Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
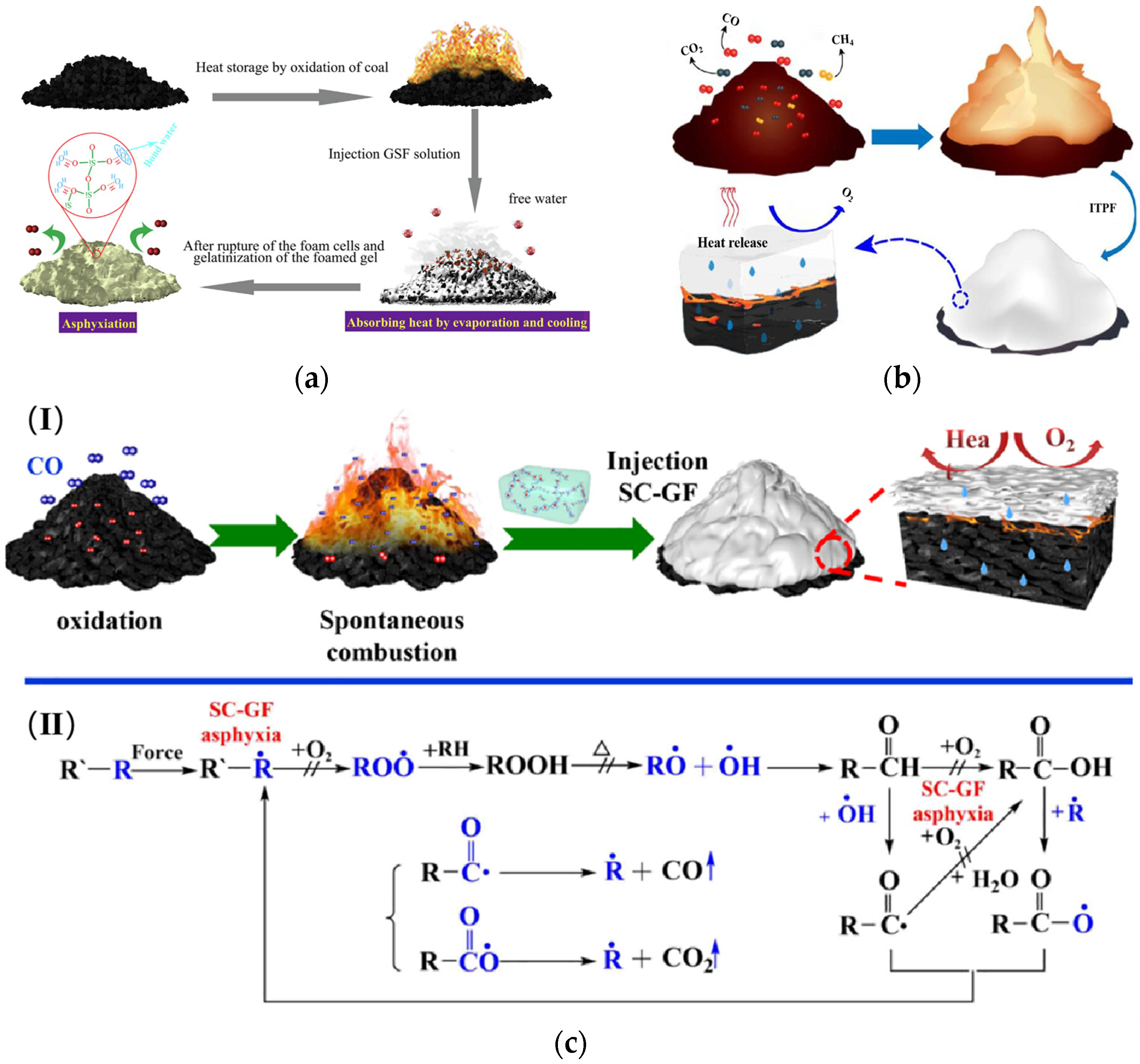
Coal is a fossil fuel with great economic potential, but meanwhile, it is threatened by coal spontaneous combustion during coal production. Gel foam extinguishing agent (gel foam) has promising applications in the prevention and management of mine coal spontaneous combustion.
- coal spontaneous combustion control
- gel foam extinguishing agent
1. Introduction
Coal is a fossil fuel with great economic potential, but meanwhile, it is threatened by coal spontaneous combustion during coal production [1][2]. Due to the complexity of the coal mining process and the particularity of the environment, coal spontaneous combustion is becoming increasingly frequent [3]. Therefore, coal spontaneous combustion prevention technologies have been widely developed in recent years.
