Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
4-Hydroxy-2-pyrones as polyketides are widespread in Nature and possess versatile bioactivity that makes them an attractive target for synthesis and modification. Also these pyrones bear several electrophilic and nucleophilic centers that determine their application in organic synthesis.
- 2-pyrone
- 4-hydroxy-2-pyrone
- polyketide
1. Introduction
2-Pyrones are an important class of heterocyclic compounds of interest as valuable reagents in organic synthesis and an essential pharmacophore in many biologically active products [1,2,3,4,5,6,7,8,9,10]. Among them, 4-hydroxy-2-pyrones occupy a special place because these molecules are both polyketide structures and pyrans [3,5,6,7,8] (Figure 1). These substances can exist in two tautomeric forms, namely, 4-hydroxy-2-pyrone and 2-hydroxy-4-pyrone. The former is a major tautomer as the result of effective conjugation. This structural feature makes a wide range of synthesis methods available for their synthesis that are typical for both 2-pyrones and 4-pyrones and that were not covered in recent reviews [1,4].
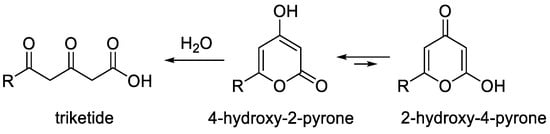
Figure 1. Structural features of 4-hydroxy-2-pyrones.
4-Hydroxy-2-pyrones are polyfunctional molecules that bear several electrophilic and nucleophilic centers that determine their application in organic synthesis. These heterocycles are attractive building blocks for the preparation of biologically important pyran structures, aromatics, polymers, azaheterocycles and acyclic structures via ring-opening transformations or pyran ring modifications [11,12,13,14,15,16,17,18,19,20,21]. Two molecules that receive the most attention are triacetic acid lactone (4-hydroxy-6-methyl-2H-pyran-2-one) [11] and dehydroacetic acid (3-acetyl-4-hydroxy-6-methyl-2H-pyran-2-one) [12,13], which are already produced industrially and show high reactivity. Additionally, triacetic acid lactone is considered a bioprivileged molecule and a potential platform molecule because it can be prepared from carbohydrates using biological methods [22]. This approach allows for the sustainable preparation of valuable chemicals and end products based on renewable carbon sources [23]. Numerous other 4-hydroxy-2-pyrones are also produced using polyketidases [6] and can lead to various structures that can also be attributed to bioprivileged molecules. Thus, the development of the synthesis of 4-hydroxy-2-pyrones as a renewable feedstock for the chemical industry is a serious task for sustainable chemistry.
The 4-hydroxy-2-pyrone fragment provides these molecules with promising and diverse biological and physical properties [1,5,6,7,8,9]. The main strategy and feature of the search for new bioactive substances includes their isolation from natural sources. Plenty of 4-hydroxy-2-pyrones have been described as varying in the nature and complexity of their substituents, number and position to which they are attached. These pyrones were found in bacteria, microbes, plants, insects, fungi and animals [1,2,3,4,5,6,7,8,9,10] and are involved in many types of biological processes, such as defense against other organisms and as signaling function [1,5,6,7,8,9], as well as representing key intermediates for biochemical transformations of complex natural molecules [6].
The wide distribution of 4-hydroxy-2-pyrones as secondary metabolites and diverse biological activity makes them an attractive target for synthesis and the design of new bioactive compounds. At the same time, the major methods of preparation are isolation from natural sources and the use of biotechnologies.
2. Natural 4-hydroxy-2-pyrones and Their Applications
Several 4-hydroxy-2-pyrones, which have their own names, include the five main classes of 4-hydroxy-2-pyrones, but we cannot cover this rapidly developing area and all observed metabolites bearing the 4-hydroxy-2-pyrone moiety in this review [3,7,8,75]. The known natural 4-hydroxy-2-pyrones can be classified according to the nature of the substituents and positions of substitution at the pyran ring. The first class includes the simplest 6-substituted 4-hydroxy-4-pyrones bearing alkyl, styryl and aromatic substituents (Figure 2). A feature of these molecules is the presence of the free C-3 position, which can be modified and leads to several new 4-hydroxy-2-pyrones [11]. The simplest natural 4-hydroxy-2-pyrones are triacetic acid lactone and tetracetic acid lactone, which were first isolated in 1967 from Penicillium stipitatum. These substances were important for understanding the pathways the polyketide formation in nature [76,77] and stimulated the development of the synthesis of lactone from carbohydrates [22]. Another simple 6-alkyl-4-hydroxy-2-pyrone, namely, fistupyrone (4-hydroxy-6-isovaleryl-2-pyrone), is produced by Streptomyces and demonstrates inhibition of the infection of Alternaria brassicicola in the leaves of seedlings of Chinese cabbage [78]. Among this series of compounds, hispidin, which is 6-styryl-4-hydroxy-2-pyrone, bears the catechol fragment and can be distinguished as a low molecular compound with valuable biological activities, such as anti-oxidative, anti-inflammatory, cytotoxic, anti-platelet aggregation, anti-diabetic, anti-dementia and anti-viral effects [79,80]. This pyrone was isolated for the first time from Inonotus hispidus fungi in 1889 and was later found in various fungi used in traditional medicine [79]. Furthermore, hispidin contains a conjugated system as a natural yellow-brown pigment and can be used for dyeing [81]. Hydroxylation at the C-3 position occurs in luminescent mushrooms Neonothopanus nambi, leading to fungal luciferin ((E)-6-(3,4-dihydroxystyryl)-3,4-dihydroxy-2H-pyran-2-one) [82]. This process is followed by light emission. Also, hispidin and bisnoryangonin undergo transformations in mushrooms to give a large and diverse range of biogenerated styrylpyrones, which have a role similar to that of flavonoids in plants [80]. Several products of the dimerization or oligomerization