion techniques have made notable progress in improving coverage of the plasma proteome, the considerable 10 orders of magnitude dynamic range present in analyzed plasma samples remains a substantial challenge when applying proteomics to biomarker discovery and clinical applications in T. gondii infection. Even if researchers remove 95% of high-abundance proteins, including albumin and IgG, from plasma samples through high-abundance protein depletion, the remaining high-abundance proteins still significantly interfere with the detection of low-abundance proteins, such as secreted proteins and their PTMs. These low-abundance proteins might be crucial for host–parasite interactions but could undergo rapid degradation.
2.3.5. Top-Down Proteomics
Top-down proteomics is a powerful approach for studying intact proteins, including the characterization of protein isoforms and PTMs. With advancements in high-resolution mass spectrometers, particularly those equipped with Orbitrap or FT-ICR and data analysis tools, such as ProSight and TopPIC, top-down proteomics is well-suited for the characterization of complex PTMs, including glycosylation, phosphorylation, and acetylation, which are associated with key processes like invasion, gene expression, translation modulation, and immune evasion in T. gondii, at the protein level. Top-down proteomics also plays a crucial role in structural proteomics, which enhances our understanding of protein folding, conformational changes, and the study of PPIs at the intact protein level. There is growing interest in applying top-down proteomics to clinical research and diagnostics since it has the potential to identify T. gondii invasion and host interactions with specific protein isoforms, truncated forms, and PTMs for biomarker discovery.
Top-down proteomics, an evolving MS technology, requires specialized expertise and resources in terms of time and cost. Analyzing the complex and diverse proteome of T. gondii demands advanced sample preparation techniques and high-resolution mass spectrometers, posing challenges in confidently identifying intact proteins. As technology becomes more accessible and computational tools improve, the use of top-down proteomics in T. gondii research will likely increase. These techniques will certainly assist researchers in identifying potential therapeutic targets and specific antibodies against toxoplasmosis.
2.3.6. Multi-Omics Integration
Multi-omics integration has also been applied to investigate host–pathogen interactions and infectious diseases. Jean Beltran et al. discussed proteomic methods and their application in studying host–pathogen interactions, highlighting how multi-omics approaches, including proteomics, genomics, transcriptomics, and metabolomics, contribute to a systems-level understanding of infectious diseases [47]. It has paved the way for the development of potential therapies and vaccines against toxoplasmosis and continues to be an active area of research.
In T. gondii research, multi-omics integration has played a pivotal role. For instance, Kloehn et al. utilized multi-omics analysis to explore the distinct functions of subcellular acetyl-CoA pools in T. gondii. This approach unveiled a deeper understanding of the parasite’s physiological and metabolic adjustments [48].
2.3.7. Bioinformatics
Despite the breadth of ToxoDB, there exists a diverse set of external bioinformatics tools that could be integrated to construct a custom pipeline for reanalyzing existing data in T. gondii proteomics. Functional and comparative genomics have emerged as well-explored perspectives for analyzing proteomics, providing insights by linking functions to proteins in our dataset based on knowledge from model organisms [49]. While pipelines for analyzing proteomics datasets from various perspectives, including epigenetics, have been developed [50], the majority of studies focus on predicting new antigens for diagnosis or vaccine development [51][52][53][54][55]. Multi-epitopes antigens are designed using bioinformatics tools like the ANTIGENpro database (http://scratch.proteomics.ics.uci.edu (accessed on 11 December 2023)), NetCTL 1.2 server (http://www.cbs.dtu.dk/services/NetCTL/ (accessed on 11 December 2023)), IFN epitope server (http://crdd.osdd.net/raghava/ifnepitope/scan.php (accessed on 11 December 2023)) and the ABCpred server (http://www.imtech.res.in/raghava/abcpred/ (accessed on 11 December 2023)). Notably, Goodswen et al. have recently introduced an advanced bioinformatics pipeline that integrates proteomics data from ToxoDB along with various external tools to predict potential vaccine candidates [56].
One of the aims of parasite bioinformatics studies at the proteomics level is to determine the protein–protein interactome [57]. A significant proportion of proteomics studies in the field attempt to propose an interaction network from experimental results. Although it is possible to predict these interactions from an input dataset using the STRINGdb tool integrated into ToxoDB, this prediction typically fails in the absence of additional information for uncharacterized genes [58].
3. Interactome Analysis
3.1. Affinity Purification
In this approach, T. gondii proteins are tagged with a specific affinity tag (such as FLAG or HA tags) and expressed in the parasite. The tagged proteins and their interacting partners can then be selectively isolated using affinity purification techniques, followed by identification using MS. Yakubu et al. utilized affinity purification combined with proteomics to reveal that Protein Arginine Methyltransferase 1 (PRMT1) significantly contributes to arginine monomethylation in T. gondii [27]. Anghel et al. applied differential affinity chromatography followed by MS to investigate cellular and molecular targets of nucleotide-tagged ruthenium complexes in T. gondii and Trypanosoma brucei [59].
3.2. Proximity Labeling Techniques
By labeling molecules that are close to a specific protein or molecular complex of interest, the labeled proteins can be isolated and identified using MS. Proximity labeling techniques offer a high-throughput approach to studying various biomolecular interactions with spatial and temporal precision. Shkel et al. utilized enzymes to covalently label nearby biomolecules, enabling their identification using MS. Their advantage lies in capturing weak or transient interactions, making them crucial for investigating organelle interactomes and macromolecular complexes [60]. Back et al. used proximity labeling to identify novel IMC proteins enriched in daughter buds and revealed that IMC29 plays an important role in Toxoplasma endodyogeny [61].
3.3. BirA*-Mediated Proximity-Dependent Biotin Identification (BioID)
BioID is a specific proximity labeling technique that uses a mutant form of the biotin ligase enzyme (BirA*) as the labeling enzyme. The protein of interest is fused to BirA*, and when the fusion protein is expressed in cells, BirA* biotinylates lysine residues on proteins within its spatial proximity. These biotinylated proteins can be isolated using streptavidin-based purification and then identified using MS [62].
3.4. Ascorbate Peroxidase-Mediated Proximity Labeling (APEX)
One notable benefit of APEX compared to traditional BioID is its considerably faster labeling rate, operating within minutes as opposed to hours. When combined with quantitative proteomic methods, this helps detect the rapid alterations in protein interactions over time or as a reaction to cellular disturbances [63][64]. With BioID and APEX techniques, Pan M et al. identified 46 proteins, including 20 known and 26 new GRAs. These GRAs, mainly in coccidian parasites, might not be vital for in vitro growth but could play roles in animal infections [65].
