Atrial fibrillation (AF) is the most common sustained arrythmia and one of the strongest risk factors and causal mechanisms of ischemic stroke (IS). Acute IS due to AF tends to be more severe than with other etiology of IS and patients with treated AF have reported to experience worse outcomes after endovascular treatment compared with patients without AF. As cardioembolism accounts for more than a fifth of ISs and the risk of future stroke can be mitigated with effective anticoagulation, which has been shown to be effective and safe in patients with paroxysmal or sustained AF, the screening of patients with cryptogenic IS (CIS) for AF is paramount.
- atrial fibrillation
- hemorrhagic stroke
- ischemic stroke
1. Introduction
2. Primary and Secondary Prevention of IS in Patients with AF
2.1. Timing of OAC Initiation after IS and Its Impact on Short-Term Outcomes
2.2. LAA
| Early vs. Late Initiation of Anticoagulation after Ischemic Stroke | |||||
|---|---|---|---|---|---|
| Trial | Intervention | Primary Outcome | Time Frame | Study Sites | Sample Size |
| OPTIMAS (NCT03759938) |
Early (within 4 days) vs. standard (7 to 14 days) initiation of DOAC | Stroke (ischemic or hemorrhagic) or systemic embolism. | 3 months | United Kingdom | 3478 |
| Left atrial appendage closure after ischemic stroke | |||||
| Occlusion-AF (NCT03642509) |
LAAC vs. DOAC within 180 after ischemic stroke | Stroke (ischemic or hemorrhagic), systemic embolism, major bleeding, or all-cause mortality. | 5 years | Scandinavia | 750 |
| ELAPSE | LAAC and DOAC vs. DOAC alone | Ischemic stroke, systemic embolism, and cardiovascular death. | 4 years | Not yet provided | 482 |
| LAAOS-4 (NCT05963698) |
LAAC and OAC vs. OAC alone | Ischemic stroke or systemic embolism. | 4 years | Not yet provided | 4000 |
| Other secondary prevention after ischemic stroke | |||||
| INTERCEPT (NCT05723926) |
Bilateral carotid filter implants and OAC vs. OAC alone | Ischemic stroke. | UNK | Not yet provided | 200 |
| STABLED (NCT03777631) |
