Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Civil
Moisture is an important factor for ASR and its availability in concrete has often been appraised with the aid of relative humidity (RH). Its sufficiency leads to improved kinetics and higher ultimate ASR-induced expansion. Numerous RH thresholds have been reported to be required to initiate and sustain the reaction. the tests from which the thresholds has been reported differ in terms of the temperature, sample size, reactivity level of aggregates and aggregate type (Fine versus Coarse). All of these factors can be hypothesized to influence the role of moisture in the reaction.
- alkali–silica reaction
- moisture
- relative humidity
- temperature
1. Moisture in Concrete
Moisture plays a vital role in the durability of concrete [42]. Its availability beyond a specific limit could be favourable for the alkali–silica reaction, freezing and thawing [21], carbonation [43], etc. Available water in concrete can be categorized into chemically bound water, physically bound water, and capillary (free) water [44]. Physically bound and free water are responsible for the transportation mechanism in concrete pore structures, and their distribution in this space is influenced by the moisture content, while chemically bound water is non-evaporable and fixed in the hydrate phases [45]. The moisture content available in concrete can therefore be interpreted as the volumetric ratio of the sum of physically bound and free water to the pore space [44]. This moisture content has been measured using several methods.
The gravimetric method is the most dependable and accurate technique for measuring moisture in concrete, especially when representative samples can be collected from the body under investigation. However, the destructive nature of the test renders it unsuitable in some circumstances [46]. Some other relevant methods are itemized in Table 1, but most of these methods do not measure moisture in concrete directly, thus limiting their use. Other challenges include cost, calibration challenges, and ease of usage. Nevertheless, the relative humidity and degree of capillary saturation have been popularly used for such measurements.
Table 1. Moisture measurement techniques.
| Test | NDT | Location | Influencing Factor | Output |
|---|---|---|---|---|
| Gamma densitometry [47] | Yes | Different depth | Geometry | Density of concrete |
| Electrical resistance [48] | Yes | Surface | Degree of maturity | Electrical conductivity |
| Hygrometry [49] | Yes | Location of choice | Calibration, temperature | Relative humidity |
| Chemical reactions [50] | No | Representative sample | Evaporation | Reaction with water |
| Thermalized neutrons [51] | Yes | Surface | Chemical composition | Thermal neutron detector |
| Microwave absorption [52] | Yes | Few inches deep | Mix proportion | Electromagnetic waves |
| Infrared thermography [53] | Yes | No contact | Concrete density | Surface temperature |
| Gravimetric [54] | No | Representative sample | Depth of collection, incomplete drying | Surface temperature |
The choice of relative humidity or degree of capillary saturation measurement is dependent on the type of moisture (water vapour or liquid water) under consideration. The assessment of frost damage in concrete can be further emphasized with the use of the degree of capillary saturation, which illustrates the number of filled pores. Whereas, in describing concrete deterioration involving chemical reactions influenced by the activity of moisture in the pores, the relative humidity measurement can be more efficient [55].
The RH considers the amount of water vapour in the air. The internal RH of early-age concrete is hugely dependent on the water-to-cement ratio (w/c), and the readings can be obtained with hygrometers [56]. The RH is assumed to be 100% at complete saturation after casting (liquid-like condition). According to Zhang et al. [57], this condition exhibits a continuous liquid network, and the RH of the air is close to 100% [58]. After some time, the RH starts dropping due to hydration and/or evaporation. As hydration continues, the formerly continuous liquid network gets breached, secluding the pore water and leading to the decrement in the internal RH.
The degree of capillary saturation (DCS) of concrete, on the other hand, can be obtained through the ratio of the volume of water available in the concrete to the volume taken up when the concrete is subjected to capillary suction until equilibrium is reached [55]. A handful of strategies have been adopted to measure the DCS in the literature, most of which are focused on obtaining the weights of the sample before and after drying as well as the weight after capillary water absorption, which can be achieved by submerging the sample or with one surface of the sample in contact with water [59,60]. The procedure (weighing and drying) for DCS can limit its use for in situ measurements, unlike that for RH [61,62].