3.5. Yeast Two Hybrid (Y2H)
Yeast two-hybrid (Y2H) assays are useful to identify PPIs in cells. Two proteins, one “bait” and one “prey,” are put into yeast cells. If there is an interaction, it is proven by the activation of reporter genes. Using Y2H assays, Lai et al. discovered significant interactions between T. gondii surface antigens (SAG2 and SAG1) and specific human proteins. They identified 20 and 39 positive clones interacting with SAG2 and SAG1, respectively. Notably, Homo sapiens zinc finger protein strongly interacted with SAG2, while Homo sapiens lysine-rich coil-coiled protein showed a strong interaction with SAG1 [66][67].
4. ToxoDB in T. gondii Proteome
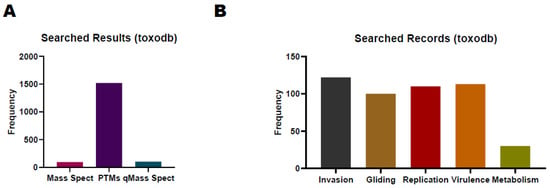
The ToxoDB database (https://toxodb.org/toxo/app/ (accessed on 10 October 2023)) is a valuable resource for the proteome study of T. gondii, allowing researchers to access experimental data related to T. gondii proteins, which include MS data, protein expression profiles, and post-translational modification information [68]. According to the current release, the ToxoDB-65_TgondiiME49_AnnotatedProteins database, which contains 8322 protein-coding genes, can be used for proteomics database searching. Researchers can also cross-reference proteomic data with other omics data to gain a more comprehensive understanding of T. gondii biology. There are 96, 1521, and 104 records about T. gondii research for MS, PTMs, and quantitative MS, respectively, in ToxoDB (Figure 2).

Figure 2. Summary of proteomics and biological processes searching results in ToxoDB. (A) T.gondii proteomics research counts in ToxoDB. (B) Search results of typical biological processes of T.gondii in ToxoDB.
4.1. Stage-Specific Proteomes
The first T. gondii proteome was generated from the tachyzoite stage [69]. In that work, three proteomic platforms were designed: two-dimensional electrophoresis, one-dimensional electrophoresis gel coupled with LC-MS/MS, and MudPIT analysis. By combining these methods, the authors identified 2252 non-redundant expressed proteins, which were approximately 29% of the annotated genes. Notably, 57% of the genes exhibiting evidence of transcription through the Expressed Sequence Tag method failed to yield detectable peptides, suggesting potential factors such as low translation levels, protein instability, transient protein expression, or even the existence of non-coding RNAs. In contrast, a comparison of the proteins identified in this study with mRNA expression levels, determined by microarray analysis, revealed a substantial number of proteins were detected even in cases when mRNA expression levels were negligible or minimal. This observation suggests the presence of highly stable proteins over time, with 204 and 632 instances in cases of minimal mRNA expression, respectively.
In 2013, the proteome of partially sporulated oocysts was published [70]. The generation of the oocyst-stage proteins was of high relevance due to the limited number of studies carried out on this stage. Moreover, the lack of identification of specific stage proteins hindered progress on this important stage for environmental dissemination of T. gondii. The analysis compared the oocyst proteome with transcriptomic data obtained in unsporulated (day 0) and sporulated (day 10) oocysts. At day 0, the analysis identified proteins associated with upregulated genes related to metabolism, cellular transport, and cell fate, while day 10 showed enrichment in proteins associated with protein synthesis, cell rescue, defense, and virulence. On the other hand, the comparison of this proteome with that of tachyzoites identified 154 oocyst-specific proteins, many associated with hypothetical genes. Oocyst-specific functional genes tend to be enriched in metabolic functions, comprising the largest category.
4.2. PTM Proteomes
The proteomes of tachyzoites, bradyzoites, and oocysts can offer information about the genes of interest that potentially provide prior knowledge about their expression levels in the different stages. However, proteomes that investigate various PTMs can provide valuable insights for researchers interested in the study of a specific gene. There are more than 400 protein PTMs, which in turn present crosstalk with each other. In T. gondii, information from a PTM could help formulate a hypothesis regarding the function and/or regulation of the protein of interest. Knowing which residues have incorporated specific PTMs provides essential information for generating site-specific mutants. In ToxoDB, there is data obtained from three PTM proteomes: phosphorylation, acetylation, and ubiquitinylation. Beyond the scope of ToxoDB, other more specific proteomes analyze PTMs in various contexts, including histone PTMs [71], acetylation in T. gondii deficient in acetyl-coA [48], crotonylation [72], phosphorylation in kinase mutants or different stages [36][73][74][75][76][77][78], lipidation [79][80], Cysteine S-nitrosylation [81].
4.3. PTM Crosstalk and Proteome
A study on combined methylation, phosphorylation, and acetylation of proteins conducted in a lung cancer model showed a large number of proteins that presented the three PTMs [82][83]. Taking a functional perspective, PTMs increase the diversity of functional units of protein origin within a cell. PTMs are still a field with great potential for future exploration in T. gondii. A crosstalk study with ubiquitination and the other PTMs showed that less than 10% of the proteins overlapped between ubiquitin and arginine methylation, 21% exhibited a combination of acetylation with ubiquitin, 25% of the SUMO proteome was ubiquitinated, while 78% of the phosphorylated proteins were also ubiquitinated [84]. Among the proteins detected with combinations of different PTMs, the histone acetyltransferase EP300 and the chaperone Hsp90 [82] stand out.
5. Subcellular Localization Associated Proteome
For researchers delving into an uncharted protein of interest, one of the key considerations for its characterization and the potential revelation of its function lies in determining its subcellular localization. This becomes even more crucial in omics studies, where a multitude of genes or proteins are involved, and establishing associations among them often requires them to inhabit the same cellular compartments. Beyond proteomes focused on subcellular locations, some proteomics studies also analyze the presence of T. gondii proteins within specific cellular structures. For example, T. gondii conoid proteome was performed [85]. This structure is relevant for invasion in T. gondii and closely related parasites, but is absent in other apicomplexans, such as Plasmodium spp. In the work, 200 proteins located in the cytoskeleton conoid and the apical region of T. gondii were identified. Another work that provides very interesting data is the proteome of extracellular proteins and vesicles (exosomes/ectosomes) [86]. In the work, 512 proteins were detected in the excretory/secretory, 210 in the ectosome, and 285 in the exosome fraction. The authors also analyzed the supernatant obtained from the purification of vesicles, identifying 421 proteins in that fraction.
