Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cancer remains one of the leading killers world-wide. New drugs to treat cancer that exploit the immune system to attack cancer have been developed called immune checkpoint inhibitors (ICIs). As use of these potent anti-cancer therapeutics have grown, researchers have noticed an unsettling association with use of ICIs and cardiovascular complications known as immune-related adverse events (irAEs).
- cardiovascular
- anti-PD-1
- anti-CTLA-4
- immunotherapy
- immune-related adverse event
1. Introduction
Cancer remains one of the leading causes of death worldwide, second only to cardiovascular disease. Over the past decades, advancements in anti-cancer therapies have increased the likelihood of positive outcomes and reduced morbidity and mortality for many types of cancer. Immunotherapy, one of the most promising recent developments in anti-cancer therapy, uses the immune system to target and destroy cancer. Despite the immense promise of this class of anti-cancer therapeutics, concerns have been raised regarding the association between immunotherapy and adverse cardiovascular events. These concerns have led to the investigation of potential cardiovascular toxicity resulting from the use of immunotherapy.
2. Introduction to Immunotherapy
2.1. Immune Response to Cancer Cells
Once a dendritic cell (DC) encounters an antigen, the DC processes and presents the antigen to T cells in the lymph node (LN). For a T cell to become activated, the DC must present the antigen via the peptide: MHC class I/II complex interacting with the T-cell Receptor (TCR) on the T cell (signal 1) [1]. After this interaction, CD28 on the T cell binds to the B7 family (CD80/CD86) on the DC to provide a co-stimulatory signal (signal 2). Once co-stimulation occurs, cytokines, such as IL-12, are then released by the DC to activate the T-cell (signal 3) fully. The activated T cells then exit the LN and travel to the site of infection or tumor site, where the T cell will provide help in the context of a T helper (Th) cell or induce cytotoxic effects in the context of a CD8+ cell [2]. Another example of the immune response against cancer cells is through Natural Killer (NK) cells. NK cells are innate immune cells that induce a cytotoxic effect on their target using perforin and granzymes, similar to CD8+ T cells. However, NK cells can recognize and kill their target cells in an MHC-independent manner [3]. M1 macrophages exhibit anti-tumor effects via intrinsic phagocytosis and release Reactive Oxygen Species (ROS) and Nitric Oxide (NO), which have cytotoxic effects on tumor cells [3].
2.2. Mechanisms of Tumor Immune System Evasion
Although the immune system has anti-tumor effects, cancer cells can also adapt and evade the immune system. Some examples of immune cells that contribute to cancer cell growth and survival are T regulatory (Treg) cells, M2 macrophages, and myeloid-derived suppressor cells (MDSC) [3]. Tregs are an immunosuppressive subset of CD4+ cells that can contribute to tumor growth using various approaches. Specifically, Tregs constitutively express CTLA-4, thereby inhibiting co-stimulatory signals by CD80/CD86. They also secrete IL-2, providing a proliferative and survival autocrine signal, and they secrete inhibitory cytokines, such as IL-10 and TGF-b, inducing the development of tolerogenic DCs within the Tumor Microenvironment (TME) [3]. In addition, Tregs express PD-1 and PD-L1, further inhibiting the immune response within the TME. Tumor cells can also escape recognition by T cells by downregulating MHC-I and exhibiting antigen loss [3].
2.3. Role of Immune Checkpoint Inhibitors in Cancer Therapy
Immunotherapy is a type of therapy that utilizes the immune system to treat cancer. Immunotherapy can be categorized as passive or active. Passive immunotherapy targets the tumor and involves administering immune-cell factors, resulting in an immediate effect [4]. Because the immunological memory is not initiated, continued dosing may be required for passive immunotherapy. Active immunotherapy acts directly on the immune system, induces an immunological memory, and produces a lasting, durable response [4]. Examples of passive and active immunotherapies include DC immunotherapy, vaccines, monoclonal antibodies (mAb) that target tumor-specific or overexpressed antigens and cytokines (e.g., IL-2, IFN-g, and TNF-a), and Adoptive Cell Therapies (ACT) (e.g., Tumor-Infiltrating Lymphocyte (TIL) therapy and Chimeric Antigen receptor (CAR) T cells) [5].