Catheter ablation and OAC vs. OAC alone 1 to 6 months after ischemic stroke | Ischemic stroke, systemic embolism, all-cause death, and hospitalization for heart failure. | 3 years | Japan | 250 |
| OCEANIC-AF (NCT05643573) |
FXIa inhibitor (asundexian) vs. apixaban mainly as primary prevention but also includes patients with prior ischemic stroke or TIA | Stroke (ischemic or hemorrhagic) or systemic embolism. | 3 years | America, Europe, Asia, Australia | 18,000 |
| LIBREXIA-AF (NCT05757869) |
FXIa inhibitor (milvexian) vs. apixaban mainly as primary prevention but also includes patients with prior ischemic stroke or TIA | Stroke (ischemic or hemorrhagic) or systemic embolism. | 4 years | America, Europe, Asia, Australasia, Africa | 15,500 |
| Oral anticoagulation resumption intracerebral hemorrhage | |||||
| ASPIRE (NCT03907046) |
Apixaban vs. aspirin 15 to 180 days after ICH | Stroke (ischemic or hemorrhagic) or all-cause mortality. Time frame 3 years. |
3 years | United States | 700 |
| ENRICH-AF (NCT03950076) |
Edoxaban vs. either no antithrombotic therapy or antiplatelet monotherapy | Stroke (ischemic or hemorrhagic) or major hemorrhage. | 2 years | America, Europe, Asia, Africa | 1200 |
| PRESTIGE-AF (NCT03996772) |
DOAC vs. no anticoagulation 15 to 180 days after ICH | Stroke (ischemic or hemorrhagic). | 3 years | Europe | 350 |
| STATICH (NCT03186729) |
Anticoagulant treatment vs. no anticoagulant treatment 1 to 180 days after ICH | Fatal or non-fatal symptomatic recurrent ICH. | 2 years | Scandinavia | 500 |
| Left atrial appendage closure after intracerebral hemorrhage | |||||
| A3ICH (NCT03243175) |
Apixaban vs. LAAC vs. no intervention at least 14 days from ICH | Fatal or non-fatal major cardiovascular/cerebrovascular ischemic or hemorrhagic events. | 2 years | France | 300 |
| STROKECLOSE (NCT02830152) |
LAAO vs. medical therapy after 4 to 52 weeks after ICH | Stroke (ischemic or hemorrhagic), systemic embolism, life-threatening or major bleeding, or all-cause mortality. | 5 years | Europe | 750 |
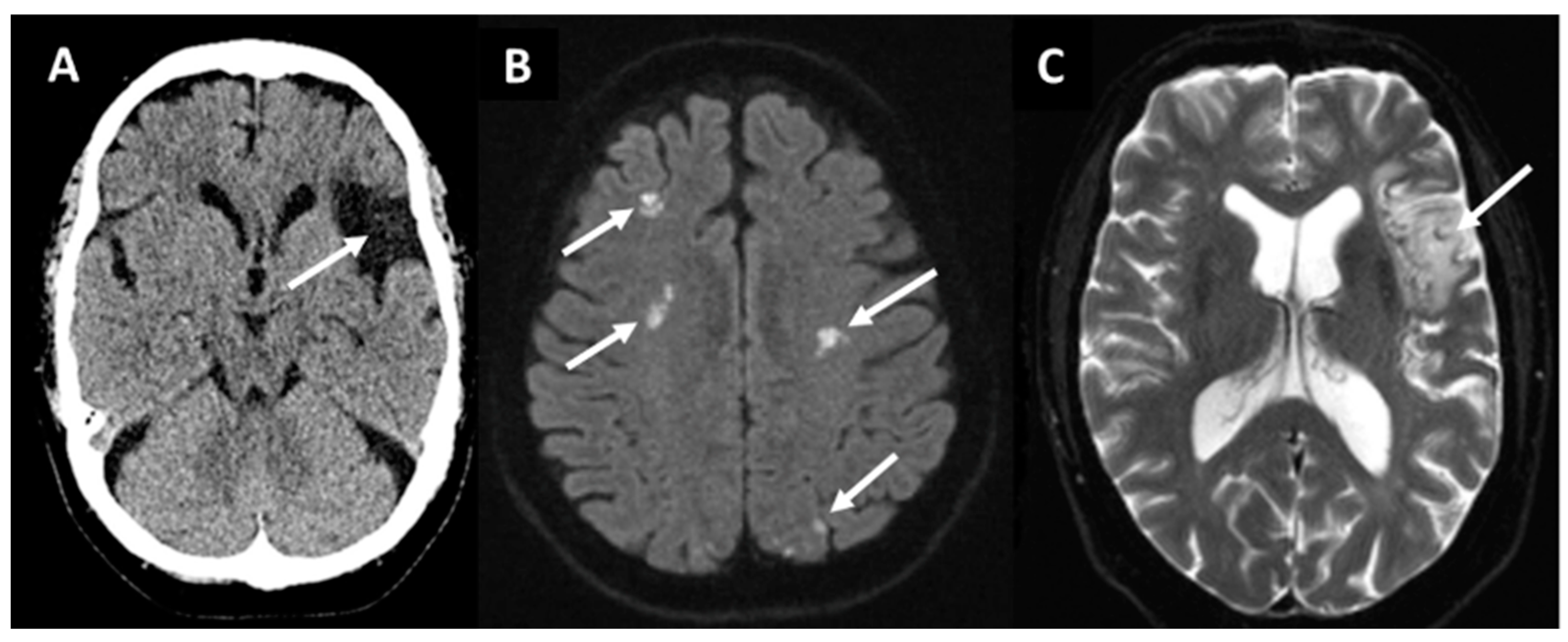