Nowadays, research on preventing and controlling coal spontaneous combustion focuses on three areas. It includes coal natural theory, prevention and control technologies, and field practice. Different technologies, such as grouting, inert gas injection, inhibitor injection, gel injection, foam injection, etc., have been used to control coal spontaneous combustion. Based on the mechanism of action, materials are classified into physical-based, chemical-based, as well as composite retardants [4][5]. Gel foam fire extinguishing agent (gel foam) is an innovative material that combines the advantages of both gel and foam. It exhibits strong practicality and promising application prospects.
2. Study on Coal Spontaneous Combustion and Prevention Technology
2.1. The Mechanism of Coal Spontaneous Combustion
Coal spontaneous combustion is one of the central causes of fire disasters. Due to the spontaneous combustion of coal, a lot of coal resources in the world’s major coal mining areas such as South Africa, Europe, the United States, India, and China have been burned [6]. Besides that, harmful gases from coal spontaneous combustion pollute the ecosystem and harm human health, like COX, NOX, SO2, etc. [7][8].
Coal spontaneous combustion has been researched since the 17th century. Various theories have been proposed to explain this phenomenon. Among these, the coal–oxygen composite hypothesis has been recognized by most scholars [9]. Coal spontaneous combustion occurs, which is the result of the joint action between coal and oxygen [10][11][12]. According to the coal–oxygen compound theory, the reaction of reactive groups with oxygen in coal has been investigated [13][14][15]. Experimental investigations have confirmed coal adsorbs oxygen. Meanwhile, the oxygen absorbed would react with the reactive groups of the coal to produce new free radicals, thereby generating a chain of water and gas and releasing heat [16][17][18]. When the heat production rate of coal oxidation is greater than the rate of heat dissipation from the coal to the environment, it will cause the accumulation of heat and make coal oxidation continue, which eventually leads to the spontaneous combustion of coal [19][20][21]. The reaction of radicals during the spontaneous combustion of coal is a chain cycle process.
2.2. Technologies of Prevention and Control of Coal Spontaneous Combustion
2.2.1. Grouting
Grouting is one of the traditional technologies in coal mines for extinguishing fires. The mud is made proportionally from water and non-combustible solids, such as yellow mud or fly ash. It was then transported to the coal seam through an injection pipe [22]. According to the distribution of high-temperature zones in the coal seam, a mud injection system is established to produce mud, which is then transported into the coal seam by a pipeline to extinguish the fire [23]. Mud can cover the coal body, filling internal pores and insulating it from oxygen. However, the mud can easily “pull the ditch” and clog the pipe. In addition, it is difficult to pile up mud to a height, and it cannot solve the fire at a height [24]. However, due to the low cost of grouting, it is still one of the main fire prevention and extinguishing measures.
2.2.2. Inert Gas
Currently, CO2 and N2 are the main inert gases injected into the fire area. Inert gas can serve the purpose of fire prevention by diluting the oxygen in the fire zone. Inert gas has the advantages of a fast fire extinguishing speed, no pollution, etc. However, due to the severe air leakage in the mining airspace, inert gas cannot be used to extinguish the fire for a long time [25]. It was found that liquid inert gas has a better cooling effect. Currently, liquid inert gas fire prevention technology is being successfully used in many coal mines in ChinaThe liquid inert gas (CO2, N2), loaded into special tanks, is transported to the site by mining vehicles and then connected to pressurized conveying equipment. It is injected into the area along the borehole using a booster or under pressure [26]. However, a lot of heat has to be absorbed during the evaporation of liquid inert gas. In addition, transporting and handling liquid coal dioxide poses a high risk. Under this condition, Liu et al. [27] developed a new equipment called the Dry-ice Phase Transformation Generator (DPTG) (Figure 1). The principle of the DPTG is based on warm water flowing through a copper pipe and exchanging heat with dry ice. The dry ice quickly changes from a solid to a gaseous state.