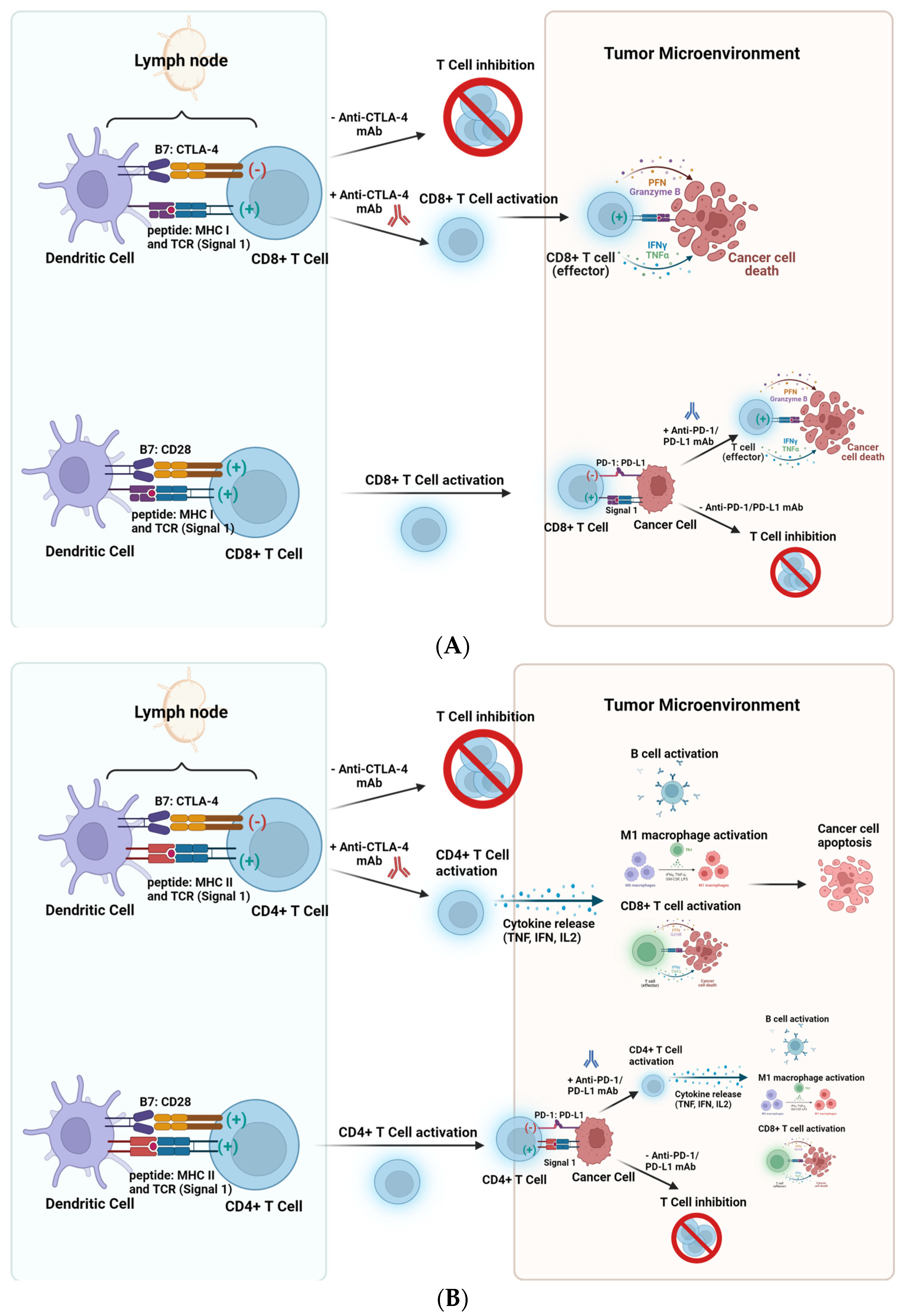
Cancer cells can evade the immune system by upregulating inhibitory receptors and ligands on their cell surface, such as CTLA-4, PD-1, PD-L1/2, Lag-3, and Tim-3 [6]. Immune checkpoint Inhibitors (ICI) are another category of cancer immunotherapy. ICIs block immune checkpoint proteins from binding with partner proteins. This research will primarily focus on three types of ICIs: anti-CTLA-4 mAb, anti-PD-1 mAb, and anti-PD-L1 mAb. Figure 1 illustrates the approaches in CD8+ and CD4+ T cell responses using these ICIs in cancer therapy.

Figure 1. Approaches using anti-CTLA-4 mAb, anti-PD-1 mAb, and anti-PD-L1 mAb in cancer therapy. (A) (Top): CTLA-4 competes with CD28 to bind to CD80 or CD86 and engagement of CTLA-4 with CD80/CD86 results in dampened immune responses. Ipilimumab (anti-CTLA-4 mAb) prevents CD8+ T-cell attenuation at the priming phase, allowing the expansion of effector T cells. (A) (Bottom): Upon binding of the TCR with peptide, MHC I (signal 1) and the engagement of B7 (CD80/CD86) with CD28 (signal 2), CD8+ T cell activation occurs. Within the Tumor Microenvironment, cancer cells can express PD-L1 and bind to PD-1 on T cells, leading to T cell inhibition. However, blocking this engagement using anti-PD-1/anti-PD-L1 mAbs enables CD8+ T cell activation and subsequent cancer cell death. (B) (Top): CTLA-4 competes with CD28 to bind to the B7 family and engagement of CTLA-4 with CD80/CD86 results in dampened CD4+ T cell responses. Anti-CTLA-4 mAb prevents CD4+ T-cell attenuation at the priming phase, allowing the expansion of CD4+ T cells. (B) (Bottom): Upon binding of the TCR with peptide, MHC II (signal 1) and the engagement of B7 (CD80/CD86) with CD28 (signal 2), CD4+ T cell activation occurs, resulting in cytokine secretion and immune cell activation. Within the Tumor Microenvironment, cancer cells can express PD-L1 and bind to PD-1 on T cells, leading to CD4+ T cell inhibition. However, blocking this engagement using anti-PD-1/anti-PD-L1 mAbs enables B cell activation, M1 macrophage polarization and CD8+ T cell activation and subsequent cancer cell death. (Figure 1 was created using Biorender).
Under normal conditions, the role of PD-1 and CTLA-4 is to prevent autoimmunity and limit immune activation to prevent bystander damage. Blocking these inhibitory receptors through therapeutic antibodies for cancer treatment has been associated with a wide range of side effects that resemble autoimmune reactions. This research will focus on the immune-related adverse events seen in the cardiovascular system after the administration of ICIs.
3. Mechanisms of Cardiovascular Injury
A growing body of clinical evidence demonstrates a mechanistic association between immunotherapy and averse cardiovascular events. However, due to the various types of injuries or acceleration of underlying pathological cardiovascular conditions, it is unlikely that there is one putative mechanism which drives cardiovascular injury in immunotherapy. Just as there are several manifestations of irAEs affecting the heart and vasculature, there are several potential mechanisms hypothesized which may drive injury during or following treatment with immunotherapy, including checkpoint inhibitors.
3.1. Mechanisms of Cardiac Injury
Cardiac injury following immunotherapy, particularly with checkpoint inhibitors, may result from the unchecked activity of immune cells interacting with cardiac tissue. It has been known for almost three decades that inhibition of immune checkpoints can promote inappropriate lymphocytic targeting of healthy, non-cancerous tissue. CTLA-4 deficient mice have been shown to develop cardiac lymphocyte infiltration, resulting in lethal myocarditis [7]. Another study conducted on mice with generated CTLA4-/- cells showed a large increase in granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-4, and IFN-g. This was a large increase compared to mice with normal CTLA-4 expression. These mice developed severe myocarditis [7]. This study again points to the importance of CTLA-4 in homeostasis in cardiac tissue. Individuals deficient in this regulator or those receiving immunotherapy targeting this regulator will be at risk. Measuring increasing levels of these cytokines may provide a useful way to gauge if the patient is at risk for myocarditis.
CTLA-4 is not the only protein receptor to be experimentally implicated in the development of cardiac compilations. PD-1 knockout models in mice have demonstrated dilated cardiomyopathy following the development of autoantibodies to cardiac troponin I or potentially other cardiac proteins, such as myosin or b1 adrenergic receptors [8]. Targeting these proteins by self-antigens could result in inappropriate activation of voltage-gated L-type Ca++ channels, resulting in myocardial stress. However, this finding is questioned, as similar PD-1-deficient BALB/c mice did not develop spontaneous dilated cardiomyopathy [9]. Heart tissue of patients who died of myocarditis following therapy with PD-1 inhibitors revealed T cell receptors identical to those found in cloned T cells in tumors, as well as the tumors demonstrating striated muscle-specific antigens [10]. The involvement of PD-1 suppression in developing cardiac dysfunction and injury is further supported experimentally in mice. Upregulation of PD-1 by IFN-g in a cytotoxic T-cell-induced myocarditis model was cardioprotective [11], suggesting that loss of PD-1 may increase susceptibility to T-cell-mediated cardiac injury. Interestingly, knockout of PD-1 in mice was not associated with myocardial infiltration of immune cells [12], unlike CTLA-4 deficient mice. Excessive activation of cytokine release and subsequent immune dysregulation has been proposed as a mechanism that could explain ICI-induced MACE and cardiac injury; however, cytokine release syndrome is more often associated with the usage of CAR T cell therapy [13]. Despite this, it is known that pro-inflammatory cytokines, including TNF-a, IL-6, and IL-12, can lead to cardiotoxicity through various mechanisms, including dysregulated b-adrenergic signaling [14] and their role cannot be discounted.
3.2. Mechanisms of Vascular Injury
It is also important to consider vascular injury when considering the risk of adverse cardiovascular events and the underlying mechanisms that may drive irAE. A retrospective review found that patients had an increased risk of adverse vascular events (AVEs) when receiving ICI therapy like those receiving chemotherapy. They concluded that caution should be exercised for patients who already show risk factors of having AVEs [15]. The investigators hypothesized that baseline inflammatory state, as measured by a neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein (CRP), would be elevated in patients with AVEs compared to controls [15]. The results from the study indicated baseline NLR was elevated in patients who developed AVEs compared to those without MACE or other irAEs. However, there were no differences noted between baseline CRP concentrations and the authors concluded that NLR may be useful in the screening and diagnosing of AVEs in cancers treated with ICIs.
Perhaps most concerning is the potential for ICIs to accelerate the development of atherosclerosis. Atherosclerosis is the accumulation of lipid-containing cells underneath large artery endothelium [16]. Specifically, endothelial macrophages and helper T cells are recruited across the arterial wall via leukocyte adhesion molecules E-selectin, intracellular adhesion molecule-1 (ICAM-1), and vascular adhesion molecule-1 (VCAM-1) by way of NF-kB regulation [17]. Monocytes, or macrophages, then phagocytose many low-density lipoproteins (LDL) utilizing a multitude of receptors, including oxLDL, and oxidize the lipid, killing the macrophage and inducing a metamorphosis into a foam cell [16][17]. Once a foam cell forms, neighboring T lymphocytes are hypothesized to encourage lesion progression via cytokine release, such as IL-9, IL-11, and IL-12, increasing the plaque size [17]. After a long enough period of plaque expansion and foam cell accumulation, the atheroma, or atherosclerotic plaque, contains a lipid or necrotic core covered by a layer of fibrotic cap consisting of both smooth muscle cells and extracellular matrix [16]. The fate of this plaque is determined by the stability or thickness of the base of the atherosclerotic lesion, which contains foam cells and T lymphocytes [16]. A stable plaque with a thick enough shoulder to prevent disruption of the necrotic core will likely remain clinically silent until the plaque occludes the vascular lumen enough to cause arterial stenosis [16]. However, a vulnerable plaque with a weak shoulder is potentially fatal due to plaque rupture contributing to ACS or thrombosis [16].
In fact, during observation of patients with melanoma being treated with ICI therapy, a four-fold increase in risk for MI, coronary revascularization, and ischemic stroke was observed [18]. Additionally, a three-fold increased total plaque volume progression rate, from 2.1 to 6.7, was observed in calcified and non-calcified atherosclerotic plaques [18]. In terms of how the atherosclerosis acceleration occurs, a murine model demonstrated that PD-1-deficient myeloid progenitor cells upregulated genes involved in cholesterol synthesis and uptake, and downregulated genes promoting cholesterol metabolism, leading to overall markedly increased cellular cholesterol levels, known to increase the risk and rate of atherosclerosis [19]. Therefore, a potential mechanism for ICI-induced acceleration of atherosclerosis may lie in the blockade of PD-1, culminating in the upregulation of a pro-atherosclerotic ecosystem in patients. However, a murine-based model analyzing the effects of ICI treatment on hyperlipidemic mice noted that short-term treatment induces an activated T-cell profile without directly affecting the myeloid system [20]. Specifically, a 3.9-fold increase in plaque necrotic core area with an immunogenic profile of markedly increased cytotoxic CD3+ CD8+ T cells and a 41.9% decrease in plaque macrophage content suggest increased macrophage apoptosis promoted necrotic core formation and subsequent plaque progression [20]. There is additional evidence that dual therapy with anti-CTLA-4/anti-PD-1 induced endothelial activation via increased expression of ICAM-1 and VCAM-1, suggesting upregulation of initial phases in atherosclerosis and the potential for new plaque development in addition to atherosclerotic plaque progression [20]. Therefore, another theoretical mechanism may be the enrichment of cytotoxic T lymphocytes, specifically leading to atherogenesis and a more vulnerable plaque phenotype, placing patients at risk of fatal atherosclerotic complications [21][22].
4. Clinical Perspectives of Immunotherapy
4.1. Current Clinical Studies in Immunotherapy
Immunotherapy has become the groundbreaking research focus for cancer treatment. Although the immune system can prevent or slow the growth of tumors, cancer cells have developed methods to evade the immune system. This includes, but is not limited to, novel genetic variants that hide cancer cells from the immune system, the addition of proteins on cell surfaces to turn off immune cells, and altering surrounding normal cells that will interfere with immune system responses to cancer cells.
In the clinical context, ICIs can have relatively minimal side effects, including rash, diarrhea, and fatigue [23][24]. The growth of ICIs in the last decade has offered heightened clinical efficacy in patients with cancer. The increased use of ICIs has radically changed the oncology field and offered a more holistic perspective to personalized treatment options [25]. Improved patient outcomes have been seen with advanced anti-cancer therapies, including ICIs and combination approaches. However, several randomized clinical trials have also shown that these treatments may result in severe cardiovascular side effects like heart failure, arrhythmias, thrombotic events, and various heart diseases. While heart failure and myocardial dysfunction are the most concerning, vascular complications are also commonly reported [26]. It is crucial to screen for cardiotoxic effects accurately to ensure patient safety and avoid overly cautious diagnoses that may impede optimal cancer treatment.
4.2. Investigation Techniques: Baseline Assessment, ECG, Acute Biomarkers, Imaging
Multiple techniques are employed in investigating suspected cardiac or vascular toxicity, although an overarching stepwise guideline has yet to be established. To properly assess an individual, a baseline assessment of cardiovascular risk, including comprehensive patient history; physical exam; screening for cardiovascular diseases such as coronary artery disease, stroke, or thromboembolic events; and screening for unfavorable lifestyle choices such as tobacco smoking, obesity, alcohol use or physical inactivity [27]. If a proper baseline is established, the following toxicity measurements will be identifiable for the patient’s cardiac or vascular functionality.
Acquisition of an electrocardiogram (ECG) is the first step in most cardiovascular evaluations. In multiple assessments of ICI-induced myocarditis, the most common cardiotoxicity identified as an irAE with ICI therapy, 94.4% of confirmed myocarditis cases showed irregular ECG markings, including abnormal T waves, ST segment, conduction defects, and sinus tachycardia [28]. Another study recorded an immense array of ICI-induced ECG changes, such as prolonged QRS and QTc, decreased QRS voltage, and various conduction blocks [29]. Therefore, there remains an impetus for baseline ECG on patients before ICI treatment to properly assess ECG changes in patients with suspected cardiovascular toxicity.
Acute cardiac biomarkers such as troponin, brain natriuretic peptide (BNP)/proBNP, and LDH may indicate ongoing cardiac adverse effects [30][31][32]. Biomarkers can help detect subclinical LV dysfunction [33]. Troponin, traditionally recognized as a marker of cardiac ischemia, may show prognostic significance in ICI-related myocarditis [29]. Additionally, one meta-analysis noted that troponin levels were increased in 94% of patients with cardiotoxicity, not necessarily myocarditis, showing that troponin may help explore various aspects of cardiac adverse events [34]. Troponin levels have been noted in patients lacking clinically apparent symptoms of cardiac irAEs, which begs the question of smoldering myocarditis or subclinical cardiotoxicity in patients treated with ICIs [34]. Brain natriuretic peptide (BNP)/N-terminal proBNP (NT-proBNP) are very sensitive markers of early myocardial damage [33] An increase in cardiac LDH levels may indicate ICI-induced myocarditis [28]. However, LDH may not distinguish correctly between cases of mild myocarditis versus severe myocarditis [28].
Various imaging studies have been described to investigate ICI-related cardiotoxicity. Echocardiography, nuclear cardiac imaging, specifically multigated radionuclide angiography (MUGA), and cardiac magnetic resonance (CMR) imaging are usually recommended. Multigated acquisition scans and cardiac magnetic resonance (CMR) are more accurate and reproducible than echocardiography for repeated assessments of left ventricular ejection fraction, but due to easy availability, echocardiography is usually used and studies recommend a baseline echocardiogram to provide information on cardiac dysfunction, similarly to baseline ECG [35]. Echocardiograms prove extremely useful in evaluating left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) [35]. This information shows that ICI-induced cardiovascular toxicity may not always present as symptomatic or clinically apparent, and subclinical cardiotoxicity may not remain subclinical [33].
4.3. Importance of Recognition of Cardiovascular irAEs
While myocarditis is the most common cardiotoxicity related to immunotherapy treatment, recent studies indicate that pericardial disease, vasculitis, ACS, arrhythmias, left ventricular dysfunction, and even Takotsubo, or stress, cardiomyopathy may also represent various presentations of ICI-induced cardiotoxicity [29]. While certain MACE, such as myocardial infarction or cardiac arrest, rapidly manifest clinically and are likely to be accounted for in any clinical trial or patient chart, the presence of subclinical cardiac anomalies should not be underestimated nor ignored. Morbidity or mortality rates are likely underestimated due to a lack of investigation concerning cardiac-induced deaths within long-term, extremely ill cancer patients. In a systematic review and meta-analysis, myocarditis mortality could range from 35–50%, and pericardial disease occurs in 0.36% of patients, with a projected mortality of 21%, fourfold higher than patients not treated via immunotherapy [29]. Furthermore, increased troponin was identified in over 50% of ICI-treated patients, indicating the possibility of smoldering myocarditis present in patients without the development of fulminant or clinical myocarditis manifestation, raising the question of patient complications in a long-term setting [34]. Without more stringent guidelines in monitoring and diagnosing MACE within immunotherapy-treated patients, oncologists and patients risk unexpected cardiac complications and death based on current data.
In terms of the gravity concerning thrombotic events as an irAE within immunotherapy-treated cancer patients, multiple studies in clinical settings showed that immunotherapy patients develop more thrombotic events than the general cancer population, including greater pulmonary embolism (PE)-linked mortality [34][36]. Additionally, PE decreased overall survival in those patients who experienced a thrombotic event, whether the event itself led to death or, by an unknown mechanism, the PE provided health complications later in the patient’s life, unrelated to cancer treatment. Furthermore, mortality remained significantly greater in patients who developed VTE than those without VTE events within immunotherapy-treated patients, with additional data suggesting significantly reduced overall survival in VTE-affected immunotherapy patients [37]. Specifically, symptomatic PE posed prognostic significance, suggesting a direct impact of VTE events on mortality in post-operative settings [37]. Additionally, VTE was an independent predictor of early, all-cause mortality, even when investigators adjusted for lab abnormalities in creatinine, alkaline phosphatase, and protein levels [37]. In summary, the potentially life-threatening nature of thrombotic events as an irAE during and after immunotherapy creates an impetus for further investigation and stringent protocols to abate patient mortality.
4.4. Cardiovascular Toxicity in Pediatric Patients Treated with Immunotherapy
Immunotherapy is approved for pediatric use, however, there is less experience with immune-related adverse effects in this population. FDA approval of immunotherapy for treating pediatric patients with melanoma has largely been extrapolated from the same treatment approach used in adults. For example, the KEYNOTE-716 phase III trial evaluating the use of adjuvant pembrolizumab in high-risk node-negative melanoma enrolled two pediatric patients (between the ages of 12 and 17 years) [38]. Similarly, atezolizumab was recently approved for the treatment of patients 2 years of age or older with alveolar soft part sarcoma based on results from the ML39345 trial (NCT03141684), in which three pediatric patients were reported [39]. Nivolumab is approved for treatment of pediatric melanoma with results extrapolated from clinical trials of adjuvant nivolumab in adults with melanoma.
The majority of existing data reporting on cardiotxicity in the pediatric population can be derived from four recent phase I/II studies investigating nivolumab (ADVL1412; NCT0230445848), pembrolizumab (KEYNOTE-051; NCT0233266849), atezolizumab (iMATRIX; NCT0254160450), and avelumab (NCT0345182551). These trials were all investigating immunotherapy for recurrent and refractory pediatric tumors. In general, toxicity was reported using NCI CTCAE terminology with no pediatric consensus system for grading and reporting of immune-related adverse effects. It is noted that many cardiovascular metrics, such as blood pressure, are age-dependent and may require a different grading system than that used for adults.
In ADVL1412 (NCT0230445848), nivolumab plus ipilimumab was studied in children and young adults with recurrent/refractory solid tumors (n = 53). Under immune-related adverse events, there were two pericardial effusions reported (2/53 incidence) as the only cardiac events [40]. The ongoing KEYNOTE-051 trial examines pembrolizumab (MK-3475) in pediatric patients with solid tumors. Preliminary results of the first phase, reporting on safety and antitumor activity with optimal dosing in advanced pediatric cancer, have been published (n = 154) [41]. Cardiotoxicity was uncommon, including only hypertension (n = 2, 1%). There was one report of death due to pulmonary edema, but it was not clearly attributed to cardiogenic toxicity, highlighting the need for enhanced reporting of potential cardiovascular effects in clinical trials of immunotherapy in pediatric patients.
The iMATRIX study was a multicenter, open-label international trial of atezolizumab for treatment of patients aged < 30 years with solid tumors or lymphomas [42]. Similar to the KEYNOTE-051 trial, cardiac adverse effects were uncommon and only hypertension was reported (n = 1, 1%). Finally, avelumab was studied in a study of children and young adults < 18 years with recurrent/refractory solid tumors [43]. In this study, the only cardiovascular adverse event reported was also blood pressure abnormality, interestingly, in this study both hypotension (n = 4) and hypertension (n = 2) were reported.
In summary, approvals by the FDA for use of immunotherapy in treating pediatric patients are likely to lead to increased use in this group. Cardiovascular events seem uncommon and mainly manifested as hypertension, but pericardial effusion has been reported and the present data are limited. The pediatric population should be considered specially and would benefit from enhanced reporting of cardiovascular effects with long-term monitoring and discussion on a consensus grading system incorporating age-dependent variables.
4.5. Accurate Grading
A generalized consensus concerning a grading scale and proper categorization of cardiovascular symptomology and clinical presentation has yet to be universally utilized. This is likely due to the variety of MACE that may develop. However, the CTCAE has developed a grading of cardiac events based on (1) biomarker outcome, (2) ECG readings, (3) symptoms, and (4) generalized cardiac complications, such as arrhythmia or myocardial ischemia [44]. This grading system provides a guide on properly treating the patient based on the severity of their condition instead of a scale to determine the likelihood an irAE might occur. Grade 1 is defined as close monitoring during treatment in an asymptomatic patient with aberrant cardiac biomarkers and irregular ECG tracings, grade 2 involves minor symptoms, grade 3 is severe symptoms with steroid treatment required, and grade 4 is moderate to severe decompensation of life-threatening situations that demand IV injections of medication or intervention [28]. Utilizing this scale, a grade 1 or 2 is considered mild, whereas a grade 3 or 4 is considered severe [28]. The National Cancer Institute’s Common Toxicity Criteria and the National Comprehensive Cancer Guidelines confirm the grading scale of CTCAE for general cardiovascular complications. The CTCAE allows for proper grading of an ongoing adverse event. However, a more efficient risk assessment system is needed to inform patients and their physicians comprehensively of the next steps in management, particularly the ability to continue treatment or re-challenge.
4.6. Prevention
Immunotherapy has revolutionized cancer treatment since its inception, which makes understanding and preventing catastrophic adverse effects of utmost importance. A baseline assessment of cardiac function and analysis of cardiovascular risk factors allows for understanding a patient’s overall cardiac health and proclivity to develop a MACE [35]. Recently, a murine model-based preclinical study analyzed the benefit of TNF-α blockade in targeting early-stage cardiotoxicity based on the PD-1/PD-L1 pathway, exhibiting protective characteristics on the myocardium [8][45]. The study identified that co-administration of TNF-α blockade alongside ICI treatment led to the mitigation of cardiotoxicity, as evidenced by an echocardiogram showing a preserved LVEF of 48.7 ± 3.9 versus 42.1 ± 6.1 for anti-TNF-α/PD1 and anti-PD-1 alone, respectively [46]. Additionally, the benefit of anti-TNF-α compared to previous attempts at CD8+ T cell depletion lies in the idea that using TNF-α blockade does not negate the effects of ICI treatment; this allows for a continuation of immunotherapy while preventing cardiotoxicity, rather than stopping cancer treatment in favor of addressing cardiotoxicity and risking cancer progression [46]. Although the use of TNF-α blockade has not withstood patient-based clinical trials yet, the applications of anti-TNFα remain promising in preventing cardiotoxicity and allowing patients to continue cancer immunotherapy.
Another potential mechanism to prevent cardiac toxicity, specifically the blockade of atherosclerosis progression, may be statin prophylaxis in immunotherapy patients. A clinical study observed a greater than three-fold increase in the aortic plaque volume in melanoma patients treated with ICIs compared to controls, but lower rates of plaque progression and total aortic plaque volume in melanoma patients treated concomitantly with ICIs and a statin compared to immunotherapy alone [18]. Statin therapy becomes especially relevant when observing the effects of PD-1 deficiency leading to significantly increased cellular levels, creating the perfect environment for prevention through statins as a two-edged sword in regulating patient lipid levels and preventing atherosclerosis advancement [18][19].
4.7. Management
Managing cardiovascular toxicity in the immunotherapy setting differs from standard symptomatic events in that intravenous high-dose corticosteroids, such as methylprednisolone, are often the first step in treatment [28]. However, a recent review concerning immunotherapy-related cardiovascular complications proposed an eight-point management system to comprehensively evaluate patients, utilizing clinical presentation, ECG, serum cardio biomarkers, TTE, CMR, coronary imaging, and endomyocardial biopsy [29]. Using a multi-point diagnostic reasoning scale allows physicians to understand precisely which type of toxicity they are dealing with, whether PE, myocarditis, ACS, or VTE. Unfortunately, this proposed system recommends that all patients presenting with cardiovascular symptoms be withheld ICI treatment and admitted for telemetry in the case of cardiotoxicity or with regular venous ultrasound or CT imaging in the case of vascular compromise, allowing cancer progression in the meantime [29]. Due to the lack of understanding concerning preventing cardiovascular irAEs, high-dose corticosteroids and cessation of immunotherapy until patient stabilization remain the standard of care [47]. Another proposed option for management of cardiovascular toxicity lies in vitamin D. Based on previous studies investigating the effect of 1.25(OH)2 vitamin D3 on cardiomyocytes in a murine model of experimental autoimmune myocarditis, markedly reduced apoptosis was observed, as well as decreased caspase expression, a marker of inducible apoptosis within cells [48][49] Given this data, a prospective clinical trial proposal can be to consider vitamin D administration alongside corticosteroid treatment when caring for a patient with ICI-induced cardiovascular toxicity.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15245707
References
- Guerder, S.; Flavell, R.A. T-cell activation. Two for T. Curr. Biol. 1995, 5, 866–868.
- Hunter, M.C.; Teijeira, A.; Halin, C. T Cell Trafficking through Lymphatic Vessels. Front. Immunol. 2016, 7, 613.
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022.
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buque, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508.
- Baxter, D. Active and passive immunization for cancer. Hum. Vaccin. Immunother. 2014, 10, 2123–2129.
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461.
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547.
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483.
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895.
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755.
- Grabie, N.; Gotsman, I.; DaCosta, R.; Pang, H.; Stavrakis, G.; Butte, M.J.; Keir, M.E.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation 2007, 116, 2062–2071.
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322.
- Ceschi, A.; Noseda, R.; Palin, K.; Verhamme, K. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol. 2020, 11, 557.
- Mohan, M.L.; Vasudevan, N.T.; Prasad, S.V.N. Pro-inflammatory cytokines mediate GPCR dysfunction. J. Cardiovasc. Pharmacol. 2017, 70, 61.
- Bar, J.; Markel, G.; Gottfried, T.; Percik, R.; Leibowitz-Amit, R.; Berger, R.; Golan, T.; Daher, S.; Taliansky, A.; Dudnik, E.; et al. Acute vascular events as a possibly related adverse event of immunotherapy: A single-institute retrospective study. Eur. J. Cancer 2019, 120, 122–131.
- Fan, J.; Watanabe, T. Inflammatory reactions in the pathogenesis of atherosclerosis. J. Atheroscler. Thromb. 2003, 10, 63–71.
- Hansson, G.K. Immune mechanisms in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1876–1890.
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311.
- Strauss, L.; Mahmoud, M.A.A.; Weaver, J.D.; Tijaro-Ovalle, N.M.; Christofides, A.; Wang, Q.; Pal, R.; Yuan, M.; Asara, J.; Patsoukis, N.; et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020, 5, eaay1863.
- Poels, K.; van Leent, M.M.T.; Reiche, M.E.; Kusters, P.J.H.; Huveneers, S.; de Winther, M.P.J.; Mulder, W.J.M.; Lutgens, E.; Seijkens, T.T.P. Antibody-Mediated Inhibition of CTLA4 Aggravates Atherosclerotic Plaque Inflammation and Progression in Hyperlipidemic Mice. Cells 2020, 9, 1987.
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588.
- Kyaw, T.; Winship, A.; Tay, C.; Kanellakis, P.; Hosseini, H.; Cao, A.; Li, P.; Tipping, P.; Bobik, A.; Toh, B.H. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 2013, 127, 1028–1039.
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38.
- Chhabra, N.; Kennedy, J. A Review of Cancer Immunotherapy Toxicity: Immune Checkpoint Inhibitors. J. Med. Toxicol. 2021, 17, 411–424.
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801.
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350.
- Tajiri, K.; Sekine, I. Atherosclerotic cardiovascular events associated with immune checkpoint inhibitors in cancer patients. Jpn. J. Clin. Oncol. 2022, 52, 659–664.
- Lei, Y.; Zheng, X.; Huang, Q.; Li, X.; Qiu, M.; Liu, M. Intrinsic Differences in Immune Checkpoint Inhibitor-Induced Myocarditis: A Retrospective Analysis of Real World Data. Front. Pharmacol. 2022, 13, 914928.
- Thuny, F.; Naidoo, J.; Neilan, T.G. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur. Heart J. 2022, 43, 4458–4468.
- Blancas, I.; Martin-Perez, F.J.; Garrido, J.M.; Rodriguez-Serrano, F. NT-proBNP as predictor factor of cardiotoxicity during trastuzumab treatment in breast cancer patients. Breast 2020, 54, 106–113.
- Kittiwarawut, A.; Vorasettakarnkij, Y.; Tanasanvimon, S.; Manasnayakorn, S.; Sriuranpong, V. Serum NT-proBNP in the early detection of doxorubicin-induced cardiac dysfunction. Asia Pac. J. Clin. Oncol. 2013, 9, 155–161.
- Sawaya, H.; Sebag, I.A.; Plana, J.C.; Januzzi, J.L.; Ky, B.; Tan, T.C.; Cohen, V.; Banchs, J.; Carver, J.R.; Wiegers, S.E.; et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging 2012, 5, 596–603.
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1063–1093.
- Patel, R.P.; Parikh, R.; Gunturu, K.S.; Tariq, R.Z.; Dani, S.S.; Ganatra, S.; Nohria, A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2021, 23, 79.
- Esposito, R.; Fedele, T.; Orefice, S.; Cuomo, V.; Prastaro, M.; Canonico, M.E.; Ilardi, F.; De Stefano, F.; Fiorillo, L.; Santoro, C.; et al. An Emergent Form of Cardiotoxicity: Acute Myocarditis Induced by Immune Checkpoint Inhibitors. Biomolecules 2021, 11, 785.
- Sheng, I.Y.; Gupta, S.; Reddy, C.A.; Angelini, D.; Funchain, P.; Sussman, T.A.; Sleiman, J.; Ornstein, M.C.; McCrae, K.; Khorana, A.A. Thromboembolism in Patients with Metastatic Renal Cell Carcinoma Treated with Immunotherapy. Target. Oncol. 2021, 16, 813–821.
- Khorana, A.A. Venous thromboembolism and prognosis in cancer. Thromb. Res. 2010, 125, 490–493.
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729.
- Chen, A.P.; Sharon, E.; O’Sullivan-Coyne, G.; Moore, N.; Foster, J.C.; Hu, J.S.; Van Tine, B.A.; Conley, A.P.; Read, W.L.; Riedel, R.F.; et al. Atezolizumab for Advanced Alveolar Soft Part Sarcoma. N. Engl. J. Med. 2023, 389, 911–921.
- Davis, K.L.; Fox, E.; Isikwei, E.; Reid, J.M.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; Mackall, C.L. A Phase I/II Trial of Nivolumab plus Ipilimumab in Children and Young Adults with Relapsed/Refractory Solid Tumors: A Children’s Oncology Group Study ADVL1412. Clin. Cancer Res. 2022, 28, 5088–5097.
- Geoerger, B.; Kang, H.J.; Yalon-Oren, M.; Marshall, L.V.; Vezina, C.; Pappo, A.; Laetsch, T.W.; Petrilli, A.S.; Ebinger, M.; Toporski, J.; et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 121–133.
- Geoerger, B.; Zwaan, C.M.; Marshall, L.V.; Michon, J.; Bourdeaut, F.; Casanova, M.; Corradini, N.; Rossato, G.; Farid-Kapadia, M.; Shemesh, C.S.; et al. Atezolizumab for children and young adults with previously treated solid tumours, non-Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): A multicentre phase 1-2 study. Lancet Oncol. 2020, 21, 134–144.
- Loeb, D.M.; Lee, J.W.; Morgenstern, D.A.; Samson, Y.; Uyttebroeck, A.; Lyu, C.J.; Van Damme, A.; Nysom, K.; Macy, M.E.; Zorzi, A.P.; et al. Avelumab in paediatric patients with refractory or relapsed solid tumours: Dose-escalation results from an open-label, single-arm, phase 1/2 trial. Cancer Immunol. Immunother. 2022, 71, 2485–2495.
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768.
- Tarrio, M.L.; Grabie, N.; Bu, D.X.; Sharpe, A.H.; Lichtman, A.H. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 2012, 188, 4876–4884.
- Michel, L.; Helfrich, I.; Hendgen-Cotta, U.B.; Mincu, R.I.; Korste, S.; Mrotzek, S.M.; Spomer, A.; Odersky, A.; Rischpler, C.; Herrmann, K.; et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur. Heart J. 2022, 43, 316–329.
- Zhang, L.; Zlotoff, D.A.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zubiri, L.; Chen, C.L.; Sullivan, R.J.; et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation 2020, 141, 2031–2034.
- Hu, F.; Yan, L.; Lu, S.; Ma, W.; Wang, Y.; Wei, Y.; Yan, X.; Zhao, X.; Chen, Z.; Wang, Z.; et al. Effects of 1, 25-Dihydroxyvitamin D3 on Experimental Autoimmune Myocarditis in Mice. Cell Physiol. Biochem. 2016, 38, 2219–2229.
- Bhalla, A.K.; Amento, E.P.; Clemens, T.L.; Holick, M.F.; Krane, S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983, 57, 1308–1310.
This entry is offline, you can click here to edit this entry!