The DCS and RH focus on different properties of moisture in concrete and are affected by distinct factors. However, there exists a seemingly linear relationship between them. According to a study by Weiss [63] on concrete specimens with a w/c of 0.42, the degree of saturation increases as the RH increases. Despite the challenges involved in performing the DCS, this test seems to be more scientific; the actual amount of water in concrete can be determined. However, the RH is more suitable for use in ASR-affected concrete due to the ease of measurement. This can be performed easily with the aid of sensors. Furthermore, the ability to carry out RH measurements in situ, especially in the laboratory, without interfering with the ASR-accelerated performance tests through the drying of samples and the capillary water absorption procedures required for DCS makes the RH more suitable.
2. The Role of Moisture in the Alkali–Silica Reaction
Moisture is a critical factor for the initiation and sustenance of the ASR [64]. The moisture present in ASR-affected concrete is largely due to the mixing water (w/c) and ingress of water from external sources. Such ingress could be from rain, ambient environmental conditions, drainage, etc., or a combination of these.
The role of moisture in the reaction has been well documented [65]. Its availability serves as a transportation medium for the ions involved in the reaction and for the formation/swelling of the resulting ASR gel. The transportation of ions can be further enhanced in a highly porous and permeable medium obtainable in concrete with high w/cm [66]. As a result, the reduction in the available moisture has been used to mitigate the reaction. On the other hand, a reduction in the w/cm could potentially aid the reaction. During hydration at low w/cm, the products are less heterogeneous and less portlandite is produced. Hydroxyl ions are dissolved from the Ca(OH)2 in order to maintain equilibrium with the high alkali concentration in the pore solution as a result of the release of Na+ and K+ from the cement, leading to an abundance of ions in the pore solution [60]. According to Nilsson and Peterson [67], the reaction can be initiated even at a low RH of 80%, but progresses slowly due to the low rate of diffusion. The ASR gel swelling that can arise in this condition may not be sufficient to exert significant pressure on the surrounding paste. If the concrete is re-exposed to water or moved to a more saturated condition, the rate of the reaction is improved, and expansion is significantly improved [68,69].
The moisture needed for the ASR has been prominently measured using the RH [3,22,70]. Poyet et al. [22] examined the kinetics of the ASR and resulting expansion in cylindrical mortar samples stored in different external relative humidity conditions and, based on their studies, ASR expansion increases as RH increases. Few researchers have argued that the degree of DCS is a better means of moisture measurement for the reaction [55,71]. Nonetheless, damage due to the ASR intensifies as the DCS increases [55], and a DCS threshold of 90% has been reported to be needed [71].
2.1. Relative Humidity Threshold
The availability of a sufficient amount of water has been confirmed as a crux for the reaction [26]. Based on the discussion in the previous sections, no significant distress can be recorded at low moisture conditions. As a result, the concept of the RH threshold has been introduced for the prevention and maintenance of ASR-affected structures.
The absorption of water and expansion of the ASR gel are dependent on the RH. According to a work carried out by Bažant and Steffens [72], the absorption capacity of the gel greatly diminishes when the RH of the air in the pores falls below 85%. Furthermore, expansion is impeded at low RH levels. According to the studies in [73], no expansion was recorded when mortar samples were stored at 65% RH and 38 °C, while considerable expansion was reported in those stored at similar temperatures but at 85% RH, buttressing the claims that a minimum RH of 80–85% is needed for the reaction [74]. Some other authors have posited a threshold of 80% [21,67]. Yet, numerous RH thresholds exist in the literature. Olafsson [70] reported 80% at 23 °C, which falls to 75% at 38 °C, while Ludwig [20] reported it to be between 80–85% at a lower temperature of 20 °C.
Some researchers have worked at higher temperatures. Tomosawa et al. [25] and Kurihara and Katawaki [24] investigated the reaction at 40 °C and reported a threshold of 75%. Poyet et al. [22] reported a threshold of less than 59% at 60 °C as an expansion of 0.06% was recorded in cylindrical concrete specimens exposed to 60 °C after just 100 days. Thus, the RH threshold could depend on the temperature [22]. As indicated by Figure 3, which includes various studies on RH thresholds at different temperatures, it is evident that the moisture needed for the reaction at room temperature surpasses that required at the standard conditioning temperature of 38 °C. Moreover, the moisture requirement is even lower at a higher temperature of 60 °C. Consequently, the critical RH for ASR-induced expansion decreases with increasing temperature, confirming the claims in [22]. However, in a report by Pedneault [23], the author stated that the critical RH depends on the form of siliceous minerals of the aggregate. Given the different reactivity levels of aggregates, some aggregates are expected to have a faster rate of expansion. As a result, very highly reactive aggregates could expand at a moisture level where moderately reactive aggregates would not. Furthermore, the reactive content (fine versus coarse) of the mix could influence the kinetics of the reaction, as clearly shown in Figure 3.