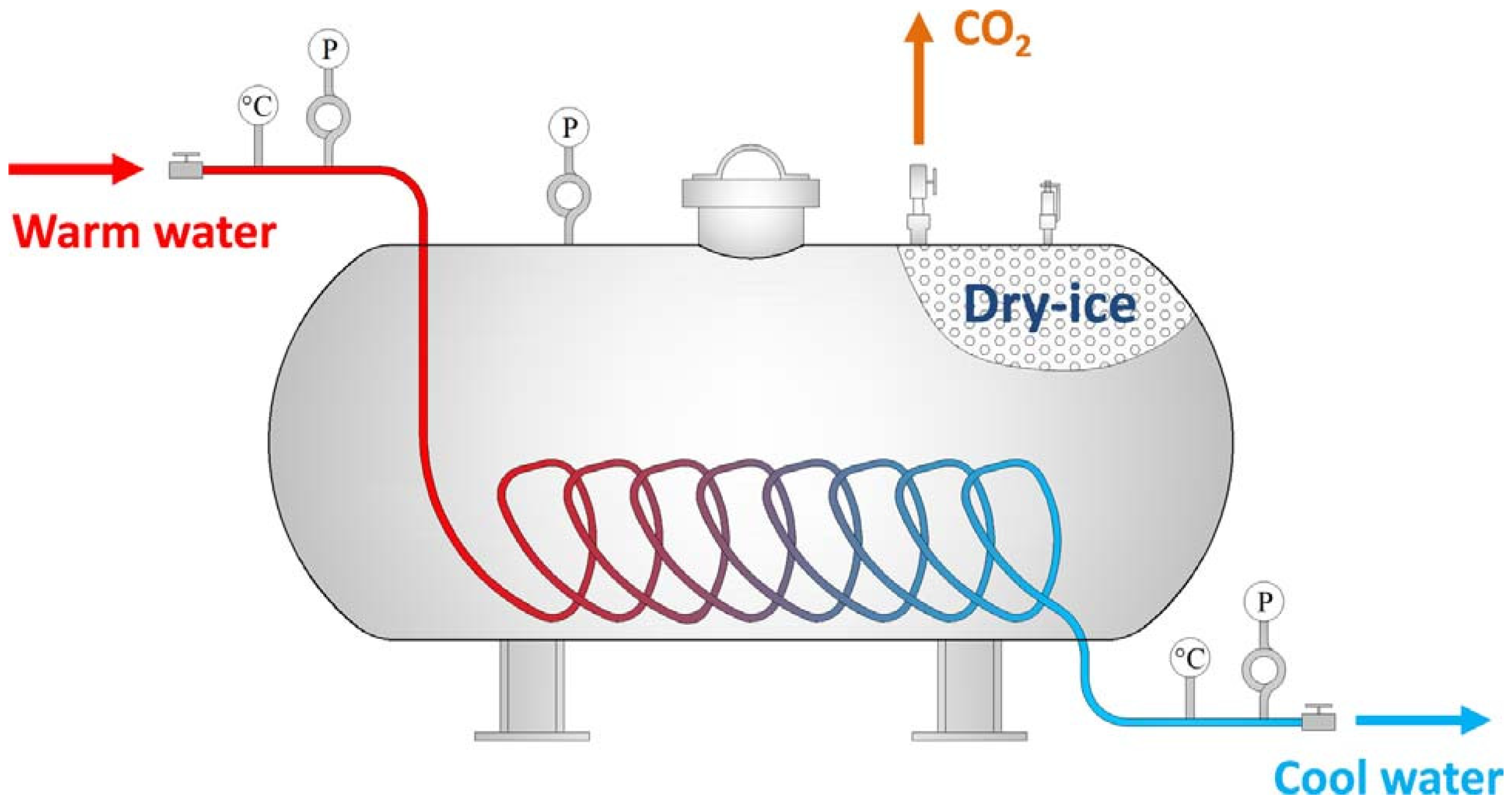
Figure 1. Schematic diagram of Dry-ice Phase Transformation Generator (DPTG) [27].
2.2.3. Inhibitor
Physical inhibitors are mainly halogen salt inhibitors, phosphate inhibitors, and ammonium salt inhibitors. The halogen salt type inhibitors mainly include CaCl2, MgCl2, NaCl, etc. [28][29][30]. These can play a better role in the low-temperature stage of coal oxidation. Physical inhibitors are less expensive and are currently the most commonly used in coal mines. Chemical inhibitors are mainly aimed at preventing the reaction between coal and oxygen, such as polyethylene glycol, anthocyanins, and catechin [31][32]. To offset the disadvantages of a single physical or chemical inhibitor, compound inhibitors offer the advantages of both: They can absorb heat to lower the temperature and effectively break the chain reaction. The compound inhibitors mainly include catechin polyethylene glycol, ascorbic acid–Rosmarinus acid, halogenated salt compound inhibitors, and so on [33][34]. Composite inhibitors have high inhibition efficiency, but the production cost is high, and the synthesis process is complicated. In practice, inhibitors are sprayed onto the coal or directly drill holes and inject inhibitor liquid into the coal wall that has begun to oxidize and heat. Wang et al. [35] examined the spraying process of the inhibitor. He sprayed nitrogen and atomized inhibitor liquid into the goaf for double fire prevention.