3. Acute Recanalization Treatment and Its Outcomes after AF-Associated IS
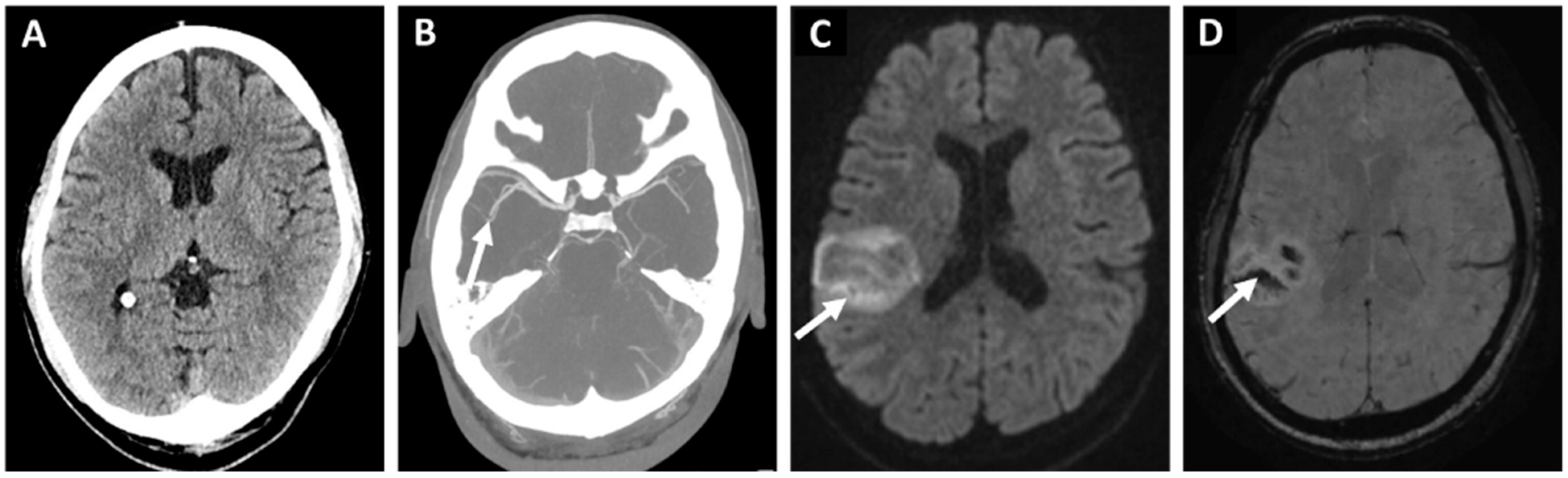
4. AF and Hemorrhagic Stroke
This entry is adapted from the peer-reviewed paper 10.3390/jcm13010030
References
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142.
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820.
- Teppo, K.; Airaksinen, K.E.J.; Jaakkola, J.; Halminen, O.; Linna, M.; Haukka, J.; Putaala, J.; Mustonen, P.; Kinnunen, J.; Hartikainen, J.; et al. Trends in treatment and outcomes of atrial fibrillation during 2007–17 in Finland. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 9, qcac086.
- Choi, S.E.; Sagris, D.; Hill, A.; Lip, G.Y.H.; Abdul-Rahim, A.H. Atrial fibrillation and stroke. Expert Rev. Cardiovasc. Ther. 2023, 21, 35–56.
- Kammersgaard, L.P.; Olsen, T.S. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc. Dis. 2006, 21, 187–193.
- Lane, D.A.; Skjøth, F.; Lip, G.Y.H.; Larsen, T.B.; Kotecha, D. Temporal Trends in Incidence, Prevalence, and Mortality of Atrial Fibrillation in Primary Care. J. Am. Heart Assoc. 2017, 6, e005155.
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke 2005, 36, 1115–1119.
- Katsanos, A.H.; Kamel, H.; Healey, J.S.; Hart, R.G. Stroke Prevention in Atrial Fibrillation: Looking Forward. Circulation 2020, 142, 2371–2388.
- Lip, G.Y.H.; Lane, D.A. Stroke Prevention in Atrial Fibrillation: A Systematic Review. JAMA 2015, 313, 1950–1962.
- Li, L.; Yiin, G.S.; Geraghty, O.C.; Schulz, U.G.; Kuker, W.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: A population-based study. Lancet Neurol. 2015, 14, 903–913.
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014, 13, 429–438.
- Lehto, M.; Haukka, J.; Aro, A.; Halminen, O.; Putaala, J.; Linna, M.; Mustonen, P.; Kinnunen, J.; Kouki, E.; Niiranen, J.; et al. Comprehensive nationwide incidence and prevalence trends of atrial fibrillation in Finland. Open Heart 2022, 9, e002140.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Gheorghe-Andrei, D.; Polychronis, E.D.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498.