Toxoplasma has a single mitochondria that is very relevant during the tachyzoite stage. Researchers observed that bradyzoites lack a functional TCA and respiratory chain [87]. The mitochondrial proteome was generated based on two proximal labeling systems already described above: one by mBirA and another modified for mitochondria using the plant ascorbate peroxidase (APEX) system [88]. The proteome data were uploaded to ToxoDB. In total, researchers identified 421 proteins: 213 in the APEX samples and 369 in the mBirA system. Notably, 36% were hypothetical and 33% were never defined in a role or location associated with mitochondria. Although some of them presented another non-mitochondrial location by other techniques, in the majority their location was confirmed. Among the new proteins identified, the authors focused on one called TgApiCox25, which was shown to be a component of the cytochrome c oxidase complex.
In the ToxoDB, two T. gondii proteomes analyze the components through subcellular fractionation. One was generated by the Laboratory of Dr. Silvia Moreno (Center for Tropical and Emerging Global Diseases, Department of Cellular Biology, University of Georgia, Athens, GA, USA). Another is based on a fractionation called hyperlexed localization of organelle proteins by isotope tagging (hyperLOPIT). LOPIT is based on protein correlation profiling, in which all proteins from a subcellular location can be marked by tandem mass tags followed by mass spectrometry [89]. In the case of T. gondii HyperLOPIT, an iodixanol density gradient was used to separate the subcellular compartments [31]. Tagged peptides from the different fractions of the gradient were analyzed. A subcellular localization map was generated based on the following compartments: apical 1, apical 2, micronemes, rhoptries 1, rhoptries 2, dense granules, IMC, tubulin cytoskeleton, Plasma Membrane (PM)—peripheral 1, endomembrane/vesicles, PM—peripheral 2, PM—integral, Golgi, endoplasmic reticulum (ER) 1, ER 2, apicoplast, mitochondria–membrane, mitochondria–soluble, nucleus–chromatin, nucleus–non-chromatin, nucleolus, 40S ribosome, 60S ribosome, cytosol, 19S proteasome, 20S proteasome and unassigned. This level of detail shows the strength of this technique in identifying proteins from different organelles and subcellular compartments.
6. Proteomics Approach in Drug Discovery Efforts
6.1. Drug Target Identification and Therapeutics
Currently, there are only a few drugs available to treat T. gondii infections and some of them have limitations such as toxicity and the potential for parasite resistance. By comparing protein expression profiles between different stages of the parasite’s life cycle and between parasite and host cells, researchers can pinpoint proteins that are specific to the parasite, making them potential targets for drug development. Proteomics has identified key drug targets, such as histone variant H2B.Z acetylation, essential for T. gondii’s fitness [38]. Innovative methods like CRISPR-based oligo recombineering (CORe) have been employed to identify chemically reactive sites for drug targeting [90]. Protein phosphatases are promising drug targets against apicomplexan parasites [91]. N-myristoyl transferase (NMT) is considered a potential target [92], and the spider peptide XYP1 demonstrates anti-T. gondii effects by interacting with membrane-associated proteins [93]. Identification of unique membrane proteins in T. gondii suggests potential candidates for therapeutic development [94].
6.2. Insights into Protein Modification and Pathway
Understanding the complex interaction between the parasite and the host is crucial for drug development, as the host’s immune response can influence the efficacy of treatment. PTMs, such as crotonylation and 2-hydroxyisobutyrylation, affect critical enzymes in energy-related pathways. Histone modifications like acetylation play essential roles in gene regulation, and parasite fitness [38]. It is not limited to histone proteins but also extends to nonhistone proteins involved in diverse cellular functions. The presence of acetylation as a regulatory mechanism in protozoan parasites offers an opportunity to explore this machinery as a target for drug development [95]. Disrupting specific protein pathways, such as plastidic iron–sulfur cluster biogenesis, affects parasite viability and cellular functions [96]. It was found that blocking palmitoylation enhances the release of invasion-related proteins, including AMA1, from apical secretory organelles. This observation suggests that AMA1 plays a role in controlling the secretion process of these proteins [80]. That information is crucial for designing drugs that disrupt key pathways or processes in the parasite’s biology while minimizing harm to the host.
6.3. Advancements in Diagnostics and Vaccines
Proteomics contributes to diagnostic tests and vaccine development against T. gondii. Multiepitope antigens and computational pipelines enhance diagnostic and vaccine strategies. Identifying immunodominant epitopes and functional proteins through proteomics aids in developing comprehensive protection strategies against toxoplasmosis [54]. A significant homology was observed in the antigenic proteome profiles between N. caninum and T. gondii, which has important implications. It suggests the feasibility of designing multicomponent vaccines that target common antigens shared by both parasites [97]. Proteomics can also play a role in vaccine development by identifying potential protein antigens that can stimulate an immune response [98].
6.4. Screening and Testing Drug Candidates
Discovering new drug candidates with anti-toxoplasma activity is a significant challenge. Proteomics facilitates the screening of potential drug compounds by assessing their impact on the parasite’s proteome. Researchers can examine how candidate drugs alter protein expression patterns, helping to identify compounds that effectively inhibit the parasite’s growth or disrupt essential pathways. The investigation of essential proteins of T. gondii as potential drug targets has been ongoing for the past two decades. While these efforts have shown promise in vitro, translating these findings into effective treatments for toxoplasmosis in humans has been challenging. The utilization of proteomics-based investigations to examine the interactions between drug candidates and proteins from both the parasite and the host can serve as an effective approach for characterizing drug targets [99]
6.5. Characterizing Drug Resistance
T. gondii has the potential to develop resistance to existing drugs, reducing their effectiveness. The presence of drug-resistant T. gondii strains poses challenges to treatment efficacy, especially in immunocompromised patients. This necessitates a better understanding of resistance mechanisms and the implementation of monitoring programs to address this public health issue [100]. Proteomics can shed light on mechanisms of drug resistance in T. gondii. By comparing protein and PTM profiles of drug-sensitive and drug-resistant strains, researchers can identify changes in protein expression that contribute to resistance, informing the design of new drugs or treatment strategies. Proteomic analysis revealed the down- or up-regulation of various proteins, with a particular focus on some key proteins, both actin and MIC8, that play important roles in invasion capability [101].