2.2.4. Foam
Foam is a kind of gas–liquid dispersed system with great importance. It is produced by physical foaming with a foaming device. The three-phase foam was first proposed to prevent the spontaneous combustion of coal in 2004 [36]. The three-phase foam gives full play to the fire protection advantages of yellow mud grouting and nitrogen injection technology. Meanwhile, it has been applied in many coal mines, such as the Longdong Coal Mine and the Geng Cun Coal Mine in Henan Province [37][38]. In addition, the preparation of three-phase foam in the coal mine site includes two processes (Figure 2): Firstly, the materials are mixed proportionally to form a slurry, and then the slurry is transported to the underground; secondly, a foaming device is used to fully mix and foam the slurry, foaming agent and nitrogen to form a three-phase foam, and the three-phase foam is transported to the area that needs to be prevented from extinguishing the fire [38]. Foam can cover the floating coal in the high place and effectively solve the defect that the grouting technology cannot be poured into the high place. At the same time, it can also be used as a carrier to transport solid phase materials and water to the coal body to improve the resistance effect [39][40].
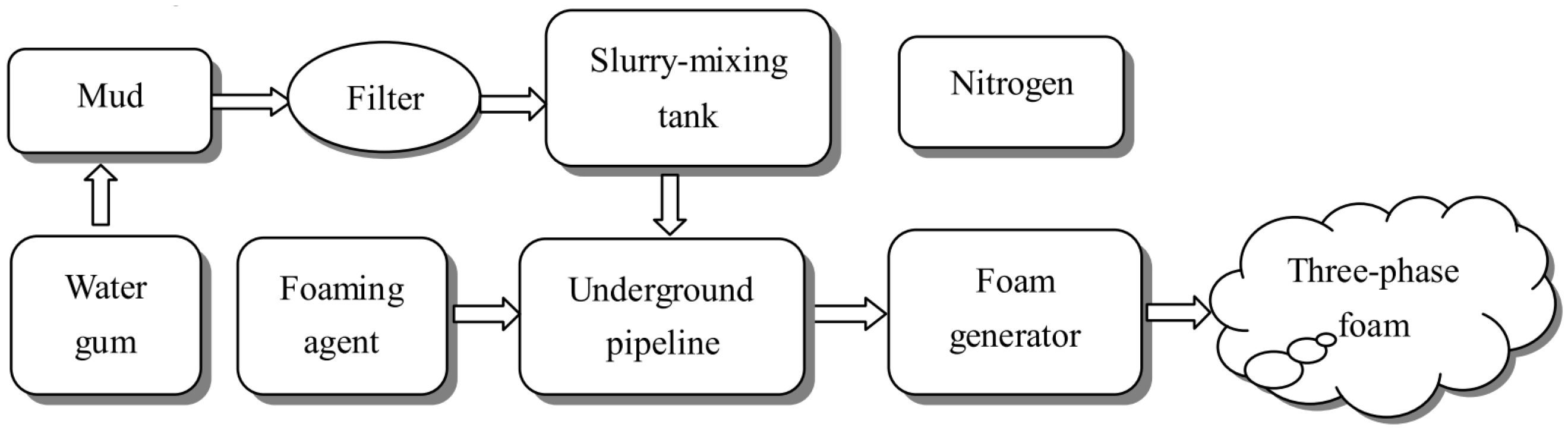
Figure 2. Craft process of infusing the three-phase foam [38].
2.2.5. Gel
The gel is a special state between solid and liquid. According to the different chemical properties of the base material for preparing gel, it can be divided into inorganic gel, organic gel, and composite gel [41][42][43]. Gel fire prevention technology is to mix the base material, additives, and water in proportion and then press into the coal seam fire area with a slurry injection system. The solution in a liquid state before gel formation can penetrate the cracks and pores of the coal body. When the solution becomes a gel, it plugs these pores and fissures. The gel has a good water retention effect and absorbs heat through water evaporation to reduce the temperature of the coal. At the same time, the gel can cover the coal body and stop the reaction process between coal and oxygen. However, the flow rate of the gel is small, the fluidity is poor, and the colloid will crack and form fissures after a long period [44].
3. Structure and Principle of Gel Foam
3.1. Structural Features of Gel Foam
Gel foam is a non-equilibrium structure with gas uniformly dispersed in the gel. It is prepared by mechanical stirring of a gelling agent, cross-linking agent, and blowing agent in a gas [45][46][47]. In China, gel foam was first used to extract oil to enhance oil recovery. And it was gradually applied to coal mine fire prevention in the 20th century [48][49]. Tian [50] built the constitutive model of gel foam with relevant factors. He carried out a systematic study on the theory and technology of gel foam in coal mines. Qin et al. [51] proposed a multi-phase gel foam combining the gel and three-phase foam. It used fly ash as the base material and explored its weak cross-linking characteristics.
Differing from gel and foam, gel foam is a mixed system with gel as the continuous phase and gas as the dispersed phase [52]. The foam transports the gel-forming material into the coal body, while the foam film contains a large amount of gas and water inside its membrane. After some time, the gel-forming material forms a colloid with a three-dimensional network structure and wraps the foam [53]. As shown in Figure 3, (a) is the structure of the gel foam, and its structural characteristics are shown in (b). The unique formation process and structure of gel foam are applied in coal mine spontaneous combustion control by combining the advantages of both gel and foam.
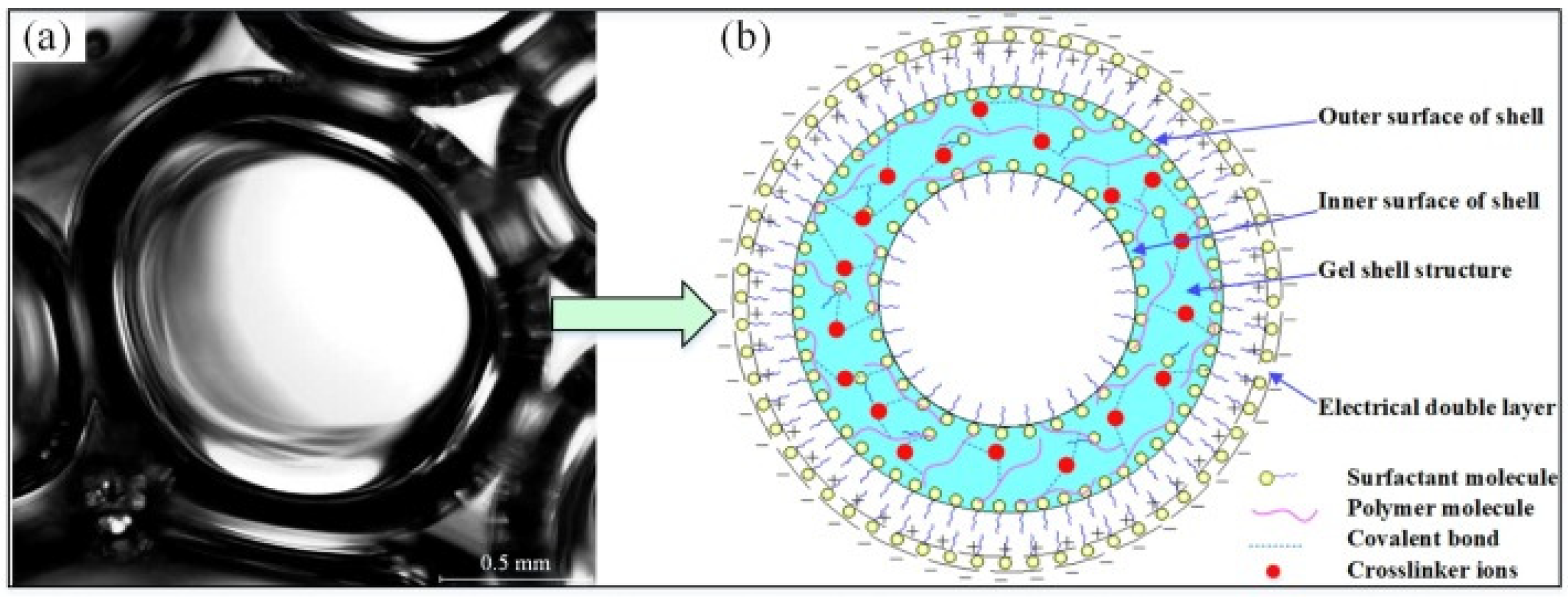
Figure 3. Schematic diagram of single bubble structure in gel-stabilized foam [53].
3.2. Principle of Gel Foam Action
Gel foam is a new material based on gel and foam. The principle of spontaneous coal combustion is controlled by preventing or delaying the oxidation process of coal. It is essential to explore the role of gel foam in spontaneous coal combustion and explain its mechanism of action. The action mechanism of gel foam has been divided into three categories based on extensive experimental characterization and analysis [54][55][56]:
- (a)
-
The cooling effect [57]
The main component of gel foam is water. When water is transported from the foam to the high-temperature area, it will evaporate quickly due to the high temperature. The temperature of the coal drops because water absorbs a lot of heat as it evaporates, as shown in Figure 4a.
- (b)
-
The plugging effect
Figure 4b demonstrates the oxygen barrier properties of the gel foam. Gel foams contain gelling and cross-linking agents, which will form a three-dimensional network structure to cover the surface of the coal body after a chemical reaction. When the moisture disappears completely, a gel film is formed, blocking oxygen from coming into contact with the coal [58][59].
- (c)
-
The inhibition effect
The inhibition of gel foam consists mainly of physical and chemical inhibition. Physical inhibition is usually to prevent the contact of oxygen with coal, using cooling and the oxygen barrier to inhibit the spontaneous combustion of coal; chemical inhibition is to prevent the oxidation reaction of coal from proceeding and to inhibit the oxidative spontaneous combustion of coal by replacing the free radicals of the coal oxygen reaction or by generating a more stable structure with them (Figure 4c) [60].
This entry is adapted from the peer-reviewed paper 10.3390/fire6120470
References
- Musa, S.D.; Zhonghua, T.; Ibrahim, A.O.; Habib, M. China’s energy status: A critical look at fossils and renewable options. Renew. Sustain. Energy Rev. 2018, 81, 2281–2290.
- Onifade, M.; Genc, B.; Carpede, A. A new apparatus to establish the spontaneous combustion propensity of coals and coal-shales. Int. J. Min. Sci. Technol. 2018, 28, 649–655.
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. Int. 2017, 24, 23453–23470.
- Li, Q.W.; Xiao, Y.; Zhong, K.Q.; Shu, C.M.; Lü, H.F.; Deng, J.; Wu, S. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel 2020, 275, 117981.
- Yu, M.; Wang, L.; Li, H. Research status and development trend of fire-extinguishing materials in Chinese coal mines. Min. Saf. Environ. Prot. 2022, 49, 22–36. (In Chinese)
- Onifade, M.; Genc, B. A review of spontaneous combustion studies—South African context. Int. J. Min. Reclam. Environ. 2019, 33, 527–547.
- Civeira, M.S.; Pinheiro, R.N.; Gredilla, A.; de Vallejuelo, S.F.O.; Oliveira, M.L.; Ramos, C.G.; Taffarel, S.R.; Kautzmann, R.M.; Madariaga, J.M.; Silva, L.F. The properties of the nano-minerals and hazardous elements: Potential environ-mental impacts of Brazilian coal waste fire. Sci. Total Environ. 2016, 544, 892–900.
- Zeng, Q.; Dong, J.; Zhao, L. Investigation of the potential risk of coal fire to local en-vironment: A case study of Daquanhu coal fire, Xinjiang region. China. Sci. Total Environ. 2018, 640, 1478–1488.
- Zhang, J.; Ren, T.; Liang, Y.; Wang, Z. A review on numerical solutions to self-heating of coal stockpile: Mechanism, theoretical basis, and variable study. Fuel 2016, 182, 80–109.
- Qin, B.T.; Zhong, X.X.; Wang, D.M.; Xin, H.H.; Shi, Q.L. Research progress of coal spontaneous combustion process characteristics and prevention technology. Coal Sci. Technol. 2021, 49, 66–99. (In Chinese)
- Wang, H.H.; Dlugogorski, B.Z.; Kennedy, E.M. Kinetic modeling of low-temperature oxidation of coal. Combust. Flame 2002, 131, 452–464.
- Lin, Q.; Wang, S.; Liang, Y.; Song, S.; Ren, T. Analytical prediction of coal spontaneous combustion tendency: Velocity range with high possibility of self-ignition. Fuel Process. Technol. 2017, 159, 38–47.
- Ma, T.; Zhai, X.W.; Xiao, Y.; Bai, Y.E.; Shen, K.; Song, B.B.; Hao, L.; Ren, L.F.; Chen, X.K. Study on the influence of key active groups on gas products in spontaneous combustion of coal. Fuel 2023, 344, 128020.
- Qi, X.; Wang, D.; Xin, H.; Qi, G. An In Situ Testing Method for Analyzing the Changes of Active Groups in Coal Oxidation at Low Temperatures. Spectrosc. Lett. 2014, 47, 495–503.
- Qi, X.; Xue, H.; Xin, H.; Wei, C. Reaction pathways of hydroxyl groups during coal spontaneous combustion. Can. J. Chem. 2016, 94, 494–500.
- Wang, D.M.; Xin, H.H.; Qi, X.Y.; Dou, G.L.; Zhong, X.X. Mechanism and relationships of elementary reactions in spontaneous combustion of coal: The coal oxidation kinetics theory and application. J. China Coal Soc. 2014, 39, 1667–1674. (In Chinese)
- Xi, Z.; Suo, L.; Xia, T. Characterization of elementary reactions inducing coal spontaneous combustion. Fuel 2024, 358, 130138.
- Wei, A.; Li, Z.; Pan, S.; Yang, Y. Experimental study on free radical reaction of coal initiated by ultraviolet light. J. China Univ. Min. Technol. 2007, 36, 582–585. (In Chinese)
- Marinov, V.N. Self-ignition and mechanisms of interaction of coal with oxygen at low temperatures. Fuel 1977, 56, 158–164.
- Clemens, A.H.; Matheson, T.W.; Rogers, D.E. Low temperature oxidation studies of dried New Zealand coals. Fuel 1991, 70, 215–221.
- Ren, W.X.; Guo, Q.; Zuo, B.Z.; Wang, Z.F.; Fang, S.Q. Characteristics and mechanism of prevention and control of coal spontaneous combustion with foam-gel. J. China Coal Soc. 2015, 40 (Suppl. S2), 401–406. (In Chinese)
- Zhao, J.; Zhang, X. Experimental study of mine grout injection plastic fire prevention materials flow properties. J. China Coal Soc. 2015, 40, 383–388. (In Chinese)
- Zhai, X.; Wu, S.; Wang, K.; Drebenstedt, C.; Zhao, J. Environment influences and extinguish technology of spontaneous combustion of coal gangue heap of Baijigou coal mine in China. Energy Procedia 2017, 136, 66–72.
- Wu, J.M.; Zhang, J.H.; Wu, Y.G. Detection on Spontaneous Combustion Area in Surface Mine and Study on Comprehensive Control Technology of Spontaneous Combustion Area. Coal Eng. 2012, 44, 53–56.
- Gao, G. The present state and prospects for nitrogen fire prevention and fire-elimination technology in coal mines of China. J. China Coal Soc. 1999, 24, 48–51. (In Chinese)
- Zhang, C.H.; Wang, J.; Zhang, Z.M. Liquid Carbon Dioxide Fire Extinguishing Equipments and Their Engendering Applications. Sci. Technol. Rev. 2013, 31, 44–48. (In Chinese)
- Liu, W.; Qin, Y.; Yang, X.; Wang, W.; Chen, Y. Early extinguishment of spontaneous combustion of coal underground by using dry-ice’s rapid sublimation: A case study of application. Fuel 2018, 217, 544–552.
- Chi, K.; Wang, J.; Ma, L.; Wang, J.; Zhou, C. Synergistic Inhibitory Effect of Free Radical Scavenger/Inorganic Salt Compound Inhibitor on Spontaneous Combustion of Coal. Combust. Sci. Technol. 2022, 194, 2146–2162.
- Lu, W.; Guo, B.; Qi, G.; Cheng, W.; Yang, W. Experimental study on the effect of preinhibition temperature on the spontaneous combustion of coal based on an MgCl2 solution. Fuel 2020, 265, 117032.
- Liu, W.Y.; Wen, H.; Xiao, Y.; Li, Y.H.; Yin, L.; Bai, G.Y. Inhibiting effects of layered double hydroxides containing the rare-earth element lanthanum on coal spontaneous combustion. Thermochim. Acta 2020, 687, 178573.
- Pan, R.; Ma, J.; Fu, D.; Li, C.; Jia, H.; Zheng, L. Experimental study on the new environmental protection chemical composite inhibitor for the inhibition of coal spontaneous combustion. J. Therm. Anal. Calorim. 2020, 139, 37–45.
- Huang, Z.; Liu, X.; Gao, Y.; Zhang, Y.; Li, Z.; Wang, H.; Shi, X. Experimental study on the compound system of proanthocyanidin and polyethylene glycol to prevent coal spontaneous combustion. Fuel 2019, 254, 115610.
- Zhong, X.; Qin, B.; Dou, G.; Xia, C.; Wang, F. A chelated calcium-procyanidine-attapulgite composite inhibitor for the suppression of coal oxidation. Fuel 2018, 217, 680–688.
- Qin, B.; Dou, G.; Wang, Y.; Xin, H.; Ma, L.; Wang, D. A superabsorbent hydrogel–ascorbic acid composite inhibitor for the suppression of coal oxidation. Fuel 2017, 190, 129–135.
- Wang, X.; Deng, H.; Deng, C.; Zhang, X.; Zhao, Q. Research on Selection of Coal Spontaneous Combustion Inhibitors and Spraying Process. China Saf. Sci. J. 2013, 23, 105–109. (In Chinese)
- Wang, D. A Novel Technology of Mine Fire Fighting-Three-phrase Foam. Saf. Coal Mines 2004, 35, 16–18. (In Chinese)
- Xie, Z.; Li, X.; Liu, M. Application of Three-phase Foam Technology for Spontaneous Combustion Prevention in Longdong Coal Mine. Procedia Eng. 2011, 26, 63–69.
- Ren, W.; Kang, Z.; Wang, D. Causes of Spontaneous Combustion of Coal and Its Prevention Technology in the Tunnel Fall of Ground of Extra-thick Coal Seam. Procedia Eng. 2011, 26, 717–724.
- Qin, B.; Lu, Y.; Li, Y.; Wang, D. Aqueous three-phase foam supported by fly ash for coal spontaneous combustion prevention and control. Adv. Powder Technol. 2014, 25, 1527–1533.
- Zhang, L.; Shu, S.; Bian, Y. Inhibition effect and mechanism of nano-aluminum hydroxide foam on coal spontaneous combustion. Thermochim. Acta 2022, 718, 179389.
- Zhou, C.; Tang, Y. A novel sodium carboxymethyl ellulose/aluminium citrate gel for extinguishing spontaneous fire in coal mines. Fire Mater. 2018, 42, 760–769.
- Huang, Z.; Sun, C.; Gao, Y.; Ji, Y.; Wang, H.; Zhang, Y.; Yang, R. RD of colloid components of composite material for fire prevention and extinguishing and an investigation of its performance. Process Saf. Environ. Prot. 2018, 113, 357–368.
- Ren, X.; Hu, X.; Xue, D.; Li, Y.; Shao, Z.; Dong, H.; Cheng, W.; Zhao, Y.; Xin, L.; Lu, W. Novel sodium silicate/polymer composite gels for the prevention of spontaneous combustion of coal. J. Hazard. Mater. 2019, 371, 643–654.
- Li, F. Research on Characteristics of the Newly Developed Thickening Gels for Prevention and Control of Coal Spontaneous Combustion. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2017. (In Chinese).
- Miller, M.J.; Khilar, K.; Fogler, H.S. Aging of foamed gel used for subsurface permeability reduction. J. Colloid Interface Sci. 1995, 175, 88–96.
- Qin, B.; Feng, L.; Jiang, W. Research progress on extinguishing foam of coal mine. Coal Sci. Technol. Mag. 2022, 43, 1–1226. (In Chinese)
- Xie, J.; Zhang, X.; Xu, S. Research Status and Development Trend of Foam Fire Prevention Materials. Coal Technol. 2023, 42, 129–132. (In Chinese)
- Zhao, L. Laboratory experimental researches on profile control by gelling foaming fluids. Oilfield Chem. 1990, 7, 347–350. (In Chinese)
- Zhao, R.; Yue, X.; Hou, J. Feasibility Study of Authigenic Gas Gelling/Foaming Fluid as Indepth Profiling Agent Injected in Single Slug. Oilfield Chem. 2005, 22, 362–365. (In Chinese)
- Tian, Z. Research on the Theory and Technology of Foamed Gel Using for Fire Prevention. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2009. (In Chinese).
- Qin, B.T.; Zhang, L.L.; Lu, Y. Experimental studies on preparation of multi-phase foamed gel for preventing spontaneous combustion of coal. J. Cent. South Univ. Sci. Technol. 2013, 44, 4652–4657. (In Chinese)
- Zheng, C. Study on Preparation and Performance of New Gel Foam for Preventing Coal Spontaneous Combustion. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2020. (In Chinese).
- Shi, L. Study on the Theory and Properties of Colloidal Foam for Controlling and Preventing Coal Spontaneous Combustion. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2019. (In Chinese).
- Zhong, D. Study on Formation Mechanism and Inhibitory Property of Gel Foam for Coal Mine Fire Prevention and Extinguishing. Ph.D. Thesis, Hunan University of Science and Technology, Xiangtan, China, 2019. (In Chinese).
- Ji, Y. Study on Mechanism of Antioxidant Foamed Gel for Preventing Spontaneous Combustion of Coal in Gob. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2022. (In Chinese).
- Cai, C. Study on Preparation and Characteristics of Highly Stable Foam Gel for Prevention and Control of Coal Spontaneous Combustion. Shandong Coal Sci. Technol. 2022, 40, 112–114120. (In Chinese)
- Xue, D.; Hu, X.; Cheng, W.; Wei, J.; Zhao, Y.; Shen, L. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications. Fuel 2020, 264, 116903.
- Chen, J.; Jia, B.; Fu, S.; Wen, Y.; Liang, Y.; Tian, F. Novel PFA-Based Inorganic Three-Phase Foam for Inhibiting Coal Spontaneous Combustion. ACS Omega 2023, 8, 24615–24623.
- Qin, B.T.; Zhang, L.L.; Lu, Y. Preparation and inhibition characteristic of multi-phase foamed gel for preventing spontaneous combustion of coal. Adv. Mater. Res. 2013, 634, 3678–3682.
- Han, C.; Nie, S.; Liu, Z.; Liu, S.; Zhang, H.; Li, J.; Zhang, H.; Wang, Z. A novel biomass sodium alginate gel foam to inhibit the spontaneous combustion of coal. Fuel 2022, 314, 122779.
This entry is offline, you can click here to edit this entry!