- Escudero-Martínez, I.; Morales-Caba, L.; Segura, T. Atrial fibrillation and stroke: A review and new insights. Trends Cardiovasc. Med. 2023, 33, 23–29.
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526.
- Sharobeam, A.; Churilov, L.; Parsons, M.; Donnan, G.A.; Davis, S.M.; Yan, B. Patterns of Infarction on MRI in Patients with Acute Ischemic Stroke and Cardio-Embolism: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 606521.
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340.
- Rubiera, M.; Aires, A.; Antonenko, K.; Lémeret, S.; Nolte, C.H.; Putaala, J.; Schabel, R.B.; Tuladhar, A.M.; Werring, D.J.; Zeraatkar, D.; et al. European Stroke Organisation (ESO) guideline on screening for subclinical atrial fibrillation after stroke or transient ischaemic attack of undetermined origin. Eur. Stroke J. 2022, 7, VI.
- Sagris, D.; Harrison, S.L.; Buckley, B.J.R.; Ntaios, G.; Lip, G.Y.H. Long-Term Cardiac Monitoring After Embolic Stroke of Undetermined Source: Search Longer, Look Harder. Am. J. Med. 2022, 135, e311–e317.
- Kalscheur, M.M.; Goldberger, Z.D. Screening for Atrial Fibrillation—Refining the Target. JAMA Netw. Open 2022, 5, e2139910.
- Hannon, N.; Daly, L.; Murphy, S.; Smith, S.; Hayden, D.; Ní Chróinín, D.; Callaly, E.; Horgan, G.; Sheehan, O.; Honari, B.; et al. Acute hospital, community, and indirect costs of stroke associated with atrial fibrillation: Population-based study. Stroke 2014, 45, 3670–3674.
- Hannon, N.; Sheehan, O.; Kelly, L.; Marnane, M.; Merwick, A.; Moore, A.; Kyne, L.; Duggan, J.; Moroney, J.; McCormack, P.M.E.; et al. Stroke associated with atrial fibrillation—Incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc. Dis. 2010, 29, 43–49.
- Lin, H.J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.S.; Kase, C.S.; Benjamin, E.J.; D’Agostino, B.R.B. Stroke Severity in Atrial Fibrillation. Stroke 1996, 27, 1760–1764.
- Jørgensen, H.S.; Nakayama, H.; Reith, J.; Raaschou, H.O.; Olsen, T.S. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996, 27, 1765–1769.
- Kimura, K.; Minematsu, K.; Yamaguchi, T. Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 679–683.
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. Long-term survival after ischemic stroke in patients with atrial fibrillation. Neurology 2014, 82, 1033–1037.
- Frost, L.; Andersen, L.V.; Vestergaard, P.; Husted, S.; Mortensen, L.S. Trend in mortality after stroke with atrial fibrillation. Am. J. Med. 2007, 120, 47–53.
- Gao, X.; Passman, R. Stroke Prevention in Atrial Fibrillation. Curr. Cardiol. Rep. 2022, 24, 1765–1774.
- Hindsholm, M.F.; Damgaard, D.; Gurol, M.E.; Gaist, D.; Simonsen, C.Z. Management and Prognosis of Acute Stroke in Atrial Fibrillation. J. Clin. Med. 2023, 12, 5752.
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.J.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132.
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; John Camm, A.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962.
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992.
- Van de Werf, F.; Brueckmann, M.; Connolly, S.J.; Friedman, J.; Granger, C.B.; Härtter, S.; Harper, R.; Kappetein, A.P.; Lehr, T.; Mack, M.J.; et al. A comparison of dabigatran etexilate with warfarin in patients with mechanical heart valves: THE Randomized, phase II study to evaluate the safety and pharmacokinetics of oral dabigatran etexilate in patients after heart valve replacement (RE-ALIGN). Am. Heart J. 2012, 163, 931–937.e1.
- Piccini, J.P.; Caso, V.; Connolly, S.J.; Fox, K.A.A.; Oldgren, J.; Jones, W.S.; Gorog, D.A.; Durdil, V.; Viethen, T.; Neumann, C.; et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): A multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022, 399, 1383–1390.
