Pathogenic detection in wastewater samples (WS) plays a key role in safeguarding public health and the environment. Accurate pathogenic detection is not only essential for preventing the spread of waterborne diseases, but also for preserving the ecological integrity of natural water bodies. It is also vital to monitor and detect pathogens in wastewater samples for assessing the effectiveness of wastewater treatment processes, which will help in determining the sufficiency of treatment facilities in eliminating pathogens before discharging treated wastewater into the environment. Pathogenic detection in wastewater can serve as an early warning system for potential disease outbreaks. By monitoring the presence of specific pathogens in wastewater, proactive measures can be taken to protect public health and mitigate the spread of diseases.
- pathogenic detection
- wastewater
- free nucleic acids
- pretreatment methods
- environmental analysis
1. Introduction
2. Overview of Pretreatment Techniques
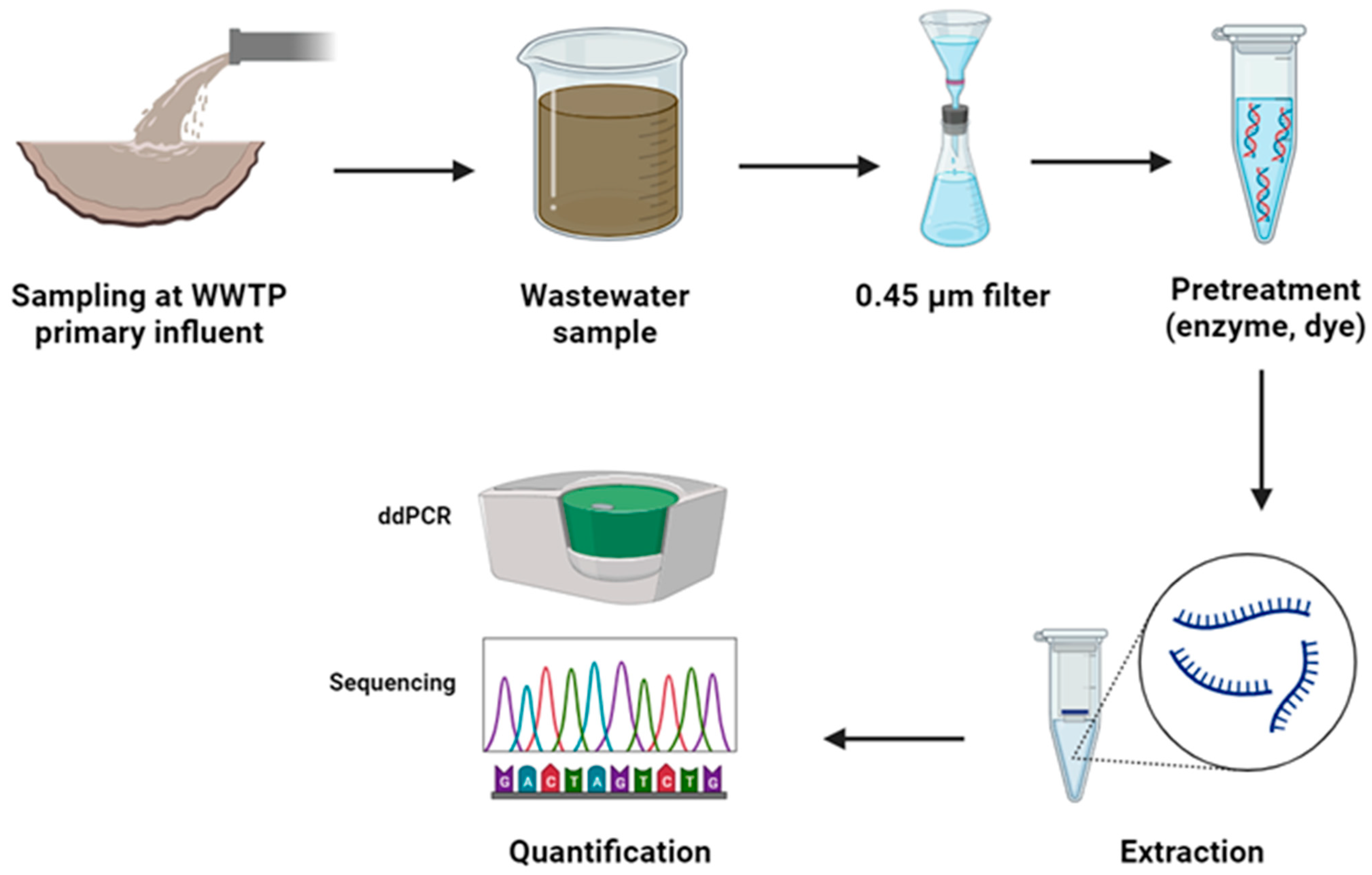
3. Physical Pretreatment Methods
3.1. Filtration
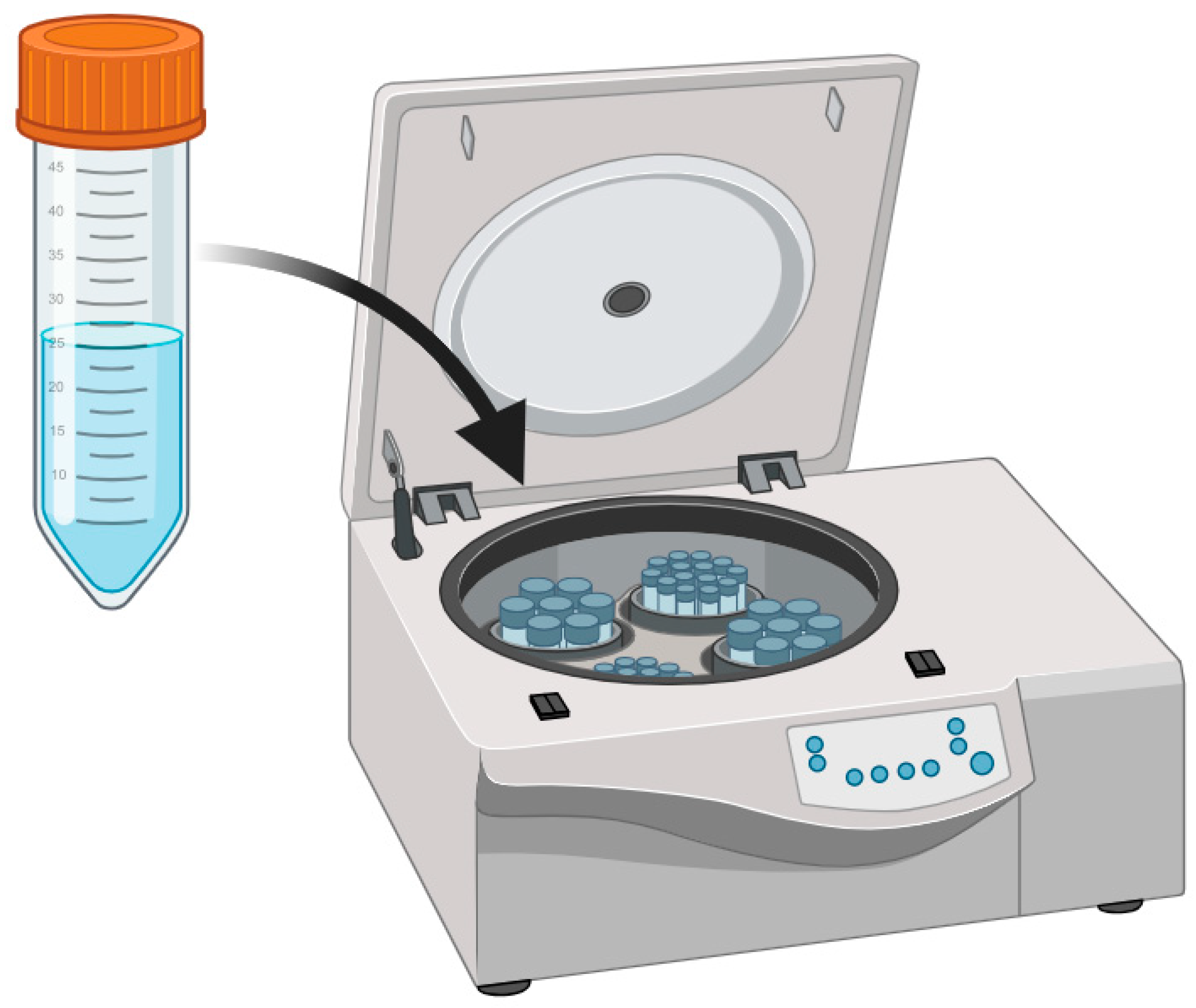
3.2. Centrifugation
3.3. Ultrasonication
