Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Magnesium alloy stents have been extensively studied in the field of biodegradable metal stents due to their exceptional biocompatibility, biodegradability and excellent biomechanical properties. Nevertheless, the specific in vivo service environment causes magnesium alloy stents to degrade rapidly and fail to provide sufficient support for a certain time. To settle these problems, this paper reviewed the protective strategies for Magnesium alloy vascular stents.
- magnesium alloy
- cardiovascular stent
- corrosion resistance
1. Introduction
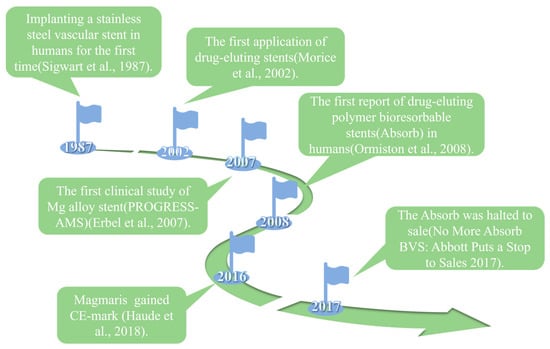
Cardiovascular disease (CVD) takes the lives of around 17.9 million people annually and stands as the primary cause of death globally [1]. One of the rapidest and most effective medical procedures for treating coronary and peripheral artery disease is to use vascular stents [2]. The entire evolution of vascular stents goes through three generations: the first-generation bare-metal stents, the second-generation drug-eluting stents, and the third-generation biodegradable drug-eluting stents (Figure 1).
The bare-metal stents (BMS) were invented and used in clinical settings first. In 1987, the clinical implantation of stainless steel stent into a human coronary artery was first reported [3]. BMS could provide arterial support and significantly improve the clinical and angiographic outcomes in patients [4]. However, implantation of bare-metal stents (BMS) might result in symptoms including in-stent restenosis (ISR) and stent thrombosis (ST) [5,6]. To address the issues of ISR and ST associated with BMS, drug-eluting stents (DES) were created. These stents are coated with polymer coating to load and deliver drugs, such as sirolimus and paclitaxel [7,8]. However, as a permanent implant in the vasculature, DES still brought the potential risks of thrombogenicity, delayed re-endothelialization, mismatches in mechanical behavior, long-term endothelial dysfunction, and chronic local inflammatory reactions [9,10].
Magnesium alloy stents were the first biodegradable metal stents to have been used in clinical applications due to their outstanding biocompatibility, biodegradability and mechanical properties. Mg alloy stents could be degraded and completely absorbed in vivo, thus avoiding the neointimal hyperplasia and stenosis caused by a long-term foreign body reaction. Mg alloys allow for faster expansion and have more consistent mechanical strength and ductility than bioabsorbable polymer stents. This improves radial support for vessels and makes stent processing and crimp–expansion deformation easier [25]. However, the rapid degradation and localized degradation of Mg alloy stents constrain their clinical application, and rapid degradation may result in restenosis and a loss of mechanical integrity. Based on the computational modelling of the corrosion process, it was found that heterogeneous corrosion leads to a significant reduction in the supporting performance of stent, but a slight reduction in mass loss compared to homogeneous corrosion [26]. In addition, the degradation of magnesium alloys could cause an elevation of the local pH and an accumulation of hydrogen, which may lead to the obstruction of blood circuits and tissue necrosis [27]. Rapid degradation can generate high concentrations of Mg ions in a local environment. These ions have the potential to cause coagulation or inflammation and hinder the process of vascular remodeling [28]. Therefore, it is very important to effectively regulate the corrosion behavior of magnesium alloys to promote the clinical application of magnesium alloy stents. This is also the key research issue for biodegradable magnesium alloy stents at present.
2. Traditional Strategy for the Protection of Magnesium Alloy Stents
According to the corrosion mechanisms of both magnesium alloys and stents, different strategies for the protection of magnesium alloy stents were attempted. Alloying is an effective way to improve the mechanical properties of metallic materials, and the addition of alloying elements can affect the corrosion behaviors of materials. Thus, how to balance the mechanical property and corrosion resistance are very important. In addition, design optimization for a magnesium alloy stent could reduce the maximum residual stress by lowering the concentration of residual stress, thereby reducing the degree of stress corrosion. The last and most important strategy is the preparation of a coating on magnesium alloy stents. The coating is a physical barrier against the penetration of corrosive media, and its effectiveness is particularly important for magnesium alloy stents that need to undergo complex deformation.
The three aspects above have improved the corrosion resistance of magnesium alloy stents to a certain extent. Next, the related research progress in these three aspects are introduced one by one.
