Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder impairing cognition and memory in the elderly. This disorder has a complex etiology, including senile plaque and neurofibrillary tangle formation, neuroinflammation, oxidative stress, and damaged neuroplasticity. The treatment options are limited, so alternative treatments such as herbal medicine could suppress symptoms while slowing cognitive decline.
- Alzheimer’s disease (AD)
- kaempferol
- quercetin
- flavonoids
- traditional Chinese medicine
- dementia
1. Introduction
Alzheimer’s disease (AD) is a debilitating neurodegenerative disorder characterized by cognitive decline and memory impairment. AD could affect 152 million individuals by 2050 [1]. The progression of AD is influenced by multiple factors, including the accumulation of beta-amyloid plaques (Aβ) and the formation of neurofibrillary tangles (NFTs). The aggregation of Aβ plaques exacerbates the disease by impairing neuronal function and triggering neuroinflammation [2][3][4][5]. Oxidative stress and the presence of neurofibrillary tangles (NFTs) also contribute to the aggregation of Aβ into senile plaques [6][7][8][9][10][11][12][13][14]. NFTs consist of hyperphosphorylated tau proteins that disrupt neuronal transport systems [15][16][17][18][19][20][21]. Neuroinflammation, in turn, exacerbates damage to neuronal integrity [22]. Symptoms of AD include memory loss, impaired learning, emotional changes, cognitive and speech deficits, shortened attention span, and impaired management of daily tasks [23][24][25].
Currently, the treatments available for AD are expensive and have minimal efficacy. Acetylcholinesterase inhibitors (AChEIs), including donepezil, and N-methyl-D-aspartate (NMDA) receptor antagonists, including memantine, are commonly prescribed for AD [26]. AChEIs inhibit the enzymatic degradation of ACh by inhibiting cholinesterase activity [27], while NMDA receptor antagonists limit calcium influx to prevent glutamate-induced cytotoxic cell death [28]. However, these drugs simply suppress symptoms and fail to halt disease progression [26], and only half of the population positively responds to these current treatments [29][30]. Herbal medicine boasts a well-documented history of safe and effective incorporation into traditional Asian diets [31][32]. Preclinical studies have demonstrated that these herbs can enhance cognitive and memory functions [33][34]. These herbs serve as dependable sources of phytochemicals, such as kaempferol and quercetin, that have limited side effects and could combat Alzheimer’s disease [35][36][37]. Specifically, these flavonoids have anti-inflammatory, neuroprotective, and anti-degenerative effects [33][38][39][40][41][42][43][44][45][46].
2. Hallmarks of Alzheimer’s Disease
Several features of AD, including Aβ plaque accumulation [47][48], tau hyperphosphorylation and neuroinflammation [49], and oxidative stress [50][51][52][53], have been identified as targets for drug development. Moreover, these deficits have been observed in studies with human patients [6][54][55][56][57][58][59][60][61][62][63]. This section will briefly explore the pathophysiology of AD, with a focus on the proposed molecular origins and outcomes of their aberrant activities.
While the origins are still debated, the literature greatly supports the roles of oxidative stress and neuroinflammation as critical drivers of neurodegeneration. Antioxidant deficits facilitate ROS production, driving oxidative stress via lipid peroxidation [57]. Consequently, mitochondrial energy production is impaired and pro-apoptotic signaling follows [57]. Glutamate-induced excitotoxicity could also facilitate oxidative stress [64][65][66][67]. Disrupted ROS clearance establishes the neuroinflammatory microglial and astrocytic hyperactivity [38][68][69][70][71][72] and favors neuronal signaling pathways that impair Aβ clearance [48][73][74]. Finally, proper mitochondrial function is required for Aβ clearance and can, in turn, maintain appropriate tau activity states [75].
Although normal Aβ levels can maintain regular neuronal function [76], failed Aβ clearance from the brain can expedite neurodegeneration by facilitating plaque accumulation and impairing neuronal communication [22][47][48][75][77][78][79]. Moreover, Aβ accumulation further promotes oxidative stress [80][81][82][83]. As Aβ plaques accumulate in the brain due to impaired clearance [84], overzealous astrocytic and microglial responses compound the neuroinflammatory environment by releasing pro-inflammatory factors, promoting neuronal apoptosis [6][49][85][86][87][88][89]. These findings were supported in postmortem tissue [6][54][55][56]. Finally, Aβ signaling significantly impairs LTP [90], facilitating neurodegeneration via low synaptic activity.
AD is one of the most common tauopathies [91]. Aβ plaque accumulation drives tau hyperphosphorylation [47][58][92][93][94][95][96][97][98][99][100], possibly by excess GSK-3β signaling [101]. Likewise, tau hyperphosphorylation also compounds Aβ toxicity [102][103], which has been supported by PET imaging in humans with memory impairment and cognitive decline [60]. These studies demonstrate that Aβ toxicity is necessary for tau hyperphosphorylation [59][60]. Specifically, accumulating Aβ binds to NMDAR, generating excess calcium levels to activate calpain-mediated microtubule-associated protein cleavage [65][104][105]. These events impair mitochondrial function, invoking pro-apoptotic signaling [65][106]. Tau hyperphosphorylation dismantles axonal microtubules to degenerate the axon [15][107][108][109][110], impairing synaptic plasticity [102][103][111][112]. Hyperphosphorylated tau spreads throughout the hippocampus in AD models [113], and uptake may be mediated by clathrin-induced endocytosis [114]. Risk factors such as sleep apnea may potentiate the spread of tau in this manner [115]. Ultimately, these events result in neuronal death and compromise neuroplasticity, thereby driving neuroinflammation and impairing cognitive function.
3. Anti-AD Mechanisms of Quercetin and Kaempferol
Given the limited therapeutics available to AD patients, it is essential to explore alternative treatments, such as plant-derived phytochemicals. Flavonoids, including kaempferol and quercetin, belong to the class of polyphenols commonly found in various herbs. Notably, kaempferol and quercetin possess lipophilic properties [50], which facilitate their easy entry into cells. These phytochemicals are abundant, with an average daily consumption of approximately 23 mg of flavonoids in a typical diet [116][117]. Kaempferol and quercetin produce several beneficial properties, including anti-inflammatory, antioxidant, anti-Aβ, anti-tau, and pro-neuroplastic effects [37][38][39][57][74][118][119][120][121][122][123][124][125][126][127][128]. Moreover, they have demonstrated cognitive and memory-enhancing effects in animal studies [37].
3.1. Quercetin
Quercetin, the most prevalent flavonoid, is found in several traditional medicinal herbs and is commonly found in fruits and vegetables, including berries, onions, and leeks [118][129][130][131][132][133][134][135][136][137][138][139]. Quercetin intake constitutes approximately 60–75% of total flavonols [140][141], and 25 mg of quercetin is found in the average diet [38]. Quercetin is commonly investigated for its potential anti-neurodegenerative efficacy and is considered safe [51][142]. Quercetin is a 15-carbon flavonoid with two benzene rings connected via a 3-carbon shape (Figure 1) [38][130][143].
Quercetin produces anti-inflammatory effects via multiple signaling pathways, including Nrf2, paraoxonase-2 (PON2), JNK, PKC, and NF-kB [51][118][128][144][145][146][147]. Quercetin dose-dependently protected HT22 hippocampal neurons from glutamate-induced apoptosis by limiting ROS production, impairing the calpain-mediated cleavage of cytoskeletal proteins, and preserving mitochondrial membrane potential [65]. Quercetin also inhibits NO release by inhibiting iNOS activity [33][38][148], which could reduce excess glutamate signaling and minimize the risk of glutamate-induced cytotoxicity in hippocampal neurons in a similar fashion to kaempferol and its derivatives [149]. Moreover, quercetin inhibits COX-2 and TLR4 activity to reduce inflammatory responses [6][39][148]. Interestingly, quercetin may have epigenetic mechanisms by inhibiting lysine acetyltransferase (KAT) activity [150][151] and increasing lysine deacetylase (KDAC) activity [152], suggesting that the flavonoid can bidirectionally regulate autophagy [153], neuroinflammation, and apoptosis [154]. Quercetin also inhibits acetylcholinesterase (AChE) [155], which can enhance alertness and cognitive function in AD patients.
The anti-Aβ effects of quercetin are well studied in AD and related models and have yielded promising therapeutic properties. The hydrophobic groups of quercetin can inhibit the formation of Aβ fibrils [120][121][122][123][156]. Chronic quercetin treatment also slowed Aβ aggregation by potentiating AMPK signaling and inhibiting mitochondrial ROS production, leading to improved memory and object recognition in APPswe/PS1dE9 [80][157]. Quercetin treatment also inhibits the BACE1-mediated cleavage of APP into Aβ by inhibiting NF-kB [74]. Consequently, mitochondrial membrane permeability is restored, and cellular survival is favored over oxidative stress [158]. This anti-neurodegenerative effect could be due to the free radical-quenching structure of the catechol group, reducing neuroinflammation, lipid peroxidation, mitochondrial stress, and DNA damage [38][51]. Elevated SOD, GPx, and Na+-K+ ATPase activity could also be due to quercetin’s anti-Aβ effects [44][78].
In many studies, quercetin and its derivatives reduced tau hyperphosphorylation [23][58][132][159]. In rodent HT22 hippocampal neurons, chronic quercetin treatment inhibited tau phosphorylation at four sites by reducing p-Cdk5 levels, limiting calpain activity, and dramatically reducing Ca2+ influx [58]. In 3xTgAD mice, chronic quercetin inhibited Aβ pathology, reduced NFT levels, and prevented astrocytic and microglial hyperactivity in the amygdala and hippocampus [132][160], showing that the anti-Aβ and anti-tau mechanisms of quercetin depend on its anti-inflammatory effects. Consequently, these mice demonstrated improved learning and memory and decreased anxiety [132], while combined exercise and quercetin treatment robustly improved spatial memory in AD rodents [161]. Studies also found that quercetin enhanced cell viability and morphology by reducing MDA and ROS levels and increasing antioxidant SOD and GSH activity [159][162], limiting NF-κB signaling, restoring mitochondrial membrane potential to baseline, inhibiting tau hyperphosphorylation, and regulating Akt/PI3K/GSK-3β signaling pathway [159][163]. Taken together, these data show that quercetin has a multimodal mechanism of action in treating AD. Of note, the anti-tau and consequent pro-neuroplastic effect of quercetin is further explored in Section 5, but the primary anti-inflammatory, anti-Aβ, ant-tau, and pro-neuroplastic effects of this flavonoid are all dependent on each other.