In summary, recent advances in high-throughput proteomics techniques show potential for identifying parasite-specific markers, especially very low abundance proteins and proteins that have small differences in protein structure but have significant functional consequences in increasing drug specificity and reducing toxicity. Utilizing high-throughput proteomics techniques can significantly reduce the expenses associated with screening and validating drug targets. This cost reduction is particularly advantageous for neglected diseases with limited market potential, such as toxoplasmosis. With the applications of these cutting-edge proteomic technologies in T. gondii research, the resolution of these challenges in drug development appears to be on the horizon.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens13010033
References
- Jones, J.L.; Kruszon-Moran, D.; Wilson, M.; McQuillan, G.; Navin, T.; McAuley, J.B. Toxoplasma gondii infection in the United States: Seroprevalence and risk factors. Am. J. Epidemiol. 2001, 154, 357–365.
- Frenal, K.; Dubremetz, J.F.; Lebrun, M.; Soldati-Favre, D. Gliding motility powers invasion and egress in Apicomplexa. Nat. Rev. Microbiol. 2017, 15, 645–660.
- Xia, J.; Kong, L.; Zhou, L.J.; Wu, S.Z.; Yao, L.J.; He, C.; He, C.Y.; Peng, H.J. Genome-Wide Bimolecular Fluorescence Complementation-Based Proteomic Analysis of Toxoplasma gondii ROP18’s Human Interactome Shows Its Key Role in Regulation of Cell Immunity and Apoptosis. Front. Immunol. 2018, 9, 61.
- Yang, Z.; Hou, Y.; Hao, T.; Rho, H.S.; Wan, J.; Luan, Y.; Gao, X.; Yao, J.; Pan, A.; Xie, Z.; et al. A Human Proteome Array Approach to Identifying Key Host Proteins Targeted by Toxoplasma Kinase ROP18. Mol. Cell. Proteom. 2017, 16, 469–484.
- Gallego-Lopez, G.M.; Guzman, E.C.; Knoll, L.J.; Skala, M. Metabolic changes to host cells with Toxoplasma gondii infection. bioRxiv 2023.
- Katsarou, E.I.; Billinis, C.; Galamatis, D.; Fthenakis, G.C.; Tsangaris, G.T.; Katsafadou, A.I. Applied Proteomics in ‘One Health’. Proteomes 2021, 9, 3.
- Bautista, J.M.; Marin-Garcia, P.; Diez, A.; Azcarate, I.G.; Puyet, A. Malaria proteomics: Insights into the parasite-host interactions in the pathogenic space. J. Proteom. 2014, 97, 107–125.
- Muselius, B.; Durand, S.L.; Geddes-McAlister, J. Proteomics of Cryptococcus neoformans: From the Lab to the Clinic. Int. J. Mol. Sci. 2021, 22, 12390.
- Christopher, J.A.; Stadler, C.; Martin, C.E.; Morgenstern, M.; Pan, Y.; Betsinger, C.N.; Rattray, D.G.; Mahdessian, D.; Gingras, A.C.; Warscheid, B.; et al. Subcellular proteomics. Nat. Rev. Meth. Primers 2021, 1, 32.
- Jiao, F.; Zhang, D.; Jiang, M.; Mi, J.; Liu, X.; Zhang, H.; Hu, Z.; Xu, X.; Hu, X. Label-free proteomic analysis of placental proteins during Toxoplasma gondii infection. J. Proteom. 2017, 150, 31–39.
- Zhang, D.; Sun, X.; Ren, L.; Yang, C.; Liu, X.; Zhang, H.; Jiang, Y.; Hu, X. Proteomic profiling of human decidual immune proteins during Toxoplasma gondii infection. J. Proteom. 2018, 186, 28–37.
- Weiss, L.M.; Fiser, A.; Angeletti, R.H.; Kim, K. Toxoplasma gondii proteomics. Expert. Rev. Proteom. 2009, 6, 303–313.
- Yakubu, R.R.; Nieves, E.; Weiss, L.M. The Methods Employed in Mass Spectrometric Analysis of Posttranslational Modifications (PTMs) and Protein-Protein Interactions (PPIs). Adv. Exp. Med. Biol. 2019, 1140, 169–198.
- Morlon-Guyot, J.; El Hajj, H.; Martin, K.; Fois, A.; Carrillo, A.; Berry, L.; Burchmore, R.; Meissner, M.; Lebrun, M.; Daher, W. A proteomic analysis unravels novel CORVET and HOPS proteins involved in Toxoplasma gondii secretory organelles biogenesis. Cell. Microbiol. 2018, 20, e12870.
- McMurtrey, C.; Trolle, T.; Sansom, T.; Remesh, S.G.; Kaever, T.; Bardet, W.; Jackson, K.; McLeod, R.; Sette, A.; Nielsen, M.; et al. Toxoplasma gondii peptide ligands open the gate of the HLA class I binding groove. eLife 2016, 5, e12556.
- Wang, Z.X.; Zhou, C.X.; Elsheikha, H.M.; He, S.; Zhou, D.H.; Zhu, X.Q. Proteomic Differences between Developmental Stages of Toxoplasma gondii Revealed by iTRAQ-Based Quantitative Proteomics. Front. Microbiol. 2017, 8, 985.
- Xie, H.; Sun, H.; Dong, H.; Dai, L.; Xu, H.; Zhang, L.; Wang, Q.; Zhang, J.; Zhao, G.; Xu, C.; et al. Label-free quantitative proteomic analyses of mouse astrocytes provides insight into the host response mechanism at different developmental stages of Toxoplasma gondii. PLoS Negl. Trop. Dis. 2023, 17, e0011102.
- Nelson, M.M.; Jones, A.R.; Carmen, J.C.; Sinai, A.P.; Burchmore, R.; Wastling, J.M. Modulation of the host cell proteome by the intracellular apicomplexan parasite Toxoplasma gondii. Infect. Immun. 2008, 76, 828–844.