4. Chemical Pretreatment Methods
4.1. Precipitation
4.2. Organic Extraction
4.3. Dye Treatment Method
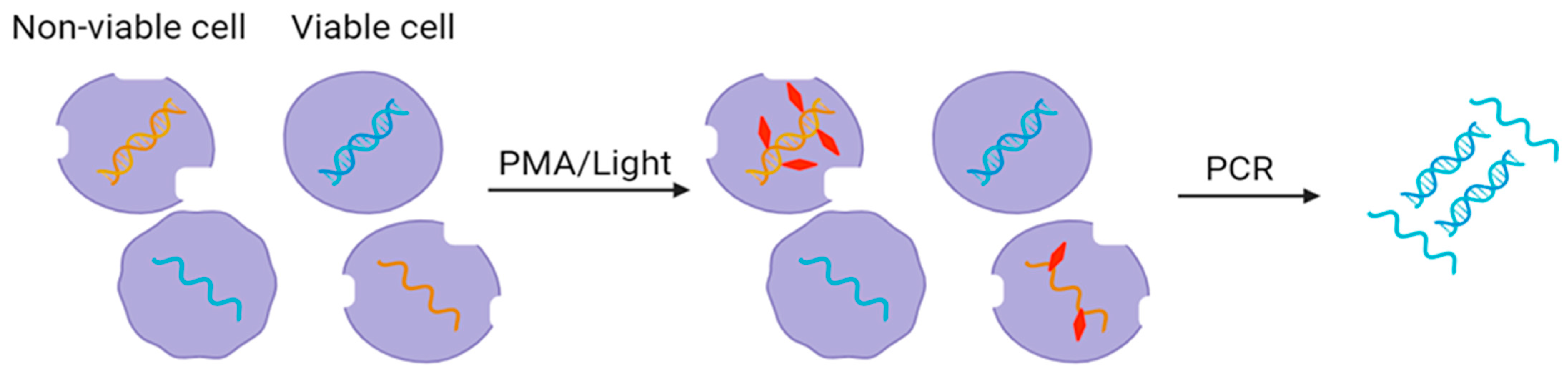
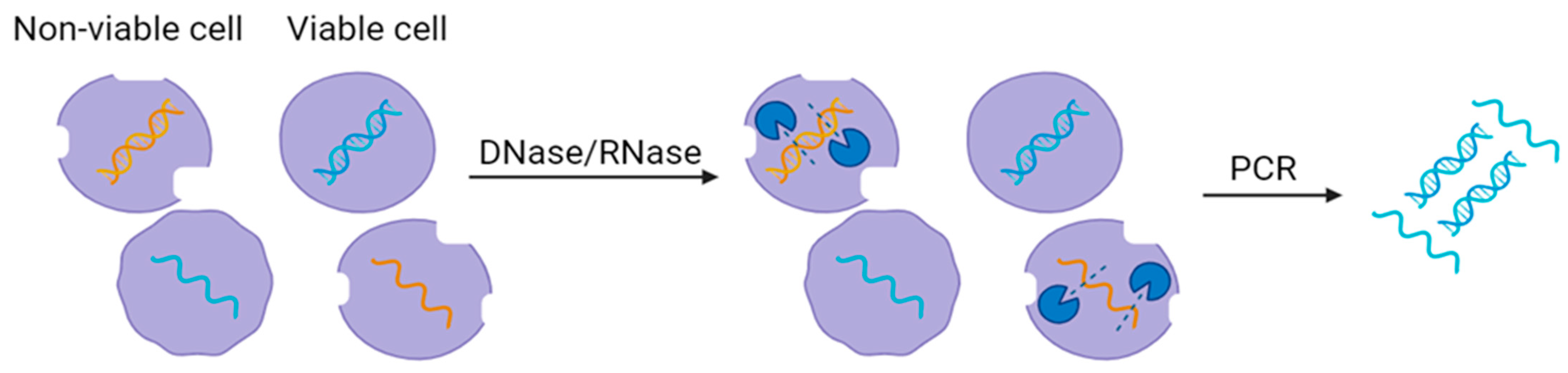
5. Enzymatic Pretreatment Methods
This entry is adapted from the peer-reviewed paper 10.3390/applmicrobiol4010001
References
- Rani, M.; Paul, B.; Bhattacharjee, A.; Das, K.; Singh, P.; Basu, S.; Pandey, S.; Tripathi, D.; Kumar, A. Chapter 13—Detection and removal of pathogenic bacteria from wastewater using various nanoparticles. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 311–322.