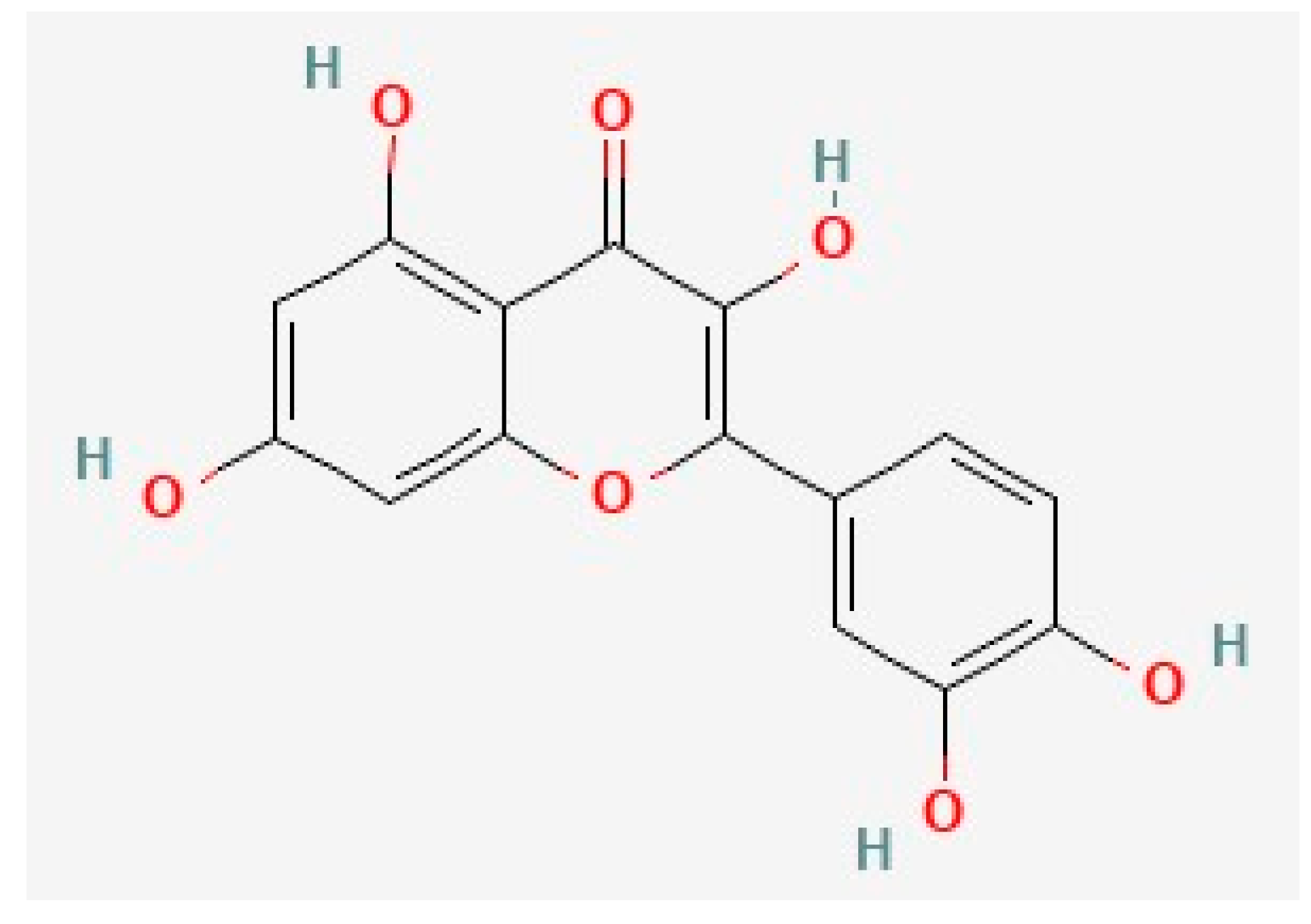
Figure 1. The chemical structure of quercetin, deduced from PubChem [164].
3.2. Kaempferol
Kaempferol is a common 15-carbon polyphenol (Figure 2) that shares significant structural similarity with quercetin. It is one of the most common flavonoids and is found in a variety of common foods, including fruits and vegetables [129][130][165][166][167][168][169][170]. Multiple preclinical and clinical studies have supported the anti-AD activity of kaempferol [57][149][171][172][173][174]. Kaempferol has pro-neuroplastic, anti-Aβ, anti-tau, anti-inflammatory, and antioxidant properties [29][44][57][171][175][176][177][178][179]. Notably, kaempferol also inhibits AChE like quercetin [180], but this mechanism is beyond the scope of this research.
Like quercetin, kaempferol and its metabolites reduce inflammation and have potent antioxidant properties [181][182][183][184]. Kaempferol also directly modulates neuroinflammation by impairing microglial TLR4 and NF-kB signaling and inhibiting the release of NO, iNOS, PGE2, IL-1β, TNF-α, and IFN-γ [167][185]. Kaempferol also reversed BBB damage [36][186][187]. Kaempferol can also modulate neuroinflammation by regulating epigenetic factors such as SIRT1, a subtype of KDAC [188][189][190]. Kaempferol also prevents cytotoxic damage to PC12 neurons by upregulating SIRT [191]. Other immune factors modulated by kaempferol include COX-2, lipoxygenases, prostacyclin, and leukotrienes [148][187][192][193][194]. Finally, kaempferol may reduce neuroinflammation via Nrf-2 signaling [185].
Like quercetin, kaempferol and its derivatives reverse Aβ-induced damage [29][120][122][124][125][149][195]. Kaempferol-3-O-rhamnoside (K-3-Rh), a kaempferol derivative, limited total Aβ burden and toxicity by disrupting β-sheet formation and impairing Aβ plaque formation in human SH-SY5Y cells [195][196]. However, kaempferol antagonized fibrilization with lower potency compared to quercetin and morin [120][122]. In rodent neuroblastoma cells, kaempferol 3-O-(6″-acetyl)-β-glucopyranoside (KAG) robustly inhibited Aβ-mediated cytotoxic cell death and ROS generation [149]. KAG reversed Aβ-mediated oxidative stress and increased cell survival by regulating caspase-3, Bax, and Bcl-2 signaling [44][64][149][197][198][199][200]. Kaempferol dose-dependently and sex-dependently limited Aβ-induced mitochondrial toxicity in neurons, improving rodent memory in the Y-maze test [57][134][201]. Of note, studies regarding kaempferol’s direct influence on tau are limited; thus, more research is necessary. However, due to its similar phenolic structure to quercetin [165][166], the researchers hypothesize that kaempferol could also reduce tau hyperphosphorylation.
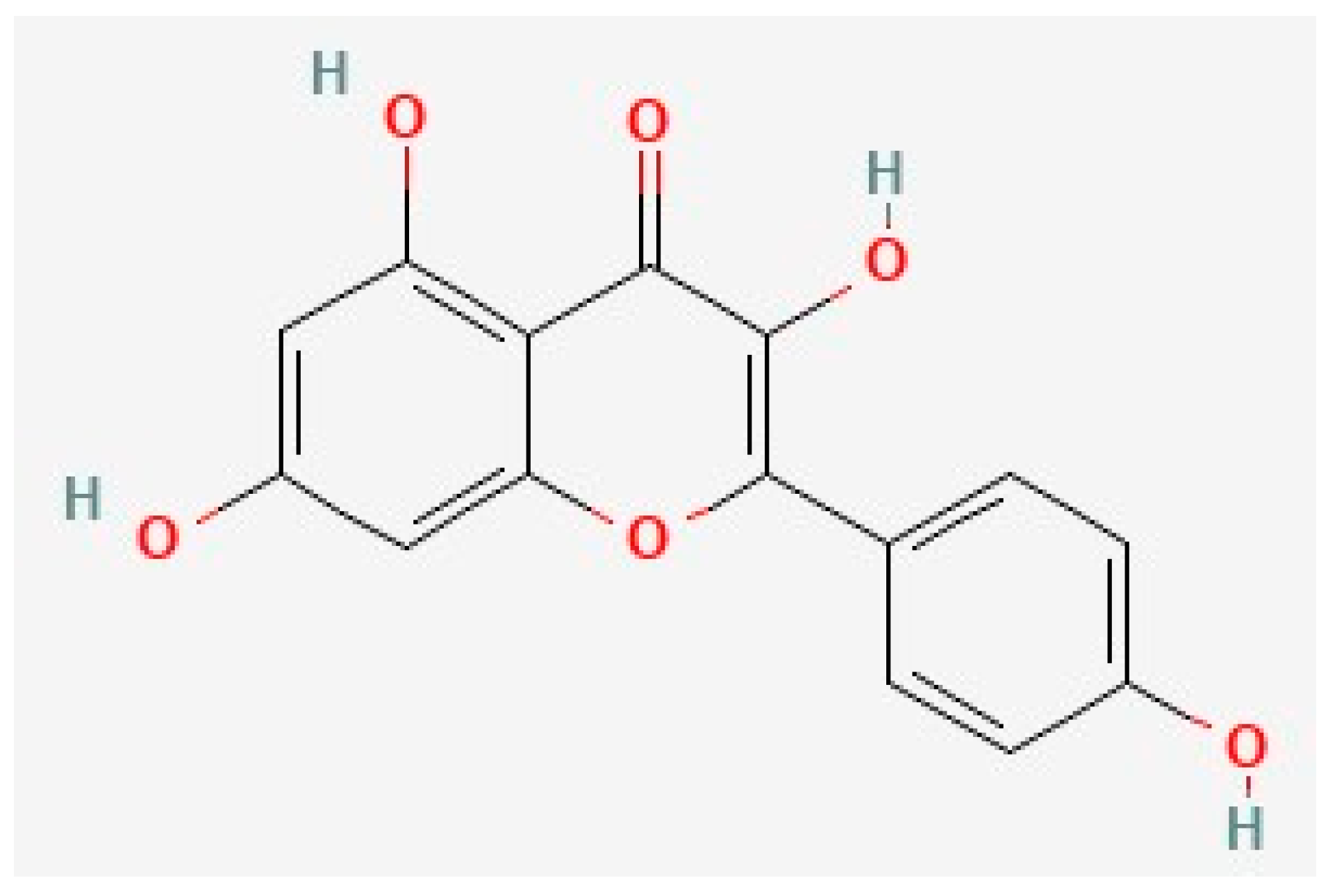
Figure 2. The chemical structure of kaempferol, deduced from PubChem [202].
4. Kaempferol, Quercetin, and Neuroplasticity
The aberrant brain changes described in Section 3 can impair memory and cognitive function by creating deficits in neuroplasticity. Thus, future AD treatments should also be designed to directly target signaling pathways that can counteract the etiologies of AD. Specifically, the researchers identified the PI3K/AKT signaling pathway as a critical candidate to counteract neurodegeneration. Several studies have suggested that flavonoids can alleviate learning and memory deficits by targeting this signaling pathway [29][203][204][205]. However, other pathways, including the MAPK-ERK1/2 cascade [206], have also been proposed and outlined in a recent review [207].
4.1. Neuroplasticity Deficits in AD
An ideal AD treatment should enhance the expression of plasticity-related genes such as BDNF, a neurotrophic factor that regulates neuronal plasticity and survival [208][209][210][211][212][213][214]. BDNF signaling begins with its binding to the receptor, Trkβ, activating signaling via a variety of pathways like PI3K/AKT [211][215]. Then, AKT or protein kinase B (PKB) [216] can activate the CREB-mediated transcription of BDNF [217][218]. Since Trkβ receptors mediate the pro-neuroplastic effects of BDNF [219], AD drugs must produce a direct or indirect effect on the receptor.
BDNF deficits increase the risk of AD development [220], and BDNF dysfunction due to impaired PI3K and AKT signaling can expedite neurodegeneration [7][41][221][222][223]. The PI3K/AKT signaling pathway has multiple functions, including regulating synaptic plasticity, glucose processing, cell cycle progression, cell proliferation, survival, and apoptosis [167][175][224][225][226]. Moreover, this pathway may protect neurons from Aβ toxicity [224], oxidative stress [227], and neuroinflammation [217]. GSK-3β is downstream of PI3K/AKT, and Aβ can specifically lead to its hyperactivity [7]. However, BDNF and CREB are also vulnerable to Aβ signaling [228] as CREB is regulated by the PI3K/AKT/GSK-3β pathway [211][212][215][229][230][231].
Thus, the Aβ-mediated signaling cascade that degenerates the neuron is as follows (Figure 3A): Aβ binding to NMDAR inhibits PI3K/AKT signaling by activating GSK-3β-mediated tau hyperphosphorylation and CREB downregulation [93][97][210][211][223][229][230][232][233][234][235][236][237]. Consequently, the impaired CREB-mediated transcription of BDNF genes decreases plasticity and facilitates plaque accumulation, as demonstrated in postmortem tissues from humans and human neuronal cells [209][210][229][232]. The absence of protective BDNF and PI3K/AKT activity facilitates the caspase-mediated pro-apoptotic signaling cascade [6][224], degenerating the neuronal circuitry, while tau dissociation from microtubules breaks down the neuronal cytoskeleton [7][233][238][239][240][241][242].
4.2. Quercetin and Kaempferol Resolve AD-Related Plasticity Deficits
The multimodal mechanisms of kaempferol and quercetin collectively slow neurodegeneration by combating the impairments that are illustrated in Figure 3A. Specifically, the restoration of proper PI3K/AKT signaling will greatly improve synaptic plasticity deficits in AD [7]. While quercetin’s interaction with each component of this signaling pathway has already been documented [7], kaempferol’s mechanisms are still unclear. However, since kaempferol’s structure is similar to that of quercetin [165], the researchers propose that kaempferol has a nearly identical mechanism with respect to the signaling pathway in this subsection. Finally, the researchers will propose the potential outcomes of these molecular interactions.
Molecular docking studies suggested that quercetin can bind PI3K, AKT, and GSK3β [213][243][244][245][246][247][248][249]. Specifically, quercetin can bind to PI3K [245], consequently activating AKT signaling [249], or quercetin can directly bind to AKT [246]. In preclinical studies, quercetin reduced GSK-3β activity, which decreased tau hyperphosphorylation and reduced pro-apoptotic signaling [7][38][159]. Quercetin treatment in rodents also increased BDNF, Trkβ, PI3K, and AKT expression [250][251]. Consequently, quercetin enhanced neurite outgrowth in hippocampal neurons [36] and ameliorated the stress-induced downregulation of CREB and BDNF [40], suggesting that quercetin could potently replenish neuroplasticity in the AD brain. Moreover, quercetin inhibited Aβ by restoring Trkβ signaling and CREB-mediated BDNF transcription, increasing the viability of SH-SY5Y cells [252]. Finally, quercetin’s dual pro-neuroplastic and anti-inflammatory effects may also be related to the quercetin-mediated downregulation of BACE1 expression via the inhibition of NF-kB [248][253][254][255]. Taken together, these data suggest that quercetin antagonizes Aβ-induced GSK-3β signaling relative to tau by activating the PI3K/AKT pathway and directly inhibiting GSK-3β [7][225][241][244][245][247]. Consequently, proper BDNF levels can be restored to replenish neuronal plasticity in the AD brain. Similar chemicals, such as epigallocatechin-3-gallate (EGCG), attenuated tau hyperphosphorylation in a similar mechanism [23][256][257][258]. Thus, quercetin clearly has dual neuroprotective and pro-neuroplastic mechanisms in cells [33][65][252], and the clinical outcomes of quercetin’s pro-neuroplastic mechanisms were supported by its memory and cognition-boosting effects in rodent models of AD and Parkinson’s disease [23][38][44][259][260][261][262][263][264].
First, kaempferol improved hippocampal plasticity following traumatic brain injury in young rodents [265] and improved memory in rodents [29][57] and Drosophila [173]. Moreover, kaempferol dose-dependently maintained cell viability following Aβ treatment in multiple studies [29][149][195][266]. This could be due to kaempferol’s inhibition of BACE1-mediated Aβ synthesis [253][254] or the activation of the PI3K/AKT signaling pathway, enhancing CREB-mediated BDNF transcription [175][211][267]. Although one molecular docking study suggested that kaempferol may have minimal affinity for GSK-3β [243], kaempferol likely inhibits GSK-3β indirectly by first binding and activating PI3K [245] or AKT [175][185][246]. Via this mechanism, kaempferol prevents tau hyperphosphorylation, protecting neuronal morphology and function [47][268][269][270][271]. Then, AKT can activate CREB-mediated BDNF transcription [217]. Supporting this pro-neuroplastic mechanism, kaempferol and its metabolite, kaempferide, produced similar effects that resulted in Trkβ signaling [171][210] and enhanced BDNF expression in Aβ-treated mice [250]. Taken together, these data suggest that kaempferol enhances neuroplasticity to reverse Aβ damage by activating the PI3K/AKT cascade, which potentiates CREB-mediated BDNF transcription. However, kaempferol produces the opposite effect on this signaling pathway in microglial cells [167] and cancer cells [272]. Thus, kaempferol’s effects on the PI3K/AKT signaling cascade are dynamic and depend on cell lineage.
Despite the lack of literature demonstrating a direct modulation of tau by kaempferol, there is plenty of evidence to support the possibility that kaempferol inhibits tau hyperphosphorylation via the PI3K/AKT pathway and by antagonizing Aβ-mediated GSK-3β signaling [29][149][195].
These data suggest a clear anti-AD mechanism of quercetin and kaempferol, as outlined in Figure 3B. First, quercetin and kaempferol could enter the cell cytoplasm due to their lipophilic polyphenolic structure. Quercetin and kaempferol scavenge ROS and activate PI3K/AKT signaling to inhibit GSK-3β. Specifically, they can bind directly to PI3K or AKT to activate protective signaling, inhibiting GSK-3β and preventing tau hyperphosphorylation. This signaling cascade reduces the formation of NFTs in the AD brain. GSK-3β inhibition can also antagonize Aβ-NMDAR interactions. Thus, downstream pro-apoptotic signaling mediators are also inhibited by quercetin and kaempferol treatment. Due to reduced NFT and amyloid plaque formation, microglial hyperactivity decreases in the absence of the burden of clearance. Thus, progressive neuroinflammatory signaling is slowed, allowing surrounding neuronal synapses to survive. After chronic quercetin treatment, progressive elevations in BDNF release rebuild damaged synapses by favoring neurotrophic signaling over cytotoxic Aβ signaling, improving memory and cognition. Of note, molecular docking studies have not supported the possibility that kaempferol and quercetin can directly bind to tau protein, supporting their indirect inhibitory mechanism via GSK-3β inhibition. Taken together, kaempferol and quercetin share multiple mechanisms that slow AD progression by first limiting ROS activity, NFT aggregation, and Aβ-mediated toxic signaling, slowing neurodegeneration.
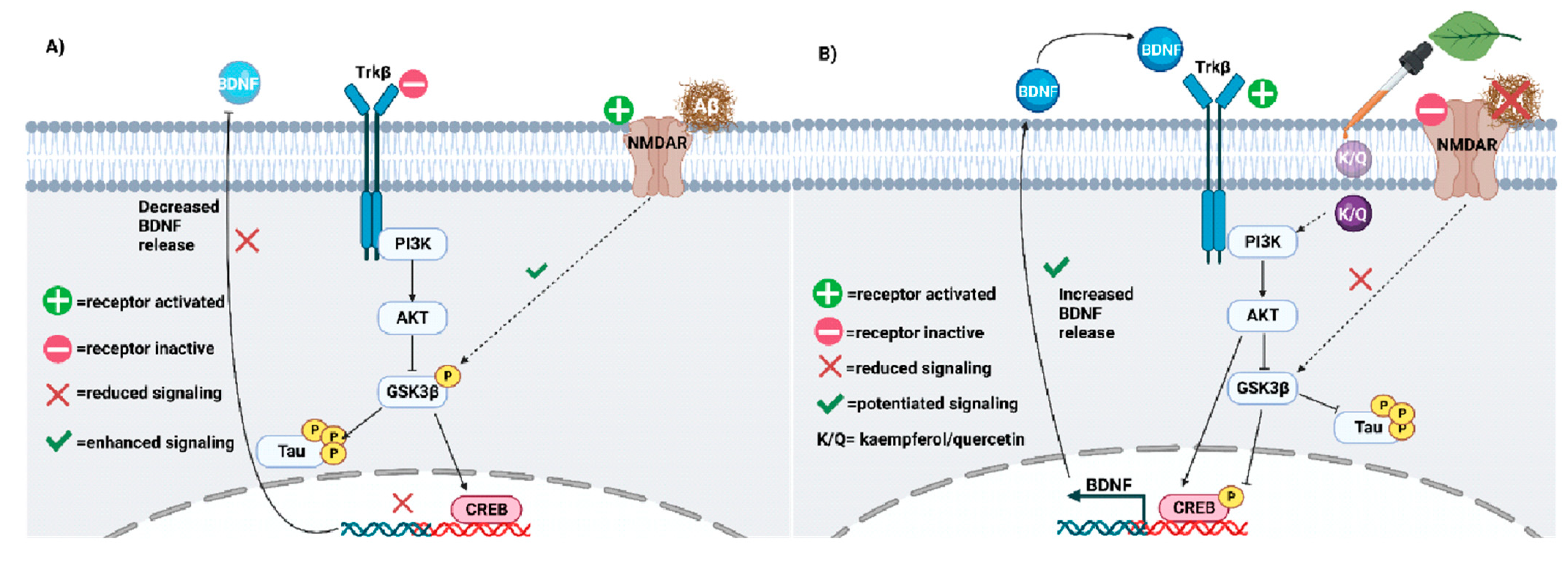
Figure 3. (A) Neuroplasticity deficits accelerate AD progression and must be treated. Impaired PI3K-AKT signaling facilitates GSK3β-mediated phosphorylation of tau. Aβ may potentiate tau hyperphosphorylation via GSK3β. (B) Kaempferol and quercetin (K/Q) invoke the PI3K/AKT pathway to antagonize Aβ and reduce tau hyperphosphorylation in neurons. As a result, neuroplasticity is increased in the AD brain [273].
5. Quercetin and Kaempferol in Common Herbs
Although data on the co-treatment of quercetin and kaempferol are still somewhat limited, the abundance of both compounds in several common herbs requires the investigation of the synergistic effects of both flavonoids, in addition to their interactions with other herbal phytochemicals. Flavonoid-rich herbs are commonly employed in traditional Chinese medicine (TCM), in which an emphasis is placed on the utility of natural treatments. Moreover, these herbs are generally safe for consumption [224]. Kaempferol is the second most common flavonoid in traditional medicinal herbs, following quercetin [225][274]. Other reviews have assessed the efficacy and safety of natural medicine in the treatment of neurodegenerative diseases [7][224], highlighting the potential medicinal properties of herbs in treating AD. Flavonoids are commonly found in herbs such as Schima wallichii Korth, Maesa membranacea, Ginkgo biloba, and many more [175][225][268]. These phytochemicals could work synergistically with each other and with other herbal components to invoke anti-AD effects. Thus, the researchers explore common herbal sources of kaempferol and quercetin, describe the anti-AD mechanisms of herbs, and propose a design for a future AD treatment based on the current evidence of these effects.
Ginkgo biloba is a quercetin- and kaempferol-rich herb proposed to treat AD [275]. G. biloba improves memory and cognition by inhibiting ROS, facilitating hippocampal neuron proliferation, halting Aβ plaque accumulation, and reducing tau hyperphosphorylation [47][276][277][278]. Moreover, this effect is associated with reduced GSK-3β activity and the increased expression of PSD-95 and synapsin-1 [47]. As seen with kaempferol and quercetin alone, G. biloba potentiates PI3K/AKT relative to CREB signaling to promote neuroplasticity [277][279][280][281][282][283]. Hippophae rhamnoides extracts are also rich in quercetin and kaempferol, and they enhanced neuronal differentiation and neurite outgrowth via PI3K/AKT and ERK signaling [284][285]. However, clinical trials have revealed the inconsistent efficacy of G. biloba on cognition and other AD-related parameters [286]. Camellia sinensis is another kaempferol- and quercetin-rich herb commonly grown to produce black and green tea [287][288]. C. sinensis extracts improved spatial memory and reduced hippocampal Aβ fibrillization in AD rodents and had greater antioxidant effects compared to other herbs [288][289]. Kaempferol and its derivatives are found in the leaves of Maesa membranacea, Schima wallichii Korth, Carthamus tinctorius, Panax ginseng, and several other herbs [175][188][225][268]. S. wallichii was neuroprotective due to the promotion of hippocampal and cortical AKT signaling [175], and M. membranacea could protect H202-treated SH-SY5Y cells [225] and hippocampal tissue [290] via the same pathway due to their kaempferol abundance. C. tinctorus is rich in kaempferol, produces a similar effect, and invokes protective AMPK signaling [188]. Finally, recent studies also suggested that other herbs such as Morenga oleifera, Cuscuta chinensis, Allium cepa, Litchi chinensis, Prakia roxburghii, Radix astragali, Acoritatan Fagopyrum tataricum, Carthami flos, Punica granatum, and Cyperi rhizoma [246][248][291][292][293][294][295][296] may also be great sources of kaempferol and/or quercetin and produce anti-AD effects.
Polyherbal cocktails, such as Chaihu shugan san (CSS) and Huangqi Sijunzi (HQSJDZ), could treat AD and its risk factors. CSS is abundant in kaempferol and quercetin and contains herbs such as Glycyrrhiza uralensis, Cyperus rotundus, and Buplerum falcatum [245]. Specifically, the antidepressant effect of CSS is mediated by increased PI3K/AKT/BDNF signaling and decreased GSK-3β and IL-2 activity [245], suggesting that polyherbal cocktails may be protected from AD development. HQSJDZ, rich in kaempferol and quercetin, had cholinergic, anti-inflammatory, and anti-GSK-3β effects [268][297]. Moreover, a cocktail of C. sinensis, Hypericum perforatum, and Bacopa monnieri produced robust antioxidant effects compared to single-herb treatment [288]. These data suggest that polyherbal treatment may be superior to single-herb therapy.
Due to the well-documented effects of quercetin and kaempferol on Aβ, GSK-3β, PI3K/AKT, and multiple pro-inflammatory molecules, it is possible that both phytochemicals, given their abundance, contribute vastly to the anti-AD effects of several herbs. Such herbs include Ginkgo biloba, Camellia sinensis, Glycyrrhiza uralensis, Cyperus rotundus, and Buplerum falcatum. According to the practice of TCM, it is possible that a multi-herb cocktail containing varying amounts of these herbs could alleviate AD symptoms, as seen with current medications, but it may also halt progression relative to a unique multi-modal mechanism. Multiple studies have suggested that the synergistic effects of polyherbal treatments produce greater anti-AD efficacy compared to single-herb treatment [245][268][288]. Thus, the research and development of future AD drugs should consider the applications of these common herbs in future drug cocktails. On the other hand, since clinical trials featuring Ginkgo biloba extracts have demonstrated controversial results on the progression of AD [286], single-herb treatments may be insufficient to treat AD.
This entry is adapted from the peer-reviewed paper 10.3390/biology12111453
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789.
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479.
- Riche, K.; Lenard, N.R. Quercetin’s Effects on Glutamate Cytotoxicity. Molecules 2022, 27, 7620.
- Yu, H.; Wu, J. Amyloid-β: A double agent in Alzheimer’s disease? Biomed. Pharmacother. 2021, 139, 111575.
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89.
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016, 136, 457–474.
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimer's Res. Ther. 2014, 6, 35.
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629.
- Bhaskar, K.; Miller, M.; Chludzinski, A.; Herrup, K.; Zagorski, M.; Lamb, B.T. The PI3K-Akt-mTOR pathway regulates Abeta oligomer induced neuronal cell cycle events. Mol. Neurodegener. 2009, 4, 14.
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356.
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel'nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3085756.
- Uddin, M.S.; Kabir, M.T. Oxidative Stress in Alzheimer’s Disease: Molecular Hallmarks of Underlying Vulnerability. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Ashraf, G., Alexiou, A., Eds.; Springer: Singapore, 2019.
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.T.; Witting, P.K. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553.
- Gao, W.; Wang, W.; Peng, Y.; Deng, Z. Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up-regulation of AKT/β-catenin cascade. Metab. Brain Dis. 2019, 34, 485–494.
- Alquezar, C.; Arya, S.; Kao, A.W. Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation. Front. Neurol. 2021, 11, 595532.
- Merino-Serrais, P.; Benavides-Piccione, R.; Blazquez-Llorca, L.; Kastanauskaite, A.; Rábano, A.; Avila, J.; DeFelipe, J. The influence of phospho-tau on dendritic spines of cortical pyramidal neurons in patients with Alzheimer’s disease. Brain 2013, 136, 1913–1928.
- Spittaels, K.; Haute, C.V.D.; Van Dorpe, J.; Geerts, H.; Mercken, M.; Bruynseels, K.; Lasrado, R.; Vandezande, K.; Laenen, I.; Boon, T.; et al. Glycogen synthase kinase-3β phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 2000, 275, 41340–41349.
- Tatebayashi, Y.; Haque, N.; Tung, Y.-C.; Iqbal, K.; Grundke-Iqbal, I. Role of tau phosphorylation by glycogen synthase kinase-3β in the regulation of organelle transport. J. Cell Sci. 2004, 117, 1653–1663.
- Jaworski, T.; Kügler, S.; van Leuven, F. Modeling of tau-mediated synaptic and neuronal degeneration in Alzheimer’s disease. Int. J. Alzheimer's Dis. 2010, 2010, 573138.
- Hoover, B.R.; Reed, M.N.; Su, J.; Penrod, R.D.; Kotilinek, L.A.; Grant, M.K.; Pitstick, R.; Carlson, G.A.; Lanier, L.M.; Yuan, L.-L.; et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 2010, 68, 1067–1081.
- Thies, E.; Mandelkow, E.-M. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J. Neurosci. 2007, 27, 2896–2907.
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2013, 79, 1–12.
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145.
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152.
- Joe, E.; Ringman, J.M. Cognitive symptoms of Alzheimer’s disease: Clinical management and prevention. BMJ 2019, 367, l6217.
- Casey, D.A.; Antimisiaris, D.; O’Brien, J. Drugs for Alzheimer’s disease: Are they effective? Pharm. Ther. 2010, 35, 208–211.
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectrums 2005, 10 (Suppl. S18), 6–9.
- Wang, R.; Reddy, P.H. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J. Alzheimer's Dis. 2017, 57, 1041–1048.
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018, 13, 1827–1832.
- Farlow, M.R.; Miller, M.L.; Pejovic, V. Treatment options in Alzheimer’s disease: Maximizing benefit, managing expectations. Dement. Geriatr. Cogn. Disord. 2008, 25, 408–422.
- Tian, J.; Shi, J.; Zhang, X.; Wang, Y. Herbal therapy: A new pathway for the treatment of Alzheimer’s disease. Alzheimer's Res. Ther. 2010, 2, 30.
- Lee, J.; Jin, C.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; Kwon, S. Herbal medicine treatment for Alzheimer disease: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e21745.
- Chen, M.M.; Yin, Z.Q.; Zhang, L.Y.; Liao, H. Quercetin promotes neurite growth through enhancing intracellular cAMP level and GAP-43 expression. Chin. J. Nat. Med. 2015, 13, 667–672.
- Zhang, X.W.; Chen, J.Y.; Ouyang, D.; Lu, J.H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493.
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921.
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776.
- Sreenivasmurthy, S.G.; Liu, J.Y.; Song, J.X.; Yang, C.B.; Malampati, S.; Wang, Z.Y.; Huang, Y.Y.; Li, M. Neurogenic traditional Chinese medicine as a promising strategy for the treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 272.
- Khan, A.; Ali, T.; Rehman, S.U.; Khan, M.S.; Alam, S.I.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M.O. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383.
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795.
- Ma, Z.X.; Zhang, R.Y.; Rui, W.J.; Wang, Z.Q.; Feng, X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/ BDNF signaling pathway. Behav. Brain Res. 2021, 406, 113245.
- Das, D.; Biswal, S.; Barhwal, K.K.; Chaurasia, O.P.; Hota, S.K. Kaempferol Inhibits Extra-synaptic NMDAR-Mediated Downregulation of TRkβ in Rat Hippocampus During Hypoxia. Neuroscience 2018, 392, 77–91.
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37.
- Yu, L.; Chen, C.; Wang, L.F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.R. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-κB and STAT3 in transient focal stroke. PLoS ONE 2013, 8, e55839.
- Azam, S.; Jakaria, M.; Kim, I.S.; Kim, J.; Haque, M.E.; Choi, D.K. Regulation of Toll-Like Receptor (TLR) Signaling Pathway by Polyphenols in the Treatment of Age-Linked Neurodegenerative Diseases: Focus on TLR4 Signaling. Front. Immunol. 2019, 10, 1000.
- Hou, Y.; Aboukhatwa, M.A.; Lei, D.L.; Manaye, K.; Khan, I.; Luo, Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology 2010, 58, 911–920.
- Kim, J.H.; Kim, H.Y.; Cho, E.J. Protective effects of kaempferol, quercetin, and its glycosides on amyloid beta-induced neurotoxicity in C6 glial cell. J. Appl. Biol. Chem. 2019, 62, 327–332.
- Zeng, K.; Li, M.; Hu, J.; Mahaman, Y.A.R.; Bao, J.; Huang, F.; Xia, Y.; Liu, X.; Wang, Q.; Wang, J.Z.; et al. Ginkgo biloba Extract EGb761 Attenuates Hyperhomocysteinemia-induced AD Like Tau Hyperphosphorylation and Cognitive Impairment in Rats. Curr. Alzheimer Res. 2018, 15, 89–99.
- Ly, P.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235.
- Latta, C.H.; Brothers, H.M.; Wilcock, D.M. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience 2015, 302, 103–111.
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 2013, 236, 136–148.
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119.
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74.
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247.
- Beach, T.G.; Walker, R.; McGeer, E.G. Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia 1989, 2, 420–436.
- Delacourte, A. General and dramatic glial reaction in Alzheimer brains. Neurology 1990, 40, 33.
- Arends, Y.M.; Duyckaerts, C.; Rozemuller, J.M.; Eikelenboom, P.; Hauw, J.J. Microglia, amyloid and dementia in alzheimer disease. A correlative study. Neurobiol. Aging 2000, 21, 39–47.
- Kim, J.K.; Choi, S.J.; Cho, H.Y.; Hwang, H.J.; Kim, Y.J.; Lim, S.T.; Kim, C.J.; Kim, H.K.; Peterson, S.; Shin, D.H. Protective effects of kaempferol (3,4’,5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. Biosci. Biotechnol. Biochem. 2010, 74, 397–401.
- Shen, X.Y.; Luo, T.; Li, S.; Ting, O.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin inhibits okadaic acid-induced tau protein hyperphosphorylation through the Ca2+-calpain-p25-CDK5 pathway in HT22 cells. Int. J. Mol. Med. 2018, 41, 1138–1146.
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193.
- Sperling, R.A.; Mormino, E.C.; Schultz, A.P.; Betensky, R.A.; Papp, K.V.; Amariglio, R.E.; Hanseeuw, B.J.; Buckley, R.; Chhatwal, J.; Hedden, T.; et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann. Neurol. 2019, 85, 181–193.
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011, 10, 829–843.
- Sakakibara, R.; Kawai, T. Cerebrospinal fluid oxidative stress markers in Alzheimer’s disease. Neurol. Clin. Neurosci. 2020, 8, 232–240.
- Willette, A.A.; Li, T.; Willette, S.A.; Larsen, B.A.; Pollpeter, A.; Klinedinst, B.S.; Moody, S.; Barnett, N.; Parvin, M.; Pappas, C.; et al. Oxidative stress biomarkers and longitudinal changes in human brain imaging across the Alzheimer’s disease continuum. Alzheimer’s Dement. 2022, 18, e068364.
- Kim, H.G.; Ju, M.S.; Shim, J.S.; Kim, M.C.; Lee, S.H.; Huh, Y.; Kim, S.Y.; Oh, M.S. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson’s disease models. Br. J. Nutr. 2010, 104, 8–16.
- Song, K.S.; Yang, E.J.; Kim, G.S.; Kim, J.A. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Mag. 2013, 9, 302–308.
- Greenwood, S.M.; Connolly, C.N. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology 2007, 53, 891–898.
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–130.
- Simpson, D.S.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743.
- Di Filippo, M.; Sarchielli, P.; Picconi, B.; Calabresi, P. Neuroinflammation and synaptic plasticity: Theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 2008, 29, 402–412.
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396.
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216.
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003.
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer's disease. Redox Biol. 2018, 14, 450–464.
- Paris, D.; Mathura, V.; Ait-Ghezala, G.; Beaulieu-Abdelahad, D.; Patel, N.; Bachmeier, C.; Mullan, M. Flavonoids lower Alzheimer’s Aβ production via an NFκB dependent mechanism. Bioinformation 2011, 6, 229–236.
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412.
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522.
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. NeuroMolecular Med. 2011, 13, 223–250.
- Kim, H.R.; Lee, P.; Seo, S.W.; Roh, J.H.; Oh, M.; Oh, J.S.; Oh, S.J.; Kim, J.S.; Jeong, Y. Comparison of Amyloid β and Tau Spread Models in Alzheimer’s Disease. Cereb. Cortex 2019, 29, 4291–4302.
- Ismail, R.; Parbo, P.; Madsen, L.S.; Hansen, A.K.; Hansen, K.V.; Schaldemose, J.L.; Kjeldsen, P.L.; Stokholm, M.G.; Gottrup, H.; Eskildsen, S.F.; et al. The relationships between neuroinflammation, beta-amyloid and tau deposition in Alzheimer’s disease: A longitudinal PET study. J. Neuroinflammation 2020, 17, 151.
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543.
- Cha, M.Y.; Han, S.H.; Son, S.M.; Hong, H.S.; Choi, Y.J.; Byun, J.; Mook Jung, I. Mitochondria-specific accumulation of amyloid beta induces mitochondrial dysfunction leading to apoptotic cell death. PLoS ONE 2012, 7, e34929.
- Moreira, P.I.; Santos, M.S.; Moreno, A.; Rego, A.C.; Oliveira, C. Effect of amyloid beta-peptide on permeability transition pore: A comparative study. J. Neurosci. Res. 2002, 69, 257–267.
- Beal, M.F. Mitochondria take centre stage in aging and neurodegeneration. Ann. Neurol. 2005, 58, 495–505.
- Li, Y.; Rusinek, H.; Butler, T.; Glodzik, L.; Pirraglia, E.; Babich, J.; Mozley, P.D.; Nehmeh, S.; Pahlajani, S.; Wang, X.; et al. Decreased CSF clearance and increased brain amyloid in Alzheimer’s disease. Fluids Barriers CNS 2022, 19, 21.
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477.
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405.
- Arai, H.; Suzuki, H.; Yoshiyama, T.; Lobello, K.; Peng, Y.; Liu, E.; Ketter, N.; Margolin, R.; Jackson, N.; Fujimoto, Y. Safety, tolerability and immunogenicity of an immunotherapeutic vaccine (vanutide cridificar ) and the QS-21 adjuvant in Japanese individuals with mild-to-moderate Alzheimer’s disease: A phase IIa, multicenter, randomized, adjuvant and placebo clinical trial. Alzheimer’s Dement. 2013, 9, 282.
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013, 369, 341–350.
- Doody, R.S.; Farlow, M.; Aisen, P.S. Alzheimer’s Disease Cooperative Study Data Analysis and Publication Committee. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 1460.
- Li, S.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597.
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204.
- Stancu, I.C.; Vasconcelos, B.; Terwel, D.; Dewachter, I. Models of β-amyloid induced Tau-pathology: The long and “folded” road to understand the mechanism. Mol. Neurodegener. 2014, 9, 51.
- Takashima, A.; Honda, T.; Yasutake, K.; Michel, G.; Murayama, O.; Murayama, M.; Ishiguro, K.; Yamaguchi, H. Activation of tau protein kinase I/glycogen synthase kinase-3beta by amyloid beta peptide (25–35) enhances phosphorylation of tau in hippocampal neurons. Neurosci. Res. 1998, 31, 317–323.
- Ferreira, A.; Lu, Q.; Orecchio, L.; Kosik, K.S. Selective phosphorylation of adult tau isoforms in mature hippocampal neurons exposed to fibrillar A beta. Mol. Cell. Neurosci. 1997, 9, 220–234.
- Zheng, W.H.; Bastianetto, S.; Mennicken, F.; Ma, W.; Kar, S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 2002, 115, 201–211.
- Ma, Q.L.; Lim, G.P.; Harris-White, M.E.; Yang, F.; Ambegaokar, S.S.; Ubeda, O.J.; Glabe, C.G.; Teter, B.; Frautschy, S.A.; Cole, G.M. Antibodies against beta-amyloid reduce Abeta oligomers, glycogen synthase kinase-3beta activation and tau phosphorylation in vivo and in vitro. J. Neurosci. Res. 2006, 83, 374–384.
- Tackenberg, C.; Grinschgl, S.; Trutzel, A.; Santuccione, A.C.; Frey, M.C.; Konietzko, U.; Grimm, J.; Brandt, R.; Nitsch, R.M. NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss. Cell Death Dis. 2013, 4, e608.
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136.
- Vogel, J.W.; Iturria-Medina, Y.; Strandberg, O.T.; Smith, R.; Levitis, E.; Evans, A.C.; Hansson, O.; Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFinder Study. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat. Commun. 2020, 11, 2612, Erratum in Nat. Commun. 2021, 12, 4862.
- Giacobini, E.; Gold, G. Alzheimer disease therapy—Moving from amyloid-β to tau. Nat. Rev. Neurol. 2013, 9, 677–686.
- Amaral, A.C.; Perez-Nievas, B.G.; Chong, M.S.T.; Gonzalez-Martinez, A.; Argente-Escrig, H.; Rubio-Guerra, S.; Commins, C.; Muftu, S.; Eftekharzadeh, B.; Hudry, E.; et al. Isoform-selective decrease of glycogen synthase kinase-3-beta (GSK-3β) reduces synaptic tau phosphorylation, transcellular spreading, and aggregation. Iscience 2021, 24, 102058.
- Mandelkow, E.-M.; Mandelkow, E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247.
- Roberson, E.D.; Scearce-Levie, K.; Palop, J.J.; Yan, F.; Cheng, I.H.; Wu, T.; Gerstein, H.; Yu, G.Q.; Mucke, L. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 2007, 316, 750–754.
- Duchen, M.R. Mitochondria and calcium: From cell signaling to cell death. J. Physiol. 2000, 529, 57–68.
- Squìer, M.K.; Miller, A.C.; Malkinson, A.M.; Cohen, J.J. Calpain activation in apoptosis. J. Cell. Physiol. 1994, 159, 229–237.
- Maher, P.; Schubert, D. Signaling by reactive oxygen species in the nervous system. Cell. Mol. Life Sci. 2000, 57, 1287–1305.
- Darling, A.L.; Uversky, V.N. Intrinsic disorder and posttranslational modifications: The darker side of the biological dark matter. Front. Genet. 2018, 9, 158.
- Barber, K.W.; Rinehart, J. The ABCs of PTMs. Nat. Chem. Biol. 2018, 14, 188–192.
- Buee, L.; Bussiere, T.; Buee-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 2000, 33, 95–130.
- Xia, C.; Makaretz, S.J.; Caso, C.; McGinnis, S.; Gomperts, S.N.; Sepulcre, J.; Gomez-Isla, T.; Hyman, B.T.; Schultz, A.; Vasdev, N.; et al. Association of in vivo AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 2017, 74, 427–436.
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimeras disease. Brain 2017, 140, 3286–3300.
- Fleeman, R.M.; Proctor, E.A. Astrocytic Propagation of Tau in the Context of Alzheimer’s Disease. FFront. Cell. Neurosci. 2021, 15, 645233.
- Wegmann, S.; Bennett, R.E.; Delorme, L.; Robbins, A.B.; Hu, M.; MacKenzie, D.; Kirk, M.J.; Schiantarelli, J.; Tunio, N.; Amaral, A.C.; et al. Experimental evidence for the age dependence of tau protein spread in the brain. Sci. Adv. 2019, 5, eaaw6404.
- Wei, Y.; Liu, M.; Wang, D. The propagation mechanisms of extracellular tau in Alzheimer’s disease. J. Neurol. 2022, 269, 1164–1181.
- Kazim, S.F.; Sharma, A.; Saroja, S.R.; Seo, J.H.; Larson, C.S.; Ramakrishnan, A.; Wang, M.; Blitzer, R.D.; Shen, L.; Peña, C.J.; et al. Chronic Intermittent Hypoxia Enhances Pathological Tau Seeding, Propagation, and Accumulation and Exacerbates Alzheimer-like Memory and Synaptic Plasticity Deficits and Molecular Signatures. Biol. Psychiatry 2022, 91, 346–358.
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S.
- Hertog, M.G.L.; Feskens, E.J.M.; Hollman, P.C.H.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011.
- Anhê, G.F.; Okamoto, M.M.; Kinote, A.; Sollon, C.; Lellis-Santos, C.; Anhê, F.F.; Lima, G.A.; Hirabara, S.M.; Velloso, L.A.; Bordin, S.; et al. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur. J. Pharmacol. 2012, 689, 285–293.
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513.
- Hanaki, M.; Murakami, K.; Akagi, K.; Irie, K. Structural insights into mechanisms for inhibiting amyloid β42 aggregation by non-catechol-type flavonoids. Bioorganic Med. Chem. 2016, 24, 304–313.
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37.
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181.
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945.
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224.
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. BioMed Res. Int. 2020, 2020, 8210578.
- Kumar, S.; Krishnakumar, V.G.; Morya, V.; Gupta, S.; Datta, B. Nanobiocatalyst facilitated aglycosidic quercetin as a potent inhibitor of tau protein aggregation. Int. J. Biol. Macromol. 2019, 138, 168–180.
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015, 28, 744–750.
- Peng, J.; Li, Q.; Li, K.; Zhu, L.; Lin, X.; Lin, X.; Shen, Q.; Li, G.; Xie, X. Quercetin Improves Glucose and Lipid Metabolism of Diabetic Rats: Involvement of Akt Signaling and SIRT1. J. Diabetes Res. 2017, 2017, 3417306.
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288.
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595.
- Xu, M.; Huang, H.; Mo, X.; Zhu, Y.; Chen, X.; Li, X.; Peng, X.; Xu, Z.; Chen, L.; Rong, S.; et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ1-42 -Induced AD-Like Mice and SH-SY5Y Cells. Mol. Nutr. Food Res. 2021, 65, e2000660.
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044.
- Babaei, F.; Mirzababaei, M.; Nassiri-Asl, M. Quercetin in Food: Possible Mechanisms of Its Effect on Memory. J. Food Sci. 2018, 83, 2280–2287.
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387.
- Nishihira, J.; Nishimura, M.; Kurimoto, M.; Kagami-Katsuyama, H.; Hattori, H.; Nakagawa, T.; Muro, T.; Kobori, M. The effect of 24-week continuous intake of quercetin-rich onion on age-related cognitive decline in healthy elderly people: A randomized, double-blind, placebo-controlled, parallel-group comparative clinical trial. J. Clin. Biochem. Nutr. 2021, 69, 203–215.
- Bayazid, A.B.; Lim, B.O. Quercetin Is An Active Agent in Berries against Neurodegenerative Diseases Progression through Modulation of Nrf2/HO1. Nutrients 2022, 14, 5132.
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N.; et al. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Neuropharmacology 2022, 12, 665031.
- Wu, Q.; Naeem, A.; Zou, J.; Yu, C.; Wang, Y.; Chen, J.; Ping, Y. Isolation of Phenolic Compounds from Raspberry Based on Molecular Imprinting Techniques and Investigation of Their Anti-Alzheimer’s Disease Properties. Molecules 2022, 27, 6893.
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303.
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203.
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75.
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 1.
- Nakagawa, T.; Ohta, K. Quercetin Regulates the Integrated Stress Response to Improve Memory. Int. J. Mol. Sci. 2019, 20, 2761.
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019, 109, 2145–2154.
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796.
- Wei, C.; Li, S.; Zhu, Y.; Chen, W.; Li, C.; Xu, R. Network pharmacology identify intersection genes of quercetin and Alzheimer’s disease as potential therapeutic targets. Front. Aging Neurosci. 2022, 14, 902092.
- García-Mediavilla, M.V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229.
- Song, K.S.; Jeong, W.S.; Jun, M. Inhibition of β-amyloid peptide-induced neurotoxicity by kaempferol 3-O-(6″-acetyl)-β-glucopyranoside from butterbur (Petasites japonicus) leaves in B103 cells. Food Sci. Biotechnol. 2012, 21, 845–851.
- Kaypee, S.; Singh, S.; Swarnkar, S.; Kundu, T.K. Emerging epigenetic therapies—Lysine acetyltransferase inhibitors. In Epigenetic Cancer Therapy; Academic Press: Cambridge, MA, USA, 2023; pp. 459–505.
- Xiao, X.; Shi, D.; Liu, L.; Wang, J.; Xie, X.; Kang, T.; Deng, W. Quercetin suppresses cyclooxygenase-2 expression and angiogenesis through inactivation of P300 signaling. PLoS ONE 2011, 6, e22934.
- Pei, Y.; Parks, J.S.; Kang, H.W. Quercetin alleviates high-fat diet-induced inflammation in brown adipose tissue. J. Funct. Foods 2021, 85, 104614.
- Son, S.M.; Park, S.J.; Fernandez-Estevez, M.; Rubinsztein, D.C. Autophagy regulation by acetylation-implications for neurodegenerative diseases. Exp. Mol. Med. 2021, 53, 30–41.
- Fiorentino, F.; Mai, A.; Rotili, D. Lysine Acetyltransferase Inhibitors From Natural Sources. Front. Pharmacol. 2020, 11, 1243.
- Liao, Y.; Mai, X.; Wu, X.; Hu, X.; Luo, X.; Zhang, G. Exploring the Inhibition of Quercetin on Acetylcholinesterase by Multispectroscopic and In Silico Approaches and Evaluation of Its Neuroprotective Effects on PC12 Cells. Molecules 2022, 27, 7971.
- Alghamdi, A.; Birch, D.J.; Vyshemirsky, V.; Rolinski, O.J. Impact of the Flavonoid Quercetin on β-Amyloid Aggregation Revealed by Intrinsic Fluorescence. J. Phys. Chem. B 2022, 126, 7229–7237.
- Ho, C.L.; Kao, N.J.; Lin, C.I.; Cross, T.L.; Lin, S.H. Quercetin Increases Mitochondrial Biogenesis and Reduces Free Radicals in Neuronal SH-SY5Y Cells. Nutrients 2022, 14, 3310.
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective Effect of Quercetin against Oxidative Stress-Induced Cytotoxicity in Rat Pheochromocytoma (PC-12) Cells. Molecules 2017, 22, 1122.
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.-Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS ONE 2016, 11, e0152371.
- Paula, P.C.; Maria, S.G.; Luis, C.H.; Patricia, C.G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287.
- Molaei, A.; Hatami, H.; Dehghan, G.; Sadeghian, R.; Khajehnasiri, N. Synergistic effects of quercetin and regular exercise on the recovery of spatial memory and reduction of parameters of oxidative stress in animal model of Alzheimer’s disease. EXCLI J. 2020, 19, 596–612.
- Dhawan, S.; Kapil, R.; Singh, B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011, 63, 342–351.
- Chen, J.; Deng, X.; Liu, N.; Li, M.; Liu, B.; Fu, Q.; Qu, R.; Ma, S. Quercetin attenuates tau hyperphosphorylation and improves cognitive disorder via suppression of ER stress in a manner dependent on AMPK pathway. J. Funct. Foods 2016, 22, 463–476.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280343, Quercetin. Retrieved 9 November 2023. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin (accessed on 6 October 2023).
- Dimitrić Marković, J.M.; Milenković, D.; Amić, D.; Popović-Bijelić, A.; Mojović, M.; Pašti, I.A.; Marković, Z.S. Energy requirements of the reactions of kaempferol and selected radical species in different media: Towards the prediction of the possible radical scavenging mechanisms. Struct. Chem. 2014, 25, 1795–1804.
- Wang, L.; Tu, Y.C.; Lian, T.W.; Hung, J.T.; Yen, J.H.; Wu, M.J. Distinctive antioxidant and antiinflammatory effects of flavonols. J. Agric. Food Chem. 2006, 54, 9798–9804.
- Park, S.E.; Sapkota, K.; Kim, H.; Kim, S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br. J. Pharmacol. 2011, 164, 1008–1025.
- Olszewska, M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J. Pharm. Biomed. Anal. 2008, 48, 629–635.
- Kiziltaş, H. Comprehensive evaluation of Reseda lutea L. (Wild Mignonette) and 7 isolated flavonol glycosides: Determination of antioxidant activity, anti-Alzheimer, antidiabetic and cytotoxic effects with in vitro and in silico methods. Turk. J. Chem. 2022, 46, 1185–1198.
- Sulfahri; Wardhani, R.; Makatita, F.A.; Iskandar, I.W. Utilization of Nypa fruit in Alzheimer’s Disease: An In Silico Approach. J. Phys. Conf. Ser. 2019, 1341, 022003.
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923.
- Uysal, M.; Celikten, M.; Beker, M.; Polat, N.; Huseyinbas, O.; Terzioglu-Usak, S.; Elibol, B. Kaempferol treatment ameliorates memory impairments in STZ-induced neurodegeneration by acting on reelin signaling. Acta Neurobiol. Exp. (Wars) 2023, 83, 236–245.
- Beg, T.; Jyoti, S.; Naz, F.; Rahul, X.; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2018, 17, 421–429.
- Zhang, N.; Xu, H.; Wang, Y.; Yao, Y.; Liu, G.; Lei, X.; Sun, H.; Wu, X.; Li, J. Protective mechanism of kaempferol against Aβ25-35-mediated apoptosis of pheochromocytoma (PC-12) cells through the ER/ERK/MAPK signalling pathway. Arch. Med Sci. 2020, 17, 406–416.
- Sun, J.; Wang, J.; Hu, L.; Yan, J. K-3-Rh Protects Against Cerebral Ischemia/Reperfusion Injury by Anti-Apoptotic Effect Through PI3K-Akt Signaling Pathway in Rat. Neuropsychiatr. Dis. Treat. 2020, 16, 1217–1227.
- Al-Brakati, A.; Albarakati, A.J.A.; Lokman, M.S.; Theyab, A.; Algahtani, M.; Menshawi, S.; AlAmri, O.D.; Al Omairi, N.E.; Essawy, E.A.; Kassab, R.B.; et al. Possible Role of Kaempferol in Reversing Oxidative Damage, Inflammation, and Apoptosis-Mediated Cortical Injury Following Cadmium Exposure. Neurotox. Res. 2021, 39, 198–209.
- Ai, R.; Zhuang, X.X.; Anisimov, A.; Lu, J.H.; Fang, E.F. A synergized machine learning plus cross-species wet-lab validation approach identifies neuronal mitophagy inducers inhibiting Alzheimer disease. Autophagy 2022, 18, 939–941.
- Zarei, M.; Mohammadi, S.; Komaki, A.; Golipour Choshali, Z. Antidepressant-like Effects of Intra-cerebroventricular Microinjection of Kaempferol in Male Rats: Involvement of 5-HT2 Receptors. Avicenna J. Neuro Psycho Physiol. 2022, 9, 23–30.
- Rita, L.; Neumann, N.R.; Laponogov, I.; Gonzalez, G.; Veselkov, D.; Pratico, D.; Aalizadeh, R.; Thomaidis, N.S.; Thompson, D.C.; Vasiliou, V.; et al. Alzheimer’s disease: Using gene/protein network machine learning for molecule discovery in olive oil. Hum. Genom. 2023, 17, 57.
- Karunakaran, K.B.; Thiyagaraj, A.; Santhakumar, K. Novel insights on acetylcholinesterase inhibition by Convolvulus pluricaulis, scopolamine and their combination in zebrafish. Nat. Prod. Bioprospecting 2022, 12, 6.
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. Prooxidant Properties of the Flavonoid, Kaempferol, in the Presence of Cu(II) Ions: A ROS-Scavenging Activity, Fenton Reaction and DNA Damage Study. Int. J. Mol. Sci. 2021, 22, 1619.
- Ajiboye, B.O.; Ojo, O.A.; Okesola, M.A.; Akinyemi, A.J.; Talabi, J.Y.; Idowu, O.T.; Fadaka, A.O.; Boligon, A.A.; de Campos, M.M.A. In vitro antioxidant activities and inhibitory effects of phenolic extract of Senecio biafrae (Oliv and Hiern) against key enzymes linked with type II diabetes mellitus and Alzheimer’s disease. Food Sci. Nutr. 2018, 6, 1803–1810.
- Shabir, I.; Pandey, V.K.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752.
- Álvarez-Berbel, I.; Espargaró, A.; Viayna, A.; Caballero, A.B.; Busquets, M.A.; Gámez, P.; Luque, F.J.; Sabaté, R. Three to Tango: Inhibitory Effect of Quercetin and Apigenin on Acetylcholinesterase, Amyloid-β Aggregation and Acetylcholinesterase-Amyloid Interaction. Pharmaceutics 2022, 14, 2342.
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424.
- Dong, X.; Zhou, S.; Nao, J. Kaempferol as a therapeutic agent in Alzheimer’s disease: Evidence from preclinical studies. Ageing Res. Rev. 2023, 87, 101910.
- Li, W.H.; Cheng, X.; Yang, Y.L.; Liu, M.; Zhang, S.S.; Wang, Y.H.; Du, G.H. Kaempferol attenuates neuroinflammation and blood brain barrier dysfunction to improve neurological deficits in cerebral ischemia/reperfusion rats. Brain Res. 2019, 1722, 146361.
- El-Kott, A.F.; Abd-Lateif, A.-E.M.; Khalifa, H.S.; Morsy, K.; Ibrahim, E.H.; Bin-Jumah, M.; Abdel-Daim, M.M.; Aleya, L. Kaempferol protects against cadmium chloride-induced hippocampal damage and memory deficits by activation of silent information regulator 1 and inhibition of poly (ADP-Ribose) polymerase-1. Sci. Total. Environ. 2020, 728, 138832.
- Lin, H.; Wang, X.; Zhao, J.; Lin, Z. Protective effect of kaempferol against cognitive and neurological disturbances induced by d-galactose and aluminum chloride in mice. J. Funct. Foods 2023, 100, 105385.
- Selvi, R.B.; Swaminathan, A.; Chatterjee, S.; Shanmugam, M.K.; Li, F.; Ramakrishnan, G.B.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget 2015, 6, 43806–43818.
- Zhou, Y.P.; Li, G.C. Kaempferol protects cell damage in in vitro ischemia reperfusion model in rat neuronal PC12 cells. BioMed Res. Int. 2020, 2020, 2461079.
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol Is an Anti-Inflammatory Compound with Activity towards NF-κB Pathway Proteins. Anticancer Res. 2015, 35, 2645–2650.
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10.
- Alam, W.; Khan, H.; Shah, M.A.; Cauli, O.; Saso, L. Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules 2020, 25, 4073.
- Sharoar, G.; Thapa, A.; Shahnawaz, M.; Ramasamy, V.S.; Woo, E.-R.; Shin, S.Y.; Park, I.-S. Keampferol-3-O-rhamnoside abrogates amyloid beta toxicity by modulating monomers and remodeling oligomers and fibrils to non-toxic aggregates. J. Biomed. Sci. 2012, 19, 104.
- Chowdhury, M.A.; Ko, H.J.; Lee, H.; Aminul Haque, M.; Park, I.S.; Lee, D.S.; Woo, E.R. Oleanane triterpenoids from Akebiae Caulis exhibit inhibitory effects on Aβ42 induced fibrillogenesis. Arch. Pharm. Res. 2017, 40, 318–327.
- Guo, Q.; Sebastian, L.; Sopher, B.L. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid-β peptide toxicity. J. Neurochem. 1999, 72, 1019–1029.
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free. Radic. Biol. Med. 2001, 30, 433–446.
- Miranda, S.; Opazo, C.; Larrondo, L.F.; Munoz, F.J. The role of oxidative stress in the toxicity induced by amyloid β-peptide in Alzheimer’s disease. Prog. Neurobiol. 2000, 62, 633–648.
- Jafari, A.; Babaei, P.; Rohampour, K.; Rashtiani, S. The Effect of Kaempferol on Autophagy and Nrf-2 Signaling in a Rat Model of Aβ1-42-induced Alzheimer’s Disease. Casp. J. Neurol. Sci. 2022, 8, 7–16.
- Xie, C.; Zhuang, X.X.; Niu, Z.; Ai, R.; Lautrup, S.; Zheng, S.; Jiang, Y.; Han, R.; Gupta, T.S.; Cao, S.; et al. Amelioration of Alzheimer’s disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nat. Biomed. Eng. 2022, 6, 76–93.
- Kaempferol: National Center for Biotechnology Information. PubChem Compound Summary for CID 5280863, Kaempferol. Retrieved 9 November 2023. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 6 October 2023).
- Kouhestani, S.; Zare, S.; Babaei, P. Effects of pure flavonoid of medlar leaves on passive avoidance learning and memory in Alzheimer model of ovariectomized rats. J. Guilan Univ. Med. Sci. 2017, 26, 62–71.
- Krishnaveni, M. Flavonoid in enhancing memory function. J. Pharm. Res. 2012, 5, 3870–3874.
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104, 40–47.
- Liu, L.; Liu, Y.; Zhen, Y.; Guo, T.; Wang, C.; Shen, L.; Li, W. Quercetin inhibits cytotoxicity of PC12 cells induced by amyloid-beta 25–35 via stimulating estrogen receptor α, activating ERK1/2, and inhibiting apoptosis. Open Life Sci. 2022, 17, 230–242.
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomed. Pharmacother. 2023, 165, 115215.
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 20–29.
- Lee, J.; Fukumoto, H.; Orne, J.; Klucken, J.; Raju, S.; Vanderburg, C.R.; Irizarry, M.C.; Hyman, B.T.; Ingelsson, M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005, 194, 91–96.
- Yan, T.; He, B.; Xu, M.; Wu, B.; Xiao, F.; Bi, K.; Jia, Y. Kaempferide prevents cognitive decline via attenuation of oxidative stress and enhancement of brain-derived neurotrophic factor/tropomyosin receptor kinase B/cAMP response element-binding signaling pathway. Phytotherapy Res. 2019, 33, 1065–1073.
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020.
- Walton, M.R.; Dragunow, M. Is CREB a key to neuronal survival? Trends Neurosci. 2000, 23, 48–53.
- Gao, Q.; Tian, D.; Han, Z.; Lin, J.; Chang, Z.; Zhang, D.; Ma, D. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of buyang huanwu decoction in the treatment of ischemic stroke. Evid. -Based Complement. Altern. Med. 2021, 2021, 1–15.
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB neurotrophic activity stimulates δ-secretase by upregulating C/EBPβ in Alzheimer’s disease. Cell Rep. 2019, 28, 655–669.
- Connor, B.; Young, D.; Yan, Q.; Faull, R.L.M.; Synek, B.; Dragunow, M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Mol. Brain Res. 1997, 49, 71–81.
- Levenga, J.; Wong, H.; Milstead, R.; LaPlante, L.; Hoeffer, C.A. Immunohistological Examination of AKT Isoforms in the Brain: Cell-Type Specificity That May Underlie AKT’s Role in Complex Brain Disorders and Neurological Disease. Cereb. Cortex Commun. 2021, 2, tgab036.
- Zarneshan, S.N.; Fakhri, S.; Khan, H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res. 2022, 177, 106099.
- Pak, M.E.; Yang, H.J.; Li, W.; Kim, J.K.; Go, Y. Yuk-Gunja-Tang attenuates neuronal death and memory impairment via ERK/CREB/BDNF signaling in the hippocampi of experimental Alzheimer’s disease model. Front. Pharmacol. 2022, 13, 1014840.
- Jain, V.; Baitharu, I.; Prasad, D.; Ilavazhagan, G. Enriched environment prevents hypobaric hypoxia induced memory impairment and neurodegeneration: Role of BDNF/PI3K/GSK3β pathway coupled with CREB activation. PLoS ONE 2013, 8, e62235.
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4.
- Rada, P.; Rojo, A.I.; Chowdhry, S.; McMahon, M.; Hayes, J.D.; Cuadrado, A. SCF/-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011, 31, 1121–1133.
- Kume, T.; Kouchiyama, H.; Kaneko, S.; Maeda, T.; Kaneko, S.; Akaike, A.; Shimohama, S.; Kihara, T.; Kimura, J.; Wada, K.; et al. BDNF prevents NO mediated glutamate cytotoxicity in cultured cortical neurons. Brain Res. 1997, 756, 200–204.
- Mercado-Gómez, O.; Hernández-Fonseca, K.; Villavicencio-Queijeiro, A.; Massieu, L.; Chimal-Monroy, J.; Arias, C. Inhibition of Wnt and PI3K signaling modulates GSK-3beta activity and induces morphological changes in cortical neurons: Role of tau phosphorylation. Neurochem. Res. 2008, 33, 1599–1609.
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636.
- Jantas, D.; Malarz, J.; Le, T.N.; Stojakowska, A. Neuroprotective Properties of Kempferol Derivatives from Maesa membranacea against Oxidative Stress-Induced Cell Damage: An Association with Cathepsin D Inhibition and PI3K/Akt Activation. Int. J. Mol. Sci. 2021, 22, 10363.
- Wu, J.; Liu, H.; Chu, T.; Jiang, P.; Li, S.T. Neuregulin-1β attenuates sepsis-induced diaphragm atrophy by activating the PI3K/Akt signaling pathway. J. Muscle Res. Cell Motil. 2019, 40, 43–51.
- Kandezi, N.; Mohammadi, M.; Ghaffari, M.; Gholami, M.; Motaghinejad, M.; Safari, S. Novel Insight to Neuroprotective Potential of Curcumin: A Mechanistic Review of Possible Involvement of Mitochondrial Biogenesis and PI3/Akt/GSK3 or PI3/Akt/CREB/BDNF Signaling Pathways. Int. J. Mol. Cell. Med. 2020, 9, 1–32.
- Tanqueiro, S.R.; Ramalho, R.M.; Rodrigues, T.M.; Lopes, L.V.; Sebastião, A.M.; Diógenes, M.J. Inhibition of NMDA Receptors Prevents the Loss of BDNF Function Induced by Amyloid β. Front. Pharmacol. 2018, 9, 237.
- Garzon, D.J.; Fahnestock, M. Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J. Neurosci. 2007, 27, 2628–2635.
- Tong, L.; Thornton, P.L.; Balazs, R.; Cotman, C.W. β-amyloid-(1–42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival is not compromised. J. Biol. Chem. 2001, 276, 17301–17306.
- Cowansage, K.K.; LeDoux, J.E.; Monfils, M.H. Brain-derived neurotrophic factor: A dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 2010, 3, 12–29.
- Rosa, E.; Fahnestock, M. CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol. Aging 2015, 36, 2406–2413.
- DaRocha-Souto, B.; Coma, M.; Perez-Nievas, B.; Scotton, T.; Siao, M.; Sánchez-Ferrer, P.; Hashimoto, T.; Fan, Z.; Hudry, E.; Barroeta, I. Activation of glycogen synthase kinase-3 beta mediates β-amyloid induced neuritic damage in Alzheimer’s disease. Neurobiol. Dis. 2012, 45, 425–437.
- Barco, A.; Pittenger, C.; Kandel, E.R. CREB, memory enhancement and the treatment of memory disorders: Promises, pitfalls and prospects. Expert Opin. Ther. Targets 2003, 7, 101–114.
- Christensen, R.; Marcussen, A.B.; Wörtwein, G.; Knudsen, G.; Aznar, S. Aβ (1–42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT2A levels. Exp. Neurol. 2008, 210, 164–171.
- Ciaramella, A.; Salani, F.; Bizzoni, F.; Orfei, M.D.; Langella, R.; Angelucci, F.; Spalletta, G.; Taddei, A.R.; Caltagirone, C.; Bossù, P. The stimulation of dendritic cells by amyloid beta 1–42 reduces BDNF production in Alzheimer’s disease patients. Brain Behav. Immun. 2013, 32, 29–32.
- Zussy, C.; Brureau, A.; Keller, E.; Marchal, S.; Blayo, C.; Delair, B.; Ixart, G.; Maurice, T.; Givalois, L. Alzheimer’s disease related markers, cellular toxicity and behavioral deficits induced six weeks after oligomeric amyloid-β peptide injection in rats. PLoS ONE 2013, 8, e53117.
- Wu, H.; Lu, D.; Jiang, H.; Xiong, Y.; Qu, C.; Li, B.; Mahmood, A.; Zhou, D.; Chopp, M. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/AKT pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J. Neurotrauma 2008, 25, 130–139.
- Hu, Y.S.; Long, N.; Pigino, G.; Brady, S.T.; Lazarov, O. Molecular mechanisms of environmental enrichment: Impairments in AKT/GSK3β, neurotrophin-3 and CREB signaling. PLoS ONE. 2013, 8, e64460.
- Nordberg, A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004, 3, 519–527.
- Caricasole, A.; Copani, A.; Caruso, A.; Caraci, F.; Iacovelli, L.; Sortino, M.A.; Terstappen, G.C.; Nicoletti, F. The Wnt pathway, cell-cycle activation and beta-amyloid: Novel therapeutic strategies in Alzheimer’s disease? Trends Pharmacol. Sci. 2003, 24, 233–238.
- Lee, C.W.; Lau, K.F.; Miller, C.C.; Shaw, P.C. Glycogen synthase kinase-3 beta-mediated tau phosphorylation in cultured cell lines. Neuroreport 2003, 14, 257–260.
- Simayi, J.; Bayinsang; Nuermaimaiti, M.; Hailati, S.; Han, M.; Reheman, Z.; Wumaier, A.; Zhou, W. A Network Pharmacology-Based Study on the Mechanism of Dibutyl Phthalate of Ocimum basilicum L. against Alzheimer’s Disease through the AKT/GSK-3β Pathway. BioMed Res. Int. 2022, 2022, 9494548.
- Li, E.; Yan, K.; Zhang, R.; Zou, P.; Li, S.; Ma, Q.; Liao, B. Kaempferol Protects Against Apoptosis in PC12 Cells Exposed to Hydrogen Peroxide by Activating Akt1. Nat. Prod. Commun. 2023, 18, 1934578X231170448.
- Zhang, S.; Lu, Y.; Chen, W.; Shi, W.; Zhao, Q.; Zhao, J.; Li, L. Network Pharmacology and Experimental Evidence: PI3K/AKT Signaling Pathway is Involved in the Antidepressive Roles of Chaihu Shugan San. Drug Des. Dev. Ther. 2021, 15, 3425–3441.
- Shi, Y.; Chen, M.; Zhao, Z.; Pan, J.; Huang, S. Network pharmacology and molecular docking analyses of mechanisms underlying effects of the cyperi rhizoma-chuanxiong rhizoma herb pair on depression. Evid. -Based Complement. Altern. Med. 2021, 2021, 5704578.
- Rasouli, H.; Hosseini Ghazvini, S.M.B.; Yarani, R.; Altıntaş, A.; Jooneghani, S.G.N.; Ramalho, T.C. Deciphering inhibitory activity of flavonoids against tau protein kinases: A coupled molecular docking and quantum chemical study. J. Biomol. Struct. Dyn. 2022, 40, 411–424.
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729.
- Gong, P.; Wang, D.; Cui, D.; Yang, Q.; Wang, P.; Yang, W.; Chen, F. Anti-aging function and molecular mechanism of Radix Astragali and Radix Astragali preparata via network pharmacology and PI3K/Akt signaling pathway. Phytomedicine 2021, 84, 153509.
- Yao, R.Q.; Qi, D.S.; Yu, H.L.; Liu, J.; Yang, L.H.; Wu, X.X. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 2012, 37, 2777–2786.
- Sadighparvar, S.; Darband, S.G.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Mobaraki, K.; Majidinia, M. Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: Possible involvement of inflammation and BDNF signalling. Exp. Physiol. 2020, 105, 1598–1609.
- Chiu, Y.J.; Teng, Y.S.; Chen, C.M.; Sun, Y.C.; Hsieh-Li, H.M.; Chang, K.H.; Lee-Chen, G.J. A Neuroprotective Action of Quercetin and Apigenin through Inhibiting Aggregation of Aβ and Activation of TRKB Signaling in a Cellular Experiment. Biomol. Ther. 2023, 31, 285–297.
- Das, S.; Sengupta, S.; Chakraborty, S. Scope of β-secretase (bace1)-targeted therapy in alzheimer’s disease: Emphasizing the flavonoid based natural scaffold for bace1 inhibition. CS Chem. Neurosci. 2020, 11, 3510–3522.
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2008, 1780, 819–825.
- Khan, H.; Singh, A.; Thapa, K.; Garg, N.; Grewal, A.K.; Singh, T.G. Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res. 2021, 1761, 147399.
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187.
- Gong, E.J.; Park, H.R.; Kim, M.E.; Piao, S.; Lee, E.; Jo, D.G.; Chung, H.Y.; Ha, N.C.; Mattson, M.P.; Lee, J. Morin attenuates tau hyperphosphorylation by inhibiting GSK3beta. Neurobiol. Dis. 2011, 44, 223–230.
- Miyai, S.; Yamaguchi, A.; Iwasaki, T.; Shamsa, F.; Ohtsuki, K. Biochemical characterization of epigallocatechin-3-gallate as an effective stimulator for the phosphorylation of its binding proteins by glycogen synthase kinase-3beta in vitro. Biol. Pharm. Bull. 2010, 33, 1932–1937.
- Ashrafpour, M.; Parsaei, S.; Sepehri, H. Quercetin improved spatial memory dysfunctions in rat model of intracerebroventricular streptozotocin-induced Alzheimer’s disease. Natl. J. Physiol. Pharm. Pharmacol. 2015, 5, 411.
- Tong-un, T.; Muchimapura, S.; Phachonpai, W.; Wattanathorn, J. Effects of quercetin encapsulated liposomes via nasal administration: A novel cognitive enhancer. Am. J. Appl. Sci. 2010, 7, 906–913.
- Sriraksa, N.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Brown, K.; Chaisiwamongkol, K. Cognitive-enhancing effect of quercetin in a rat model of Parkinson’s disease induced by 6-hyrodoxydopamine. J. Evid. Based Complement. Alternat. Med. 2011, 2012, 823206.
- Elreedy, H.A.; Elfiky, A.M.; Ahmed Mahmoud, A.; Ibrahim, K.S.; Ghazy, M.A. Effect Of Quercetin As Therapeutic And Protective Agent In Aluminum Chloride-Induced Alzheimer’s Disease Rats. Egypt. J. Chem. 2022, 65, 633–641.
- Elfiky, A.M.; Mahmoud, A.A.; Elreedy, H.A.; Ibrahim, K.S.; Ghazy, M.A. Quercetin stimulates the non-amyloidogenic pathway via activation of ADAM10 and ADAM17 gene expression in aluminum chloride-induced Alzheimer’s disease rat model. Life Sci. 2021, 285, 119964.
- Singh, N.K.; Garabadu, D. Quercetin Exhibits α7nAChR/Nrf2/HO-1-Mediated Neuroprotection Against STZ-Induced Mitochondrial Toxicity and Cognitive Impairments in Experimental Rodents. Neurotox. Res. 2021, 39, 1859–1879.
- Parent, M.; Chitturi, J.; Santhakumar, V.; Hyder, F.; Sanganahalli, B.G.; Kannurpatti, S.S. Kaempferol Treatment after Traumatic Brain Injury during Early Development Mitigates Brain Parenchymal Microstructure and Neural Functional Connectivity Deterioration at Adolescence. J. Neurotrauma 2020, 37, 966–974.
- Gu, Y.; Zhang, X.; Xu, A.; Chen, W.; Liu, K.; Wu, L.; Mo, S.; Hu, Y.; Liu, M.; Luo, Q. Protein-ligand binding affinity prediction with edge awareness and supervised attention. iScience 2022, 26, 105892.
- Zhang, S.S.; Liu, M.; Liu, D.N.; Shang, Y.F.; Du, G.H.; Wang, Y.H. Network pharmacology analysis and experimental validation of kaempferol in the treatment of ischemic stroke by inhibiting apoptosis and regulating neuroinflammation involving neutrophils. Int. J. Mol. Sci. 2022, 23, 12694.
- Zhang, W.; Lv, M.; Shi, Y.; Mu, Y.; Yao, Z.; Yang, Z. Network Pharmacology-Based Study of the Underlying Mechanisms of Huangqi Sijunzi Decoction for Alzheimer’s Disease. Evid. Based Complement. Altern. Med. 2021, 2021, 6480381.
- Matsuo, E.S.; Shin, R.W.; Billingsley, M.L.; van de Voorde, A.; O’Connor, M.; Trojanowski, J.Q.; Lee, V.M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron 1994, 13, 989–1002.
- Iqbal, K.; Liu, F.; Gong, C.X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016, 12, 15–27.
- Xia, Y.; Liu, R.; Chen, R.; Tian, Q.; Zeng, K.; Hu, J.; Liu, X.; Wang, Q.; Wang, P.; Wang, X.C.; et al. Novel multipotent AChEI-CCB attenuates hyperhomocysteinemia-induced memory deficits and Neuropathologies in rats. J. Alzheimer's Dis. 2014, 42, 1029–1039.
- Russo, M.; Milito, A.; Spagnuolo, C.; Carbone, V.; Rosén, A.; Minasi, P.; Lauria, F.; Russo, G.L. CK2 and PI3K are direct molecular targets of quercetin in chronic lymphocytic leukaemia. Oncotarget 2017, 8, 42571–42587.
- Images Created. Available online: BioRender.com (accessed on 6 October 2023).
- Fang, J.; Wang, L.; Wu, T.; Yang, C.; Gao, L.; Cai, H.; Liu, J.; Fang, S.; Chen, Y.; Tan, W.; et al. Network pharmacology-based study on the mechanism of action for herbal medicines in Alzheimer treatment. J. Ethnopharmacol. 2017, 196, 281–292.
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202.
- Longpré, F.; Garneau, P.; Christen, Y.; Ramassamy, C. Protection by EGb 761 against beta-amyloid-induced neurotoxicity: Involvement of NF-kappaB, SIRT1, and MAPKs pathways and inhibition of amyloid fibril formation. Free. Radic. Biol. Med. 2006, 41, 1781–1794.
- Tchantchou, F.; Xu, Y.; Wu, Y.; Christen, Y.; Luo, Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J. 2007, 21, 2400–2408.
- Kwon, K.J.; Lee, E.J.; Cho, K.S.; Cho, D.H.; Shin, C.Y.; Han, S.H. Ginkgo biloba extract (Egb761) attenuates zinc-induced tau phosphorylation at Ser262 by regulating GSK3β activity in rat primary cortical neurons. Food Funct. 2015, 6, 2058–2067.
- Lejri, I.; Grimm, A.; Eckert, A. Ginkgo biloba extract increases neurite outgrowth and activates the Akt/mTOR pathway. PLoS ONE. 2019, 14, e0225761.
- Rhein, V.; Song, X.; Wiesner, A.; Ittner, L.M.; Baysang, G.; Meier, F.; Ozmen, L.; Bluethmann, H.; Dröse, S.; Brandt, U.; et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc. Natl. Acad. Sci. USA 2009, 106, 20057–20062.
- Muller, W.E.; Heiser, J.; Leuner, K. Effects of the standardized Ginkgo biloba extract EGb 761(R) on neuroplasticity. Int. Psychogeriatrics 2012, 24 (Suppl. S1), S21–S24.
- Xu, Y.; Cui, C.; Pang, C.; Christen, Y.; Luo, Y. Restoration of impaired phosphorylation of cyclic AMP response element-binding protein (CREB) by EGb 761 and its constituents in Abeta-expressing neuroblastoma cells. Eur. J. Neurosci. 2007, 26, 2931–2939.
- Tchantchou, F.; Lacor, P.N.; Cao, Z.; Lao, L.; Hou, Y.; Cui, C.; Klein, W.L.; Luo, Y. Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J. Alzheimer's Dis. 2009, 18, 787–798.
- Tian, R.; Wang, H.; Xiao, Y.; Hu, P.; Du, R.; Shi, X.; Wang, Z.; Xie, Y. Fabrication of nanosuspensions to improve the oral bioavailability of total flavones from Hippophae rhamnoides L. and their comparison with an inclusion complex. AAPS Pharmscitech 2020, 21, 249.
- Xia, C.X.; Gao, A.X.; Zhu, Y.; Dong, T.T.; Tsim, K.W. Flavonoids from Seabuckthorn (Hippophae rhamnoides L.) restore CUMS-induced depressive disorder and regulate the gut microbiota in mice. Food Funct. 2023, 14, 7426–7438.
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front. Pharmacol. 2020, 10, 1688.
- Jeganathan, B.; Punyasiri, P.A.; Kottawa-Arachchi, J.D.; Ranatunga, M.A.; Abeysinghe, I.S.; Gunasekare, M.T.; Bandara, B.M. Genetic Variation of Flavonols Quercetin, Myricetin, and Kaempferol in the Sri Lankan Tea (Camellia sinensis L.) and Their Health-Promoting Aspects. Int. J. Food Sci. 2016, 2016, 6057434.
- Ishan, T.; Lalit, S.; Girdhari, G.; Gupta, G.L. Synergistic antioxidant activity of three medicinal plants Hypericum perforatum, Bacopa monnieri, and Camellia sinensis. Indo Am. J. Pharm. Res. 2014, 4, 2563–2568.
- Mahmoodzadeh, T.; Kashani, M.H.K.; Ramshini, H.; Moslem, A.; Mohammad-Zadeh, M. Effect of Camellia sinensis on Spatial Memory in a Rat Model of Alzheimer’s Disease. J. Biomed. 2016, 1, e5340.
- Onasanwo, S.A.; Adamaigbo, V.O.; Adebayo, O.G.; Eleazer, S.E. Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: Role of oxido-inflammatory and cholinergic neurotransmission pathway. Metab. Brain Dis. 2021, 36, 2445–2460.
- Rahmatkar, S.N.; Rana, A.K.; Kumar, R.; Singh, D. Fagopyrum tataricum (L.) Gaertn interacts with Gsk-3β/Nrf-2 signalling to protect neurotoxicity in a zebrafish model. J. Ethnopharmacol. 2023, 319, 117187.
- Sermkaew, N.; Plyduang, T. Self-microemulsifying drug delivery systems of Moringa oleifera extract for enhanced dissolution of kaempferol and quercetin. Acta Pharm. 2020, 70, 77–88.
- Liu, W.L.; Wu, B.F.; Shang, J.H.; Wang, X.F.; Zhao, Y.L.; Huang, A.X. Moringa oleifera seed ethanol extract and its active component kaempferol potentiate pentobarbital-induced sleeping behaviours in mice via a GABAergic mechanism. Pharm. Biol. 2022, 60, 810–824.
- Sun, Y.; Wu, A.; Li, X.; Qin, D.; Jin, B.; Liu, J.; Tang, Y.; Wu, J.; Yu, C. The seed of Litchi chinensis fraction ameliorates hippocampal neuronal injury in an Aβ25-35-induced Alzheimer’s disease rat model via the AKT/GSK-3β pathway. Pharm. Biol. 2020, 58, 35–43.
- El Gizawy, H.A.; Abo-Salem, H.M.; Ali, A.A.; Hussein, M.A. Phenolic Profiling and Therapeutic Potential of Certain Isolated Compounds from Parkia roxburghii against AChE Activity as well as GABAA α5, GSK-3β, and p38α MAP-Kinase Genes. ACS Omega 2021, 6, 20492–20511.
- Singh, S.; Singh, T.G.; Mahajan, K.; Dhiman, S. Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. J. Pharm. Pharmacol. 2020, 72, 1306–1327.
- Liu, Y.; Xue, Q.; Li, A.; Li, K.; Qin, X. Mechanisms exploration of herbal pair of HuangQi-DanShen on cerebral ischemia based on metabonomics and network pharmacology. J. Ethnopharmacol. 2020, 253, 112688.
This entry is offline, you can click here to edit this entry!