- Jungblut, P.R.; Zimny-Arndt, U.; Zeindl-Eberhart, E.; Stulik, J.; Koupilova, K.; Pleissner, K.P.; Otto, A.; Muller, E.C.; Sokolowska-Kohler, W.; Grabher, G.; et al. Proteomics in human disease: Cancer, heart and infectious diseases. Electrophoresis 1999, 20, 2100–2110.
- Beckers, C.J.; Roos, D.S.; Donald, R.G.; Luft, B.J.; Schwab, J.C.; Cao, Y.; Joiner, K.A. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. Implications for the target of macrolide antibiotics. J. Clin. Investig. 1995, 95, 367–376.
- Zhou, X.W.; Kafsack, B.F.; Cole, R.N.; Beckett, P.; Shen, R.F.; Carruthers, V.B. The opportunistic pathogen Toxoplasma gondii deploys a diverse legion of invasion and survival proteins. J. Biol. Chem. 2005, 280, 34233–34244.
- Fauquenoy, S.; Morelle, W.; Hovasse, A.; Bednarczyk, A.; Slomianny, C.; Schaeffer, C.; Van Dorsselaer, A.; Tomavo, S. Proteomics and glycomics analyses of N-glycosylated structures involved in Toxoplasma gondii--host cell interactions. Mol. Cell. Proteom. 2008, 7, 891–910.
- Al-Bajalan, M.M.M.; Xia, D.; Armstrong, S.; Randle, N.; Wastling, J.M. Toxoplasma gondii and Neospora caninum induce different host cell responses at proteome-wide phosphorylation events; a step forward for uncovering the biological differences between these closely related parasites. Parasitol. Res. 2017, 116, 2707–2719.
- Zhou, C.X.; Gao, M.; Han, B.; Cong, H.; Zhu, X.Q.; Zhou, H.Y. Quantitative Peptidomics of Mouse Brain After Infection With Cyst-Forming Toxoplasma gondii. Front. Immunol. 2021, 12, 681242.
- Sun, H.; Li, J.; Wang, L.; Yin, K.; Xu, C.; Liu, G.; Xiao, T.; Huang, B.; Wei, Q.; Gong, M.; et al. Comparative Proteomics Analysis for Elucidating the Interaction Between Host Cells and Toxoplasma gondii. Front. Cell. Infect. Microbiol. 2021, 11, 643001.
- Hanggeli, K.P.A.; Hemphill, A.; Muller, N.; Heller, M.; Uldry, A.C.; Braga-Lagache, S.; Muller, J.; Boubaker, G. Comparative Proteomic Analysis of Toxoplasma gondii RH Wild-Type and Four SRS29B (SAG1) Knock-Out Clones Reveals Significant Differences between Individual Strains. Int. J. Mol. Sci. 2023, 24, 10454.
- Yakubu, R.R.; Silmon de Monerri, N.C.; Nieves, E.; Kim, K.; Weiss, L.M. Comparative Monomethylarginine Proteomics Suggests that Protein Arginine Methyltransferase 1 (PRMT1) is a Significant Contributor to Arginine Monomethylation in Toxoplasma gondii. Mol. Cell. Proteom. 2017, 16, 567–580.
- Doliwa, C.; Xia, D.; Escotte-Binet, S.; Newsham, E.L.; Sanya, J.S.; Aubert, D.; Randle, N.; Wastling, J.M.; Villena, I. Identification of differentially expressed proteins in sulfadiazine resistant and sensitive strains of Toxoplasma gondii using difference-gel electrophoresis (DIGE). Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 35–44.
- Marugan-Hernandez, V.; Alvarez-Garcia, G.; Tomley, F.; Hemphill, A.; Regidor-Cerrillo, J.; Ortega-Mora, L.M. Identification of novel rhoptry proteins in Neospora caninum by LC/MS-MS analysis of subcellular fractions. J. Proteom. 2011, 74, 629–642.
- Rashidi, S.; Vieira, C.; Mansouri, R.; Ali-Hassanzadeh, M.; Ghani, E.; Karimazar, M.; Nguewa, P.; Manzano-Roman, R. Host cell proteins modulated upon Toxoplasma infection identified using proteomic approaches: A molecular rationale. Parasitol. Res. 2022, 121, 1853–1865.
- Barylyuk, K.; Koreny, L.; Ke, H.; Butterworth, S.; Crook, O.M.; Lassadi, I.; Gupta, V.; Tromer, E.; Mourier, T.; Stevens, T.J.; et al. A Comprehensive Subcellular Atlas of the Toxoplasma Proteome via hyperLOPIT Provides Spatial Context for Protein Functions. Cell Host Microbe 2020, 28, 752–766.E9.
- Demichev, V.; Tober-Lau, P.; Lemke, O.; Nazarenko, T.; Thibeault, C.; Whitwell, H.; Rohl, A.; Freiwald, A.; Szyrwiel, L.; Ludwig, D.; et al. A time-resolved proteomic and prognostic map of COVID-19. Cell Syst. 2021, 12, 780–794.E7.
- Herneisen, A.L.; Li, Z.H.; Chan, A.W.; Moreno, S.N.J.; Lourido, S. Temporal and thermal profiling of the Toxoplasma proteome implicates parasite Protein Phosphatase 1 in the regulation of Ca(2+)-responsive pathways. eLife 2022, 11, e80336.
- Bernard, C.; Locard-Paulet, M.; Noel, C.; Duchateau, M.; Giai Gianetto, Q.; Moumen, B.; Rattei, T.; Hechard, Y.; Jensen, L.J.; Matondo, M.; et al. A time-resolved multi-omics atlas of Acanthamoeba castellanii encystment. Nat. Commun. 2022, 13, 4104.
- Zecha, J.; Bayer, F.P.; Wiechmann, S.; Woortman, J.; Berner, N.; Muller, J.; Schneider, A.; Kramer, K.; Abril-Gil, M.; Hopf, T.; et al. Decrypting drug actions and protein modifications by dose- and time-resolved proteomics. Science 2023, 380, 93–101.
- Wang, Z.X.; Che, L.; Hu, R.S.; Sun, X.L. Comparative Phosphoproteomic Analysis of Sporulated Oocysts and Tachyzoites of Toxoplasma gondii Reveals Stage-Specific Patterns. Molecules 2022, 27, 1022.