- Bej, S.; Swain, S.; Bishoyi, A.K.; Mandhata, C.P.; Sahoo, C.R.; Padhy, R.N. Wastewater-Associated Infections: A Public Health Concern. Water Air Soil Pollut. 2023, 234, 444.
- Yesilay, G.; Dos Santos, O.A.L.; Hazeem, L.J.; Backx, B.P.; Kamel, A.H.; Bououdina, M. Impact of pathogenic bacterial communities present in wastewater on aquatic organisms: Application of nanomaterials for the removal of these pathogens. Aquat. Toxicol. 2023, 261, 106620.
- Pratap, B.; Kumar, S.; Nand, S.; Azad, I.; Bharagava, R.N.; Ferreira, L.F.R.; Dutta, V. Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 2023, 313, 137547.
- Mao, K.; Zhang, K.; Du, W.; Ali, W.; Feng, X.; Zhang, H. The potential of wastewater-based epidemiology as surveillance and early warning of infectious disease outbreaks. Curr. Opin. Environ. Sci. Health 2020, 17, 1–7.
- Zulu, N.; Idris, A.O.; Orimolade, B.O.; Nkambule, T.T.; Mamba, B.B.; Feleni, U. Approaches for the Detection of Escherichia coli in Wastewater: A Short Review. ChemistrySelect 2023, 8, e202200598.
- Al-Wohaib, F.A.; Al-Sharif, I.; Al-Zain, H.; Murad, D.; Al-Harbi, L.; Al-Mozaini, M. Evaluation of two molecular detection platforms for gastroenteritis pathogens in treated sewage water in the Eastern province of Saudi Arabia. Sci. Rep. 2022, 12, 21744.
- Delanka-Pedige, H.M.; Cheng, X.; Munasinghe-Arachchige, S.P.; Abeysiriwardana-Arachchige, I.S.; Xu, J.; Nirmalakhandan, N.; Zhang, Y. Metagenomic insights into virus removal performance of an algal-based wastewater treatment system utilizing Galdieria sulphuraria. Algal Res. 2020, 47, 101865.
- Vu, K.A.; Mulligan, C.N. Remediation of organic contaminated soil by Fe-based nanoparticles and surfactants: A review. Environ. Technol. Rev. 2023, 12, 60–82.
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130.
- Cutrupi, F.; Rossi, M.; Cadonna, M.; Poznanski, E.; Manara, S.; Postinghel, M.; Palumbi, G.; Bellisomi, M.; Nicosia, E.; Allaria, G. Evaluation of concentration procedures, sample pre-treatment, and storage condition for the detection of SARS-CoV-2 in wastewater. Environ. Sci. Pollut. Res. 2023, 1–11.
- Ahmed, W.; Simpson, S.L.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Blackall, L.L.; Bofill-Mas, S.; Bosch, A.; Brandão, J.; Choi, P.M. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022, 805, 149877.