- Klijn, C.J.; Paciaroni, M.; Berge, E.; Korompoki, E.; Kõrv, J.; Lal, A.; Putaala, J.; Werring, D.J. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: A European Stroke Organisation guideline. Eur. Stroke J. 2019, 4, 198–223.
- Verheugt, F.W.A.; Ambrosio, G.; Atar, D.; Bassand, J.P.; Camm, A.J.; Costabel, J.P.; Fitzmaurice, D.A.; Illingworth, L.; Goldhaber, S.Z.; Goto, S.; et al. Outcomes in Newly Diagnosed Atrial Fibrillation and History of Acute Coronary Syndromes: Insights from GARFIELD-AF. Am. J. Med. 2019, 132, 1431–1440.e7.
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; John Camm, A.; Kirchhof, P. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur. Heart J. 2013, 34, 2094–2106.
- Oldgren, J.; Åsberg, S.; Hijazi, Z.; Wester, P.; Bertilsson, M.; Norrving, B. Early Versus Delayed Non-Vitamin K Antagonist Oral Anticoagulant Therapy After Acute Ischemic Stroke in Atrial Fibrillation (TIMING): A Registry-Based Randomized Controlled Noninferiority Study. Circulation 2022, 146, 1056–1066.
- Fischer, U.; Koga, M.; Strbian, D.; Branca, M.; Abend, S.; Trelle, S.; Paciaroni, M.; Thomalla, G.; Michel, P.; Nedeltchev, K.; et al. Early versus Later Anticoagulation for Stroke with Atrial Fibrillation. N. Engl. J. Med. 2023, 388, 2411–2421.
- Altavilla, R.; Caso, V.; Bandini, F.; Agnelli, G.; Tsivgoulis, G.; Yaghi, S.; Furie, K.L.; Tadi, P.; Becattini, C.; Zedde, M.; et al. Anticoagulation After Stroke in Patients with Atrial Fibrillation. Stroke 2019, 50, 2093–2100.
- Paciaroni, M.; Caso, V.; Agnelli, G.; Mosconi, M.G.; Giustozzi, M.; Seiffge, D.J.; Engelter, S.T.; Lyrer, P.; Polymeris, A.A.; Kriemler, L.; et al. Recurrent Ischemic Stroke and Bleeding in Patients with Atrial Fibrillation Who Suffered an Acute Stroke While on Treatment with Nonvitamin K Antagonist Oral Anticoagulants: The RENO-EXTEND Study. Stroke 2022, 53, 2620–2627.
- Galea, R.; Seiffge, D.; Räber, L. Atrial Fibrillation and Ischemic Stroke despite Oral Anticoagulation. J. Clin. Med. 2023, 12, 5784.
- Seiffge, D.J.; De Marchis, G.M.; Koga, M.; Paciaroni, M.; Wilson, D.; Cappellari, M.; Macha, M.D.K.; Tsivgoulis, G.; Ambler, G.; Arihiro, S.; et al. Ischemic Stroke despite Oral Anticoagulant Therapy in Patients with Atrial Fibrillation. Ann. Neurol. 2020, 87, 677–687.
- Yaghi, S.; Henninger, N.; Giles, J.A.; Leon Guerrero, C.; Mistry, E.; Liberman, A.L.; Asad, D.; Liu, A.; Nagy, M.; Kaushai, A.; et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: The IAC study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1062–1067.
- Kobeissi, H.; Ghozy, S.; Seymour, T.; Gupta, R.; Bilgin, C.; Kadirvel, R.; Rabinstein, A.A.; Kallmes, D.F. Outcomes of Patients with Atrial Fibrillation Following Thrombectomy for Stroke: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e2249993.
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.D., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361.
- Dowlatshahi, D.; Butcher, K.S.; Asdaghi, N.; Nahirniak, S.; Bernbaum, M.L.; Giulivi, A.; Wasserman, J.K.; Poon, M.C.; Coutts, S.B.; Canadian PCC Registry (CanPro) Investigstors. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke 2012, 43, 1812–1817.
- Zeng, Z.; Chen, J.; Qian, J.; Ma, F.; Lv, M.; Zhang, J. Risk Factors for Anticoagulant-Associated Intracranial Hemorrhage: A Systematic Review and Meta-analysis. Neurocrit. Care 2023, 38, 812–820.