- Yakubu, R.R.; Weiss, L.M.; Silmon de Monerri, N.C. Post-translational modifications as key regulators of apicomplexan biology: Insights from proteome-wide studies. Mol. Microbiol. 2018, 107, 1–23.
- Vanagas, L.; Muñoz, D.; Cristaldi, C.; Ganuza, A.; Najera, R.; Bonardi, M.C.; Turowski, V.R.; Guzman, F.; Deng, B.; Kim, K.; et al. Histone variant H2B.Z acetylation is necessary for maintenance of Toxoplasma gondii biological fitness. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194943.
- Bouchut, A.; Chawla, A.R.; Jeffers, V.; Hudmon, A.; Sullivan, W.J., Jr. Proteome-wide lysine acetylation in cortical astrocytes and alterations that occur during infection with brain parasite Toxoplasma gondii. PLoS ONE 2015, 10, e0117966.
- Perkel, J.M. Single-cell proteomics takes centre stage. Nature 2021, 597, 580–582.
- Kelly, R.T. Single-cell Proteomics: Progress and Prospects. Mol. Cell. Proteom. 2020, 19, 1739–1748.
- Park, J.; Yu, F.; Fulcher, J.M.; Williams, S.M.; Engbrecht, K.; Moore, R.J.; Clair, G.C.; Petyuk, V.; Nesvizhskii, A.I.; Zhu, Y. Evaluating Linear Ion Trap for MS3-Based Multiplexed Single-Cell Proteomics. Anal. Chem. 2023, 95, 1888–1898.
- Srinivasan, A.; Sing, J.C.; Gingras, A.C.; Rost, H.L. Improving Phosphoproteomics Profiling Using Data-Independent Mass Spectrometry. J. Proteom. Res. 2022, 21, 1789–1799.
- Yang, Y.; Qiao, L. Data-independent acquisition proteomics methods for analyzing post-translational modifications. Proteomics 2023, 23, e2200046.
- Chan, A.W.; Broncel, M.; Yifrach, E.; Haseley, N.R.; Chakladar, S.; Andree, E.; Herneisen, A.L.; Shortt, E.; Treeck, M.; Lourido, S. Analysis of CDPK1 targets identifies a trafficking adaptor complex that regulates microneme exocytosis in Toxoplasma. eLife 2023, 12, RP85654.
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin. Proteom. 2023, 20, 32.
- Jean Beltran, P.M.; Federspiel, J.D.; Sheng, X.; Cristea, I.M. Proteomics and integrative omic approaches for understanding host-pathogen interactions and infectious diseases. Mol. Syst. Biol. 2017, 13, 922.
- Kloehn, J.; Oppenheim, R.D.; Siddiqui, G.; De Bock, P.J.; Kumar Dogga, S.; Coute, Y.; Hakimi, M.A.; Creek, D.J.; Soldati-Favre, D. Multi-omics analysis delineates the distinct functions of sub-cellular acetyl-CoA pools in Toxoplasma gondii. BMC Biol. 2020, 18, 67.
- Adomako-Ankomah, Y.; Wier, G.M.; Boyle, J.P. Beyond the genome: Recent advances in Toxoplasma gondii functional genomics. Parasite Immunol. 2012, 34, 80–89.
- Syn, G.; Blackwell, J.M.; Jamieson, S.E.; Francis, R.W. An in silico pipeline to filter the Toxoplasma gondii proteome for proteins that could traffic to the host cell nucleus and influence host cell epigenetic regulation. Mem. Inst. Oswaldo Cruz 2018, 113, e170471.
- Hammed-Akanmu, M.; Mim, M.; Osman, A.Y.; Sheikh, A.M.; Behmard, E.; Rabaan, A.A.; Suppain, R.; Hajissa, K. Designing a Multi-Epitope Vaccine against Toxoplasma gondii: An Immunoinformatics Approach. Vaccines 2022, 10, 1389.
- Ayub, F.; Ahmed, H.; Sohail, T.; Shahzad, K.; Celik, F.; Wang, X.; Simsek, S.; Cao, J. Bioinformatics-based prediction and screening of immunogenic epitopes of Toxoplasma gondii rhoptry proteins 7, 21 and 22 as candidate vaccine target. Heliyon 2023, 9, e18176.
- Onile, O.S.; Ojo, G.J.; Oyeyemi, B.F.; Agbowuro, G.O.; Fadahunsi, A.I. Development of multiepitope subunit protein vaccines against Toxoplasma gondii using an immunoinformatics approach. NAR Genom. Bioinform. 2020, 2, lqaa048.
- Rashidi, S.; Sanchez-Montejo, J.; Mansouri, R.; Ali-Hassanzadeh, M.; Savardashtaki, A.; Bahreini, M.S.; Karimazar, M.; Manzano-Roman, R.; Nguewa, P. Mining the Proteome of Toxoplasma Parasites Seeking Vaccine and Diagnostic Candidates. Animals 2022, 12, 1098.
- Hajissa, K.; Zakaria, R.; Suppian, R.; Mohamed, Z. Design and evaluation of a recombinant multi-epitope antigen for serodiagnosis of Toxoplasma gondii infection in humans. Parasit. Vectors 2015, 8, 315.
- Goodswen, S.J.; Kennedy, P.J.; Ellis, J.T. A state-of-the-art methodology for high-throughput in silico vaccine discovery against protozoan parasites and exemplified with discovered candidates for Toxoplasma gondii. Sci. Rep. 2023, 13, 8243.
- Date, S.V.; Stoeckert, C.J., Jr. Computational modeling of the Plasmodium falciparum interactome reveals protein function on a genome-wide scale. Genome Res. 2006, 16, 542–549.
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646.
- Anghel, N.; Muller, J.; Serricchio, M.; Jelk, J.; Butikofer, P.; Boubaker, G.; Imhof, D.; Ramseier, J.; Desiatkina, O.; Paunescu, E.; et al. Cellular and Molecular Targets of Nucleotide-Tagged Trithiolato-Bridged Arene Ruthenium Complexes in the Protozoan Parasites Toxoplasma gondii and Trypanosoma brucei. Int. J. Mol. Sci. 2021, 22, 10787.
- Shkel, O.; Kharkivska, Y.; Kim, Y.K.; Lee, J.S. Proximity Labeling Techniques: A Multi-Omics Toolbox. Chem. Asian J. 2022, 17, e202101240.
- Back, P.S.; Moon, A.S.; Pasquarelli, R.R.; Bell, H.N.; Torres, J.A.; Chen, A.L.; Sha, J.; Vashisht, A.A.; Wohlschlegel, J.A.; Bradley, P.J. IMC29 Plays an Important Role in Toxoplasma Endodyogeny and Reveals New Components of the Daughter-Enriched IMC Proteome. mBio 2023, 14, e0304222.
- Engelberg, K.; Bechtel, T.; Michaud, C.; Weerapana, E.; Gubbels, M.J. Proteomic characterization of the Toxoplasma gondii cytokinesis machinery portrays an expanded hierarchy of its assembly and function. Nat. Commun. 2022, 13, 4644.
- Trinkle-Mulcahy, L. Recent advances in proximity-based labeling methods for interactome mapping. F1000Research 2019, 8, 135.
- Samavarchi-Tehrani, P.; Samson, R.; Gingras, A.C. Proximity Dependent Biotinylation: Key Enzymes and Adaptation to Proteomics Approaches. Mol. Cell. Proteom. 2020, 19, 757–773.
- Pan, M.; Li, M.; Li, L.; Song, Y.; Hou, L.; Zhao, J.; Shen, B. Identification of Novel Dense-Granule Proteins in Toxoplasma gondii by Two Proximity-Based Biotinylation Approaches. J. Proteome Res. 2019, 18, 319–330.
- Lai, M.Y.; Abdul-Majid, N.; Lau, Y.L. Identification of Host Proteins Interacting with Toxoplasma gondii SAG1 by Yeast Two-Hybrid Assay. Acta Parasitol. 2019, 64, 575–581.
- Lai, M.Y.; Lau, Y.L. Screening and identification of host proteins interacting with Toxoplasma gondii SAG2 by yeast two-hybrid assay. Parasit. Vectors 2017, 10, 456.
- Harb, O.S.; Roos, D.S. ToxoDB: Functional Genomics Resource for Toxoplasma and Related Organisms. Methods Mol. Biol. 2020, 2071, 27–47.
- Xia, D.; Sanderson, S.J.; Jones, A.R.; Prieto, J.H.; Yates, J.R.; Bromley, E.; Tomley, F.M.; Lal, K.; Sinden, R.E.; Brunk, B.P.; et al. The proteome of Toxoplasma gondii: Integration with the genome provides novel insights into gene expression and annotation. Genome Biol. 2008, 9, R116.
- Possenti, A.; Fratini, F.; Fantozzi, L.; Pozio, E.; Dubey, J.P.; Ponzi, M.; Pizzi, E.; Spano, F. Global proteomic analysis of the oocyst/sporozoite of Toxoplasma gondii reveals commitment to a host-independent lifestyle. BMC Genom. 2013, 14, 183.
- Nardelli, S.C.; Che, F.Y.; Silmon de Monerri, N.C.; Xiao, H.; Nieves, E.; Madrid-Aliste, C.; Angel, S.O.; Sullivan, W.J., Jr.; Angeletti, R.H.; Kim, K.; et al. The histone code of Toxoplasma gondii comprises conserved and unique posttranslational modifications. mBio 2013, 4, e00922-13.
- Li, F.C.; Nie, L.B.; Elsheikha, H.M.; Yin, F.Y.; Zhu, X.Q. Lysine crotonylation is widespread on proteins of diverse functions and localizations in Toxoplasma gondii. Parasitol. Res. 2021, 120, 1617–1626.
- Steinberg, H.E.; Russo, P.; Angulo, N.; Ynocente, R.; Montoya, C.; Diestra, A.; Ferradas, C.; Schiaffino, F.; Florentini, E.; Jimenez, J.; et al. Toward detection of toxoplasmosis from urine in mice using hydro-gel nanoparticles concentration and parallel reaction monitoring mass spectrometry. Nanomedicine 2018, 14, 461–469.
- Bansal, P.; Antil, N.; Kumar, M.; Yamaryo-Botte, Y.; Rawat, R.S.; Pinto, S.; Datta, K.K.; Katris, N.J.; Botte, C.Y.; Prasad, T.S.K.; et al. Protein kinase TgCDPK7 regulates vesicular trafficking and phospholipid synthesis in Toxoplasma gondii. PLoS Pathog. 2021, 17, e1009325.
- Montano, H.; Anandkrishnan, R.; Carruthers, V.B.; Gaji, R.Y. TgTKL4 Is a Novel Kinase That Plays an Important Role in Toxoplasma Morphology and Fitness. mSphere 2023, 8, e0064922.
- Yang, C.; Doud, E.H.; Sampson, E.; Arrizabalaga, G. The protein phosphatase PPKL is a key regulator of daughter parasite development in Toxoplasma gondii. mBio 2023, 14, e0225423.
- Wang, Z.X.; Hu, R.S.; Zhu, X.Q.; Sun, X.L.; Elsheikha, H.M. Global phosphoproteome analysis reveals significant differences between sporulated oocysts of virulent and avirulent strains of Toxoplasma gondii. Microb. Pathog. 2021, 161 Pt A, 105240.
- Treeck, M.; Sanders, J.L.; Gaji, R.Y.; LaFavers, K.A.; Child, M.A.; Arrizabalaga, G.; Elias, J.E.; Boothroyd, J.C. The calcium-dependent protein kinase 3 of toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative phosphoproteome analysis. PLoS Pathog. 2014, 10, e1004197.
- Caballero, M.C.; Alonso, A.M.; Deng, B.; Attias, M.; de Souza, W.; Corvi, M.M. Identification of new palmitoylated proteins in Toxoplasma gondii. Biochim. Biophys. Acta 2016, 1864, 400–408.
- Foe, I.T.; Child, M.A.; Majmudar, J.D.; Krishnamurthy, S.; van der Linden, W.A.; Ward, G.E.; Martin, B.R.; Bogyo, M. Global Analysis of Palmitoylated Proteins in Toxoplasma gondii. Cell Host Microbe 2015, 18, 501–511.
- Wang, Z.; Li, J.; Yang, Q.; Sun, X. Global Proteome-Wide Analysis of Cysteine S-Nitrosylation in Toxoplasma gondii. Molecules 2023, 28, 7329.
- Grimes, M.; Hall, B.; Foltz, L.; Levy, T.; Rikova, K.; Gaiser, J.; Cook, W.; Smirnova, E.; Wheeler, T.; Clark, N.R.; et al. Integration of protein phosphorylation, acetylation, and methylation data sets to outline lung cancer signaling networks. Sci. Signal 2018, 11, eaaq1087.
- Ross, K.E.; Zhang, G.; Akcora, C.; Lin, Y.; Fang, B.; Koomen, J.; Haura, E.B.; Grimes, M. Network models of protein phosphorylation, acetylation, and ubiquitination connect metabolic and cell signaling pathways in lung cancer. PLoS Comput. Biol. 2023, 19, e1010690.
- Silmon de Monerri, N.C.; Yakubu, R.R.; Chen, A.L.; Bradley, P.J.; Nieves, E.; Weiss, L.M.; Kim, K. The Ubiquitin Proteome of Toxoplasma gondii Reveals Roles for Protein Ubiquitination in Cell-Cycle Transitions. Cell Host Microbe 2015, 18, 621–633.
- Hu, K.; Johnson, J.; Florens, L.; Fraunholz, M.; Suravajjala, S.; DiLullo, C.; Yates, J.; Roos, D.S.; Murray, J.M. Cytoskeletal components of an invasion machine--the apical complex of Toxoplasma gondii. PLoS Pathog. 2006, 2, e13.
- Ramirez-Flores, C.J.; Cruz-Miron, R.; Mondragon-Castelan, M.E.; Gonzalez-Pozos, S.; Rios-Castro, E.; Mondragon-Flores, R. Proteomic and structural characterization of self-assembled vesicles from excretion/secretion products of Toxoplasma gondii. J. Proteom. 2019, 208, 103490.
- Fleige, T.; Pfaff, N.; Gross, U.; Bohne, W. Localisation of gluconeogenesis and tricarboxylic acid (TCA)-cycle enzymes and first functional analysis of the TCA cycle in Toxoplasma gondii. Int. J. Parasitol. 2008, 38, 1121–1132.
- Seidi, A.; Muellner-Wong, L.S.; Rajendran, E.; Tjhin, E.T.; Dagley, L.F.; Aw, V.Y.; Faou, P.; Webb, A.I.; Tonkin, C.J.; van Dooren, G.G. Elucidating the mitochondrial proteome of Toxoplasma gondii reveals the presence of a divergent cytochrome c oxidase. eLife 2018, 7, e38131.
- Elzek, M.A.W.; Christopher, J.A.; Breckels, L.M.; Lilley, K.S. Localization of Organelle Proteins by Isotope Tagging: Current status and potential applications in drug discovery research. Drug Discov. Today Technol. 2021, 39, 57–67.
- Benns, H.J.; Storch, M.; Falco, J.A.; Fisher, F.R.; Tamaki, F.; Alves, E.; Wincott, C.J.; Milne, R.; Wiedemar, N.; Craven, G.; et al. CRISPR-based oligo recombineering prioritizes apicomplexan cysteines for drug discovery. Nat. Microbiol. 2022, 7, 1891–1905.
- Freville, A.; Gnangnon, B.; Khelifa, A.S.; Gissot, M.; Khalife, J.; Pierrot, C. Deciphering the Role of Protein Phosphatases in Apicomplexa: The Future of Innovative Therapeutics? Microorganisms 2022, 10, 585.
- Broncel, M.; Dominicus, C.; Vigetti, L.; Nofal, S.D.; Bartlett, E.J.; Touquet, B.; Hunt, A.; Wallbank, B.A.; Federico, S.; Matthews, S.; et al. Profiling of myristoylation in Toxoplasma gondii reveals an N-myristoylated protein important for host cell penetration. eLife 2020, 9, e57861.
- Liu, Y.; Tang, Y.; Tang, X.; Wu, M.; Hou, S.; Liu, X.; Li, J.; Deng, M.; Huang, S.; Jiang, L. Anti-Toxoplasma gondii Effects of a Novel Spider Peptide XYP1 In Vitro and In Vivo. Biomedicines 2021, 9, 934.
- Che, F.Y.; Madrid-Aliste, C.; Burd, B.; Zhang, H.; Nieves, E.; Kim, K.; Fiser, A.; Angeletti, R.H.; Weiss, L.M. Comprehensive proteomic analysis of membrane proteins in Toxoplasma gondii. Mol. Cell. Proteom. 2011, 10, M110.000745.
- Maran, S.R.; Fleck, K.; Monteiro-Teles, N.M.; Isebe, T.; Walrad, P.; Jeffers, V.; Cestari, I.; Vasconcelos, E.J.R.; Moretti, N. Protein acetylation in the critical biological processes in protozoan parasites. Trends Parasitol. 2021, 37, 815–830.
- Renaud, E.A.; Pamukcu, S.; Cerutti, A.; Berry, L.; Lemaire-Vieille, C.; Yamaryo-Botte, Y.; Botte, C.Y.; Besteiro, S. Disrupting the plastidic iron-sulfur cluster biogenesis pathway in Toxoplasma gondii has pleiotropic effects irreversibly impacting parasite viability. J. Biol. Chem. 2022, 298, 102243.
- Zhang, H.; Lee, E.G.; Yu, L.; Kawano, S.; Huang, P.; Liao, M.; Kawase, O.; Zhang, G.; Zhou, J.; Fujisaki, K.; et al. Identification of the cross-reactive and species-specific antigens between Neospora caninum and Toxoplasma gondii tachyzoites by a proteomics approach. Parasitol. Res. 2011, 109, 899–911.
- Sander, V.A.; Sanchez Lopez, E.F.; Mendoza Morales, L.; Ramos Duarte, V.A.; Corigliano, M.G.; Clemente, M. Use of Veterinary Vaccines for Livestock as a Strategy to Control Foodborne Parasitic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 288.
- Muller, J.; Hemphill, A. Toxoplasma gondii infection: Novel emerging therapeutic targets. Expert Opin. Ther. Targets 2023, 27, 293–304.
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587.
- Thabet, A.; Schmidt, J.; Baumann, S.; Honscha, W.; von Berger, M.; Daugschies, A.; Bangoura, B. Resistance towards monensin is proposed to be acquired in a Toxoplasma gondii model by reduced invasion and egress activities, in addition to increased intracellular replication. Parasitology 2018, 145, 313–325.
This entry is offline, you can click here to edit this entry!