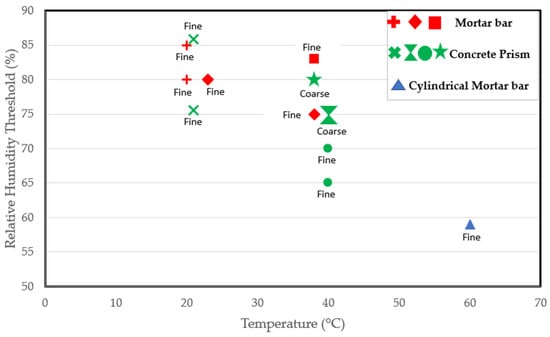
According to the studies [25,75] carried out on concrete prisms stored at 40 °C, as shown in Figure 3, specimens containing reactive coarse content have an RH threshold of 75% compared to the 65–70% RH needed for the mix prepared using a reactive fine aggregate. Hence, a lower moisture level could be sufficient to initiate the reaction in specimens involving reactive fine aggregate. After all, ASR-induced cracks propagate faster in reactive fine aggregates due to their small size. Lastly, the RH threshold required for the reaction could vary depending on the sample size and shape. After all, the rate of the reaction and ultimate ASR-induced expansion differ in concrete prisms, cubes, and cylinders incorporating reactive aggregates [77]. Thus, the varying sample types used in the available studies may play a role in the varying thresholds reported in the literature.
2.2. The Role of Alternate Drying and Wetting on the Availability of Moisture for the Alkali–Silica Reaction
Concrete exposed to natural environmental conditions is subjected to several drying and wetting cycles during its service life; these conditions have an influence on the available moisture in the concrete and the development of the ASR. According to Zhang et al. [58], when concrete is exposed to moisture (wetting), the interior RH increases rapidly within a short period of time and then reaches a stable level; this, however, is dependent on the concrete grade, permeability, size, and other properties of hardened concrete; duration of the wetting cycle; etc. Conversely, during drying, the interior RH reduces gradually [58]. According to the study by Zhang et al. [58], concrete cubes with 28 days of compressive strength of 30 MPa were subjected to a dry and wet cycle (14 and 7 days, respectively) and the internal RH at the centre of the 60 × 100 × 350 mm cubes of the samples was measured. As expected, the RH reduced during the drying cycle but failed to reach the initial moisture level when subjected to wetting. This could have been due to the duration of the wetting cycle. As a result, such a phenomenon could influence the available moisture in concrete for the ASR [15,78].
To discuss the influence of the cyclic condition on ASR, Stark et al. [26] confirmed that cyclic wetting and drying conditions could stimulate the initiation and sustenance of the reaction. Of course, there was less moisture to be absorbed by ASR gel during drying; nevertheless, the increase in temperature improved the concentration of hydroxyl ions in the pore solution, which exacerbated the attack on the poorly crystallized aggregates. As a result, expansion progressed exponentially in the following wetting cycle. Furthermore, in the next dry period, more non-expansive reaction products were formed and drying increased the extent of the cracks formed in the prior wetting cycle. Thus, minimizing wetting and drying cycles is vital in controlling the reaction [79].
Evidently, expansion due to the ASR is significantly retarded in the drying cycle, especially towards the surface of the concrete. The reaction can, however, progress actively in the inner depth but not close to the outer surface due to the moisture gradient. According to Kagimoto and Kawamura [80] in their study on lab-conditioned concrete specimens, a relative humidity of up to 80–90% (a moisture level sufficient to initiate the ASR) was measured in the middle of the concrete cylinders during drying; such variations in expansion across the depth of affected concrete were responsible for surface cracks due to the ASR [81]. A similar observation was recorded in field samples by Stark et al. [26]. It is, however, worth noting that this phenomenon could be dependent on the size of the concrete member. Slender members would experience a rather quicker drop in RH across their depth.
This entry is adapted from the peer-reviewed paper 10.3390/ma17010010
This entry is offline, you can click here to edit this entry!