2.1. Alloying Design for Magnesium Alloy Stents
Commonly employed alloying elements in magnesium alloys consist of basic metal elements such as Al, Zn, Mn, Zr, and Li, as well as rare earth elements; each alloying element has its own effect. Aluminum (Al) is a crucial element in magnesium alloys as it enhances their corrosion resistance and strength. Aluminum can form the β-phase (Mg17Al12) in magnesium alloys. Increasing its content in the basal phase facilitates the creation of a highly corrosion-resistant surface layer with a substantial aluminum content [61]. As the aluminum content increases, the β-phase increases and more β-phase precipitates along the grain boundaries, protecting the magnesium alloy matrix. By micro-alloying zinc (Zn) in the magnesium matrix, there is a decrease in the stacking fault energy and an increase in the plastic deformation capacity by effectively activating the non-basal slip. Zirconium (Zr) acts as a significant refining agent for the grains and elevates the toughness of magnesium alloys [28]. The Mg-Li (lithium) alloy is a newly developed system with ultra-high ductility and potential applications in vascular stents. However, the presence of Li causes a reduction in the mechanical strength of the alloy. Rare earth elements are added into magnesium alloys and can play the roles of solid solution strengthening by forming intermetallic phases and improving the corrosion resistance of magnesium alloys. Based on the specific alloying effects of each alloying element, three series of magnesium alloys have been attempted to be applied to the vascular stent at present. They are AZ series alloys (AZ31 and AZ91), rare earth series alloys (WE43 [37], Mg-Zn-Y-Nd (ZE21B) [62], and Mg-Nd-Zn-Zr (JDBM) [63]), and Mg-Li series alloys [64] (Figure 6).
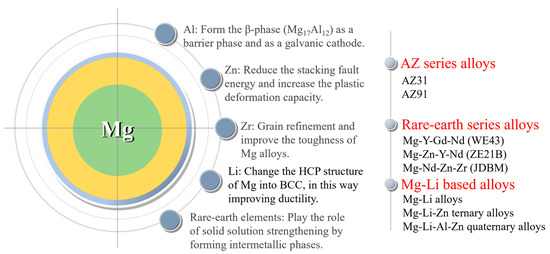
Figure 6. Magnesium alloys for stents and functions of alloying elements.
The effects and existing problems of different series of magnesium alloys used for vascular stents are reviewed below. AZ series magnesium alloys are a traditional commercially used material that possess excellent and stable comprehensive properties. In a previous study [59], 18 drug-eluting biodegradable AZ31B alloy stents were implanted into porcine coronary arteries, and there were a large number of fractures formed on the stent strut, leading to a loss of structural integrity. Despite some recoil and lumen loss, the lumen remained unobstructed after 3 months of implantation. The occurrence of fracture was related to the strong stress corrosion of the alloy. Yuen et al. [65] investigated the acceptable masses of ten magnesium alloys as degradable biomedical implant materials in their study. The results indicate that aluminum components are usually the least tolerated, and the acceptable mass for Al-containing magnesium alloys is around or below 1 g per person per year, while the limit for other magnesium alloys can exceed 10 g. It means that the element Al has a certain degree of toxicity and needs to be avoided or used with caution.
Hence, rare earth series magnesium alloys were designed and prepared, including WE43 (used as the first-in-men magnesium alloy stent by Biotronik), the Mg-Zn-Y-Nd (ZE21B) alloy developed by Zhengzhou University, China, and the Mg-Nd-Zn-Zr (JDBM) alloy developed by Shanghai Jiao Tong University, China.
The Mg-Y-Gd-Nd alloy (WE43) is a commonly used material for manufacturing biodegradable magnesium alloy stents, and Y (yttrium) and Nd (neodymium) as alloying elements can improve the corrosion resistance of the alloy. Meanwhile, studies have shown that Y, Nd, and Gd (gadolinium) are not cytotoxic [66]. The WE43 alloy stent was implanted in patients for the treatment of severe lower limb ischemia [37]. No adverse events were reported during the procedure, and no patients showed any symptoms of allergic or toxic reactions to the stent material. Magmaris (formerly known as DREAMS 2G), based on the WE43 alloy, is a balloon-expandable, sirolimus-eluting, biodegradable metal stent mounted on a rapid exchange delivery system [52]. This was the pioneer product for biodegradable metal stents, which received CE approval in June 2016. It has exhibited promising clinical outcomes so far.
The Mg-Zn-Y-Nd alloy (ZE21B) was developed with low levels of zinc and yttrium by Zhengzhou University, China, in 2010 [62]. Zinc is known to improve the corrosion resistance of magnesium alloys, leading to the cast Mg-Zn-Y-Nd alloy exhibiting a higher corrosion potential (−1.76 V) compared to the cast Mg-Y-Gd-Nd alloy (−1.95 V). The Mg-Zn-Y-Nd alloy was prepared through a sub-rapid solidification process, exhibiting superior corrosion resistance in a dynamic SBF. The mechanical properties and corrosion resistance of the Mg-Zn-Y-Nd alloy can be further improved using different processing techniques and heat treatments, for example, cyclic extrusion compression (CEC) [67]. The findings demonstrated that the grain size in the alloy was refined to 1 µm with CEC treatment. Furthermore, the second phase distributed in a grid shape along the grain boundaries, and the nanoparticles were uniformly distributed within the grain, leading to a significant improvement in mechanical properties of the alloy. The alloy also displayed uniform corrosion. The corrosion current density of the alloy was observed to decrease from 2.8 × 10−4 A/cm2 to 6.6 × 10−5 A/cm2. In 2018, Wang et al. [68] processed tube blanks of the Mg-Zn-Y-Nd alloy using hot extrusion. The annealed and drawn fine tubes exhibited superior mechanical properties. Moreover, the fine tubes demonstrated uniform corrosion in the simulated body fluid and exhibited excellent corrosion resistance. In 2021, Du et al. [69] processed fine tubes of Mg-Zn-Y-Nd alloy using a HTHE (long-time and high-temperature heat treatment, large-reduction-ratio hot extrusion) process, obtaining a refinement of the coarse secondary phase uniformly distributed in the matrix of fine tubes.