- Li, Y.; Liu, S.; Wang, Y.; Wang, Y.; Li, S.; He, N.; Deng, Y.; Chen, Z. Research on a Magnetic Separation-Based Rapid Nucleic Acid Extraction System and Its Detection Applications. Biosensors 2023, 13, 903.
- Dunbar, S.A. Nucleic acid sample preparation techniques for bead-based suspension arrays. Methods 2023, 219, 22–29.
- Yoon, T.; Kim, S.; Kim, J.H.; Park, K.S. A Syringe-Based and Centrifugation-Free DNA Extraction Procedure for the Rapid Detection of Bacteria. Chemosensors 2021, 9, 167.
- Sadia, M.; Mahmood, A.; Ibrahim, M.; Irshad, M.K.; Quddusi, A.H.A.; Bokhari, A.; Mubashir, M.; Chuah, L.F.; Show, P.L. Microplastics pollution from wastewater treatment plants: A critical review on challenges, detection, sustainable removal techniques and circular economy. Environ. Technol. Innov. 2022, 28, 102946.
- Babler, K.M.; Amirali, A.; Sharkey, M.E.; Williams, S.L.; Boone, M.M.; Cosculluela, G.A.; Currall, B.B.; Grills, G.S.; Laine, J.; Mason, C.E. Comparison of electronegative filtration to magnetic bead-based concentration and V2G-qPCR to RT-qPCR for quantifying viral SARS-CoV-2 RNA from wastewater. ACS ES&T Water 2022, 2, 2004–2013.
- Corpuz, M.V.A.; Buonerba, A.; Vigliotta, G.; Zarra, T.; Ballesteros, F., Jr.; Campiglia, P.; Belgiorno, V.; Korshin, G.; Naddeo, V. Viruses in wastewater: Occurrence, abundance and detection methods. Sci. Total Environ. 2020, 745, 140910.
- Shi, Y.; Wang, G.; Lau, H.C.; Yu, J. Metagenomic sequencing for microbial DNA in human samples: Emerging technological advances. Int. J. Mol. Sci. 2022, 23, 2181.
- Yao, L.; Zhu, W.; Shi, J.; Xu, T.; Qu, G.; Zhou, W.; Yu, X.-F.; Zhang, X.; Jiang, G. Detection of coronavirus in environmental surveillance and risk monitoring for pandemic control. Chem. Soc. Rev. 2021, 50, 3656–3676.
- Sajid, M. Advances in on-site analytical sample preparation for analysis of environmental waters: A review. Trends Environ. Anal. Chem. 2022, 36, e00175.
- Kumblathan, T.; Liu, Y.; Uppal, G.K.; Hrudey, S.E.; Li, X. Wastewater-based epidemiology for community monitoring of SARS-CoV-2: Progress and challenges. ACS Environ. Au 2021, 1, 18–31.
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953.
- Michael-Kordatou, I.; Karaolia, P.; Fatta-Kassinos, D. Sewage analysis as a tool for the COVID-19 pandemic response and management: The urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J. Environ. Chem. Eng. 2020, 8, 104306.
- Bai, Z.; Xu, X.; Wang, C.; Wang, T.; Sun, C.; Liu, S.; Li, D. A comprehensive review of detection methods for Escherichia coli O157:H7. TrAC Trends Anal. Chem. 2022, 152, 116646.
- Flores-Ramírez, A.; Ortega-Cuenca, J.; Cuetero-Martínez, Y.; de Los Cobos, D.; Noyola, A. Viability and removal assessment of Escherichia coli and Salmonella spp. by real-time PCR with propidium monoazide in the hygienization of sewage sludge using three anaerobic processes. Waste Manag. 2023, 161, 254–262.
- Monteiro, S.; Rente, D.; Cunha, M.V.; Marques, T.A.; Cardoso, E.; Vilaça, J.; Coelho, N.; Broco, N.; Carvalho, M.; Santos, R. Discrimination and surveillance of infectious severe acute respiratory syndrome Coronavirus 2 in wastewater using cell culture and RT-qPCR. Sci. Total Environ. 2022, 815, 152914.