The Mg-Nd-Zn-Zr (JDBM) alloy was designed by Shanghai Jiao Tong University (SJTU) for biomedical applications [63]. Nd was used as the main alloying element for strengthening, which was revealed by diminishing the stacking fault energy of the basal plane and producing a pinning effect on the base slip [28]. Additionally, Zr serves as a potent grain refiner in magnesium alloys with better biocompatibility [70]. The extruded JDBM alloy exhibited superior corrosion resistance with slight uniform corrosion. It was found that the corrosion rate of JDBM alloy in Hank’s solution was significantly slow at 0.28 mm/year compared to the AZ31 alloy at 1.02 mm/year [71], which was covered by a more compact and protective layer that prevented the pitting corrosion at the early stage of immersion. Furthermore, bare JDBM stents were implanted into the common carotid artery of New Zealand white rabbits to evaluate the safety, efficacy and degradation behavior of the stent [72]. The results indicated that the bare JDBM stent was effective and safe, and complete re-endothelialization occurred within 28 days. The majority of the JDBM stent struts underwent in situ replacement by degradation products in 4 months.
In addition to these, the Mg-Li alloy was developed through mechanisms such as solid solution strengthening, grain refinement and dispersion strengthening, showing superior corrosion resistance in the meantime. Bian et al. [73] used a high ductility (>40%) Mg-8.5 Li (wt.%) alloy (without rare earth metals and aluminum) to fabricate the stent. A finite element analysis verified the impacts of plastic deformation and residual stress arising from the expansion process on the degradation of this stent. While the stent showed a favorable degradation rate in vitro (0.15 mm/year), different results were found in vivo (>0.6 mm/year). Further, the stents were well-tolerated by the adjacent tissues in pigs, and no thrombosis was reported. Furthermore, a range of Mg-Li based alloys have been developed, for example, Mg-Li-Zn ternary alloy [74] and Mg-Li-Al-Zn quaternary alloy [75]. The current research on Mg-Li alloys in cardiovascular stents mainly focuses on the mechanical properties and corrosion resistance, and the toxicity and biocompatibility still need to be further verified.
Above all, the addition of alloying elements has improved the mechanical properties and corrosion resistance to a certain extent, but it has not well met the clinical demand for magnesium alloy stents. Therefore, other methods should be adopted.
2.2. Optimization of Magnesium Alloy Stent Designs
Generally, the radial strength, recoil and non-uniform expansion (a reduced dog-boning effect) during expansion as well as the in-stent restenosis of the stents are influenced by the design of the stents [76]. For magnesium alloy stents, design optimization is another way to improve its corrosion resistance, due to the decrease in the stress concentration and stress corrosion. Finite element analysis (FEA), a simple and efficient method, has been widely used in recent years for the structural design and optimization of magnesium alloy stents. Wu et al. [55] found that the width of the strut was increased by roughly 48%, resulting in improved safety performance (specifically a 29% decrease in maximum principal stress after tissue recoil and a 14% decrease in maximum principal strain during expansion) and a 24% increase in scaffolding ability. The strut width in the final optimized design was not uniform: the mass of the inner straight sections was reduced to a greater extent, while the mass of the outer curved sections was increased. This resulted in reduced maximum strain and a more uniform distribution of strain. Thereafter, a three-dimensional (3D) FEA model combined with a degradable material model was proposed [77], which was used to analyze three different stent designs made from the AZ31 alloy, crimped and expanded in arterial vessels, through the ABAQUS explicit solver. It was verified that the expectation that the design for magnesium alloy stent with more mass and optimized mechanical properties could increase the stenting time. In addition, Wu et al. [53] compared the corrosion resistance of AZ31B alloy stent samples with two designs (an optimized one [55] and a conventional one), which underwent balloon expansion and subsequently immersed in D-Hanks’ solution for a 14-day degradation test. It indicated that the optimized designed stents exhibited superior corrosion resistance to the stents with the conventional design due to less stress distribution in the former ones. The congruity between the numerical simulation and experimental data demonstrated the efficiency of the FEA numerical modelling tool in the design optimization of novel biodegradable magnesium alloy stents.
Increasing the strut size (e.g., a larger strut width) and decreasing the uniformly distributed principal stress are two effective ways of improving the mechanical properties of magnesium alloy stents. Although an augmented strut width could offer adequate radial support to prolong the uniform corrosion time, it might also escalate maximum principal stress during stent expansion and recoil, which could lead to stress corrosion. Degradation failure of magnesium alloy stents typically occurs first at the location of stress concentration. Therefore, the initial phase in designing an optimized stent design is to decrease the maximum principal stress or strain. Conventional stent design usually maintains a constant stent width. For stents with low ductility materials, changing the mass distribution of the stent is also an effective way to optimize the performance of the stent.
2.3. Coatings on Magnesium Alloy Stents
Making a coating is an important strategy to improve the corrosion resistance and biocompatibility of magnesium alloy stents. The application of the coating would not bring about any alterations to the microstructure of the magnesium alloy matrix or the stent structure. However, it alters the surface properties, resulting in outstanding corrosion resistance and biocompatibility for the magnesium alloy stent. There have been many kinds of coatings on magnesium alloys. Although a single coating typically does not attain these objectives simultaneously, composite coatings comprising various single-layer coatings can present fresh perspectives for investigations of magnesium alloy stents. When preparing a coating on magnesium alloy stent for surface modification, a chemical conversion film is commonly used to enhance the corrosion resistance of the substrate and improve the adhesion between the substrate and the coating. An outer polymer coating is then prepared to covered the chemical conversion film to further enhance the corrosion resistance and biocompatibility.
2.3.1. Inner Chemical Conversion Coating
Chemical conversion treatment can create a protective layer of metal oxides or other compounds on the surface of magnesium alloys, which acts as a physical barrier to effectively separate the magnesium substrate from the corrosive medium. This treatment improves the adhesion of the final deposited coating to the substrate and then enhances the corrosion resistance and biocompatibility. The commonly used chemical conversion coatings to protect magnesium alloy stents include a micro-arc oxidation coating, phosphate conversion coating, magnesium hydroxide coating and magnesium fluoride coating.
Micro-arc oxidation (MAO), also referred to as plasma electrolytic oxidation (PEO), is an electrochemical process for the controlled oxidation of metal materials to attain surfaces with a particular morphology, thickness, and composition, improving the corrosion resistance and biological properties of the metal materials [78]. The typical MAO coating consists of an inner and outer layer. The compact and uniform inner layer functions as a barrier, impeding the penetration of the solution into the substrate. The presence of oxygen bubbles during the growth of the coating and the thermal stress caused by the quick solidification of the molten oxide in a relatively cold electrolyte could result in a rough outer layer with micropores and microcracks [79]. Therefore, although the inner dense layer of the MAO coating enhances the corrosion resistance, the outer layer permits increased absorption of the corrosive electrolyte into the MAO coating, thus decreasing the coating’s resistance to corrosion [80].
The phosphate conversion coating has shown high biocompatibility, excellent and robust adhesion, a reduced degradation rate and inhibited the negative side effects of magnesium alloy implants in animal models [81]. Phosphate conversion coatings such as magnesium phosphate [82], zinc phosphate [83,84], and calcium phosphate [85,86] have been reported in many studies on the corrosion resistance of magnesium alloys as an environmentally friendly surface modification technique. Zai et al. [87] compared the corrosion resistance and biocompatibility of various phosphate conversion coatings, including magnesium phosphate (Mg-P), calcium phosphate (comprising Ca-P and CaMg-P) and zinc phosphate (comprising Zn-P, ZnMg-P, ZnCa-P, and ZnCaMg-P). Magnesium alloy substrates, as well as magnesium phosphate and calcium phosphate conversion coatings, showed a mixed form of corrosion involving filiform and pitting during extended immersion in Hanks’ solution. Conversely, the primary form of corrosion in the zinc phosphate conversion coating was pitting. ZnMg-P provided superior anti-corrosion performance to the other coatings due to its highly stable structure that effectively inhibited the propagation of filiform corrosion. Based on the results of the cell viability test, the calcium phosphate conversion coating displayed superior biocompatibility compared to zinc phosphate and magnesium phosphate conversion coatings, as well as the bare magnesium alloy substrate. Mao et al. [88] prepared a uniform Mg3(PO4)2 coating on the surface of the JDBM alloy using a chemical transformation method in a mixed phosphate solution of 5% NaH2PO4 and 3% Na3PO4 at a ratio of 1:1, which improved both the corrosion resistance and biocompatibility of the alloy. The lamellar-structured phosphate coating exhibited an exceptional affinity for cells by facilitating cell adhesion and spreading. The magnesium phosphate conversion coating was observed to comprise an outer layer generated through precipitation and an inner layer grown in situ [89].
The degradation product of magnesium in the human body, Mg(OH)2, exhibits superb biocompatibility without toxicity. The hydrothermal method [90,91] can be used to prepare a uniform and compact hydroxide layer on the surface of magnesium alloys, which has strong adhesion with the matrix and greatly reduces the degradation rate of magnesium alloys. However, as the degradation process of magnesium alloy advances, it leads to the formation of porous Mg(OH)2 on the surface. The Mg(OH)2 coating contains micro-pores and microcracks that function as transport channels for corrosive media. Therefore, these coatings do not provide long-term and effective protection for magnesium alloys. In order to improve the corrosion resistance of the hydroxide coating, the layered double hydroxide (LDH) coating was further developed. The Mg-Al LDH has been considered an effective agent to retard the corrosion reaction. The atomic structure comprises brucite-like octahedral layers that are positively charged by the substitution of a few Mg2+ with Al3+ ions [92]. The Mg-Al LDH incorporating carbonate was effective at capturing Cl− anions in a corrosive environment within the LDH interlayer, thus enabling the layer to shield the magnesium alloy from corrosion [93]. The Mg-Al LDH coating on magnesium alloys showed favorable corrosion resistance both in vitro and in vivo, with significant cell adhesion, migration and proliferation [91]. The layered double hydroxide (LDH)/poly-dopamine (PDA) composite coating prepared on the surface of the AZ31 alloy could significantly improve the corrosion resistance of the alloy [94]. However, it was found that the LDH coating was not always superior to the single hydroxide coating. Zhang et al. [95] fabricated three kinds of hydroxide coatings with nanosheet structures, Mg(OH)2, Mg-Fe LDH, and FeOOH, on the magnesium alloy treated with PEO. The coatings effectively closed the micropores formed during the PEO treatment. Compared with PEO-treated magnesium alloy, the corrosion resistance and biocompatibility of the magnesium alloy with hydroxide coating was significantly enhanced, and the trend was as follows: FeOOH > Mg-Fe LDH > Mg(OH)2 > PEO coatings. The FeOOH coating can be used as a novel potential coating for the surface modification of magnesium alloy implants. The coatings above all can improve the corrosion resistance of magnesium alloys, but whether they can undergo the deformation of magnesium alloy stents during implantation can be suspected.
The fluoride conversion coating, which possesses a uniform and controllable thickness and relatively high density, has the potential to considerably enhance the corrosion resistance and inhibit the degradation of magnesium alloys. Mao et al. [96] prepared a uniform and compact MgF2 film by chemical conversion on the surface of JDBM using hydrofluoric acid (HF). The MgF2 film could effectively improve the corrosion resistance of JDBM, while significantly decreasing the hemolysis rate of the alloy. In order to reduce the pollution of HF to the laboratory environment, Mao et al. [97] developed an eco-friendly and simple method to prepare a nano-scale MgF2 film on JDBM through a chemical conversion treatment of the alloy in a 0.1 M potassium fluoride (KF) solution. The film had a uniform and dense physical structure that significantly reduced the corrosion rate. Thereafter, JDBM stents coated with MgF2 film were implanted into rabbit abdominal aorta, which confirmed the excellent tissue compatibility without thrombosis or restenosis. Li et al. [98] investigated the degradation and the related mechanism of the AZ31B alloy with the fluoride conversion coating. After the alloy with the fluoride conversion coating was immersed in Hank’s solution, MgF2 in the coating dissolved into F ions and Mg ions. Due to the low solubility of MgF2, Mg(OH)2 formed at a sluggish rate, resulting in an even coating that is resistant to corrosion. As H2O and Cl− enter the alloy substrate, the alloy starts to degrade, leading to the formation of Mg(OH)2 and H2. The degradation of the magnesium alloy with the fluoride conversion coating proceeded gradually, migrating inward layer by layer. The HF-treated magnesium alloy stents exhibited excellent corrosion resistance without expansion compared to the bare stents. However, after stent expansion, small fragments and cracks appeared on the surface of HF-treated magnesium alloy stents, leading to an accelerated corrosion rate [56]. Cardiovascular stents are constantly subjected to cyclic loading due to heartbeats, and microcracks on the surface of the stent severely affect implantation stability. Thus, the fluoride conversion coating still could not satisfy the high requirement for the corrosion resistance of magnesium alloy stents, and further treatment is still needed to improve the corrosion resistance and biosafety of the magnesium alloy stents. The inorganic base layer has been utilized as a pretreatment coating to form a composite coating with a polymer, which can improve the corrosion resistance and biocompatibility of magnesium alloy stents.
2.3.2. Outer Polymer Coating
Compared with the inner inorganic coating, polymer coatings could endow magnesium alloy stents with superior corrosion resistance and biocompatibility. At the same time, polymer coatings can serve as a drug delivery platform, fulfilling various medical functional requirements. However, when a polymer coating is prepared on the surface of the magnesium alloy directly, the corrosion medium that permeates through the polymer coating would lead to rapid corrosion of magnesium matrix and hydrogen release. It could lead to gas accumulation underneath the coating, causing the coating to crack and fail [57,99]. Therefore, magnesium alloys often need to be pretreated before the polymer coating preparation, which provides a physical barrier to the substrate and at the same time improves the bonding between the substrate and the outer polymer coating. There are a large number of polymer coatings for magnesium alloy stents, owning excellent deformability.
-
Polylactic acid (PLA) coating
Polylactic acid is a hydrophobic aliphatic polyester, which is a thermoplastic, biodegradable, and biocompatible synthetic polymer with an exceptional strength and modulus. It is classified as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA) of the United States and is already used in industrial packaging and many medical devices [100,101].
The PLA coating significantly reduces the degradation rate of magnesium alloy stent, thus providing radial support to the vascular wall over a 6-month period [102]. However, the bonding between PLA and the magnesium alloy is weak, and the surface of the alloy is usually treated with fluorination to improve adhesion [56]. The MgF2 layer on the surface of magnesium alloys is smooth and compact, but with some micropores. The preparation of PLLA coatings using ultrasonic atomization spraying could well cover these pores and provide a good physical barrier [103]. The fluoride-treated magnesium alloy stents could remain unchanged in the neutral axis direction after crimping and dilating with a balloon catheter, while the coating appeared brittle and flaky at the deformed radius. In contrast, the PLLA coating prepared outside the fluoride-treated magnesium alloy stents had a homogeneous and pinhole-free appearance on the surface and did not show cracks, even after curling and dilation. Animal experiments also showed that fluoride-treated magnesium alloy stents with the PLLA coating exhibited better corrosion resistance and longer support compared to the fluoride-treated magnesium alloy stents, while also exhibiting excellent biocompatibility [104]. The composite coating prevented the penetration of erosion ions into the magnesium matrix to improve the corrosion resistance and reduce the corrosion rate. The PLA coating could eliminate the prior porous defects through a critical remelting treatment, which significantly improved the corrosion resistance of the magnesium alloy stents [105].
It has been found that the addition of certain specific nanoparticles to PLA coatings could improve the corrosion resistance and show better biocompatibility. Shi et al. [106] added 2% Mg(OH)2 particles to PLLA coatings to improve protective ability of the coating. The incorporation of Mg(OH)2 particles decreased the hydrophobicity and enhanced the water absorption of the PLLA. As a result of polymer swelling induced by water ingress, numerous defects/channels were created in the polymer coating due to the expansion of the coating volume during the swelling process. This allowed H2 to diffuse more easily through the composite coating compared to the dense PLLA coating. As a result, gas pockets did not form beneath the coating, preventing it from peeling off the substrate. Taking a similar strategy, Park et al. [107] integrated silica nanoparticles that were surface-functionalized with hexadecyltrimethoxysilane (mSiNPs) into a PLLA coating (Figure 7). The mSiNPs that were exposed contributed to the hydrophobicity of the coating, which could interfere with initial water penetration; in addition, the embedded particles could extend the water transport path to increase the time delay to contact with the magnesium alloy substrate. The delay impeded the degradation of the magnesium alloy, resulting in minimal generation of H2 and a low level of magnesium ions released. Meanwhile, the released silicon ions are considered a driving factor in angiogenesis as they activate the synthesis of vascular endothelial growth factor (VEGF) and its receptor (VEGF receptor 2). This leads to the heightened proliferation, migration, motility and differentiation of endothelial cells.
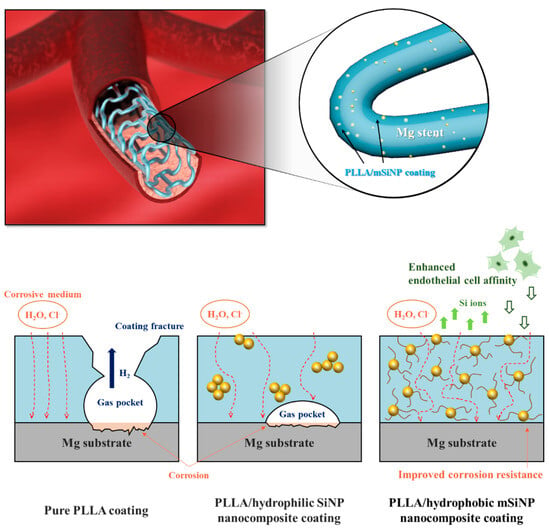
Figure 7. Schematic illustration of the biofunctional PLLA/mSiNP nanocomposite coating on a magnesium alloy stent [107].
However, the PLA coating is relatively hard and brittle, and the coating could peel and crack on the surface after the stent expands, causing serious localized corrosion and deeper pits [108].
-
Poly (lactic-co-glycolic acid) (PLGA) coating
PLGA is another coating for magnesium alloys to reduce the degradation rate and enhance cell adhesion [109], which is approved by the FDA and European Medicine Agency (EMA) in various drug delivery systems for humans [110].
As a single coating, PLGA may not effectively improve the corrosion resistance of the magnesium alloy as expected, which is related to the bulk erosion of PLGA [111]. As corrosion medium easily diffuses into and through the PLGA coating, both PLGA and the magnesium alloy begin to degrade simultaneously. Once bulk erosion has started, by-products of polymer degradation can react with the corroded magnesium ions or magnesium hydroxide, forming soluble magnesium lactates or magnesium glycolates. These can prevent the formation and growth of a dense and thick layer of magnesium hydroxide, and the polymer may provide little or no protection for the following time [99]. When the PLGA coating was applied to a magnesium alloy stent alone, there were several wrinkles, creases and partial detachment that appeared after expansion process, which could not provide good protection for the stent [112].
-
Polycaprolactone (PCL) coating
Polycaprolactone is an aliphatic polyester consisting of hexanoic repeat units. Its broad applications and intriguing attributes, including controlled degradability, miscibility with other polymers, biocompatibility, and potential use of monomers derived from renewable sources, render it a highly valuable polymer [113]. PCL inhibits gas evolution on the base metal and is a promising candidate as a coating material for controlling the degradation rate and mechanical strength of magnesium alloys [100]. PCL has been approved by FDA for use in a wide range of biomedical products, such as drug delivery, bone graft substitution, and tissue engineering applications [114,115,116].
The glass transition temperature (Tg) of PCL was measured using differential scanning calorimetry (DSC) to be (−64.5 ± 3.9 °C) [108], which implies that the PCL coating is in a rubbery and flexible state in the biological environment, and the coating maintains its macroscopic integrity after deformation of the stent and does not undergo localized corrosion. The dense coating exerts a long-lasting decelerating impact on corrosion by establishing diffusion barriers and autoinhibition of the corrosion process [117]. Compared to the PLA-coated high purity magnesium (HPM), the PCL-coated HPM showed a higher Ecorr and lower Icorr [57]. The PCL coating could improve the cell adhesion and tissue growth around the magnesium alloy implant by decreasing its corrosion rate [116,118].
-
Poly (trimethylene carbonate) (PTMC) coating
PTMC is a popular soft material used in scaffold applications for the regeneration of soft tissue, as well as a hydrophobic segment in amphiphilic block copolymers for drug delivery [119]. The degradation process of PTMC is slower than that of PLLA and other aliphatic polyesters. A study showed that enzymatic degradation played a crucial role in the surface erosion [120]. Meanwhile, PTMC possesses elasticity and softness, and so it can be used as a surface coating material for magnesium alloy stents.
It has been found that a uniform thin PTMC coating on magnesium alloys eroded from the surface to the interior when exposed to the biological environments [121]. As a result, it created a protective pathway that impeded electrolyte diffusion from the blood to the magnesium alloy, thus minimizing substrate corrosion. PTMC hydrolysis is a nearly neutral ionic process and maintains a physiological pH during degradation. PTMC allows a minimal amount of electrolyte penetration through the coating to interact with the magnesium alloy substrate beneath. The remaining PTMC preserves the stability of this thin corrosion layer, regardless of whether the product layer is Mg(OH)2 or MgH2, to prevent further corrosion and dissolution (Figure 8). The addition of graphene oxide (GO) into PTMC coatings has the potential to enhance the water barrier efficacy of composite coatings. This improvement is due to the two-dimensional GO structure, which elongates the solution’s penetration path through the labyrinth effect, and subsequently restrains hydrogen accumulation beneath the polymer layer, leading to better corrosion resistance [122,123].
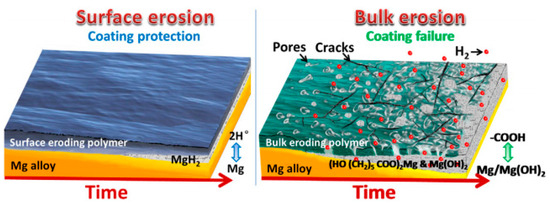
Figure 8. Schematic diagram of surface and bulk erosions [121].
PTMC coated samples showed good cytocompatibility and hemocompatibility, with very few platelets adhering on the surface. Compared with PLGA, PTMC coatings have a more stable and sustained drug release capacity and can inhibit the proliferative of HUVSMCs for a long period of time due to the much slower surface erosion behavior and degradation rate [124]. Atorvastatin calcium (ATVC) was loaded into the PTMC delivery coating on the magnesium alloy surface, which was able to promote the rapid endothelialization of HUVECs and regulate growth of HUVSMCs, further preventing endothelial hyperplasia and inflammatory responses [125]. However, PTMC lacks functional groups, which limits further functional modifications, for example, the conjunction of bioactive components and immobilization on metal surfaces. Chen et al. [126] developed amino-grafted PTMC polymers that were immobilized on the surface of magnesium alloys through the reaction of amino and carboxyl groups, and the immobilized polymeric coatings could potentially offer improved resistance to detachment during clinical delivery processes, including stent dilatation.
-
Polyurethane (PU) coating
Polyurethane is an elastic polymer. The polar urea groups in polyurethane urea enhance hydrogen bonding in the hard segments, which act as strong physical crosslinkers. Solid polyurethane coatings possess good elastomeric mechanical properties due to their high molecular weight, low crystallinity and low glass transition temperature (Tg < −46 °C) [127], making them a potentially biodegradable polymer coating for magnesium alloy stents. Gu et al. [128] investigated hemocompatibility, the dynamic degradation behavior and drug release of poly(carbonate urethane) urea (PCUU) and poly(ester urethane) urea (PEUU) coatings on magnesium alloy stents. Compared with PEUU-coated, PLGA-coated and bare magnesium alloy stents, the PCUU-coated stents showed better corrosion resistance and reduced thrombotic deposition. Compared to the PLGA coating, Arg-PEUU and Arg-Leu-PEUU have better bonding to magnesium alloys while exhibiting better corrosion resistance and biocompatibility [129,130]. The advantage of corrosion resistance could be attributed to the surface degradation nature of the amino acid based polyester urea urethane family [131], which resulted in a slow degradation rate in simulated body fluid. Arg-PEUU and Arg-Leu-PEUU coatings reduced both platelet adhesion and the hemolysis rate, had better cell adhesion to HUVECs, stimulated NO release from HUVECs, and had the ability to delay thrombosis and restenosis.
-
Silane coating
Silane coatings for magnesium alloys have been found to be valid, ecofriendly, and economical. Liu et al. [132] investigated a one-step reaction in which a cross-linked 3-amino-propyltrimethoxysilane (APTES) silane physical barrier layer was introduced onto the surface of a ZE21B alloy prior to electrostatic spraying of the rapamycin-eluting PLGA coating. Solid polysiloxane networks with exposed amine functional groups were formed by in situ APTES polycondensation, providing an effective physical barrier and strong bonding function. The APTES-treated magnesium alloy showed very favorable compatibility with HUVECs and HUVSMCs. Animal experiments confirmed that APTES-treated Mg-Zn-Y-Nd stents implanted into porcine coronary arteries for 6 months showed excellent tissue compatibility and re-endothelialization capacity without severe signs of injury, thrombosis, or restenosis of the vascular wall. After that, a simple two-step reaction was used to introduce anticorrosive silane pre-treatment on the ZE21B alloy before coating with PLGA [133] (Figure 9). The first step was to immerse the NaOH-activated ZE21B alloy in bistriethoxysilylethane (BTSE) to form a cross-linked silane coating layer with enhanced corrosion resistance, and the second step was to treat the BTSE-modified ZE21B alloy with APTES to immobilize the amino functional groups to form hydrogen bonds with the outer PLGA coating. Compared to the APTES pretreatment, the cross-linked bilayer BTSE-APTES pretreatment showed better corrosion protection and biocompatibility.
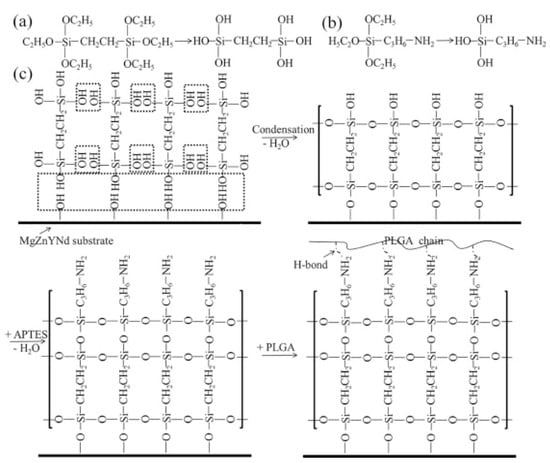
Figure 9. Chemical structure of BTSE (a) and APTES (b); (c) surface modification procedure on the ZE21B alloy [133].
3. Research Trends and Outlook of Magnesium Alloy Stents
3.1. Research Trends of Magnesium Alloy Stents
Based on the corrosion mechanism of magnesium alloy stents, together with their deformation during usage and features of the service environment, further comprehensive exploration and studies are necessary on magnesium alloy stents. This calls for adapting coatings to the deformation of the stents, preparing rapid endothelialization coatings to enhance the service environment of the stent, and constructing coatings with self-healing functions, with the aim of developing bioabsorbable magnesium alloy stents with controlled degradation (Figure 10).
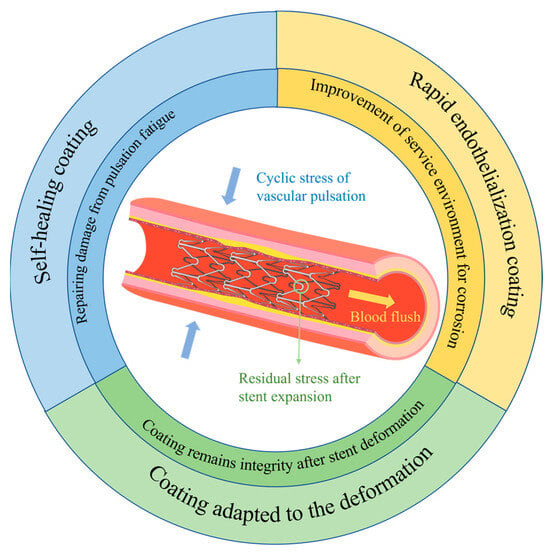
Figure 10. Further exploration and study of magnesium alloy stents.
3.2. Outlook
The magnesium alloy stent is one of the three types of biodegradable metal stents developed at present, and it is the earliest biodegradable metal stent applied in the clinic. Clinical results have shown long-term biosafety, but the physical support performance of the stent in the acute stage after surgery is not stable enough. It is necessary to further optimize the mechanical properties of the stent platform, the short-term protective effect of the coating, the rapid endothelialization function of the endothelialization-promoting coating, and the self-healing function in the stent so as to realize the effective support of magnesium alloy stents at the initial stage of implantation and the gradual loss of support after 3–6 months of implantation, that is, controllable degradation.
This entry is adapted from the peer-reviewed paper 10.3390/ma17010068
This entry is offline, you can click here to edit this entry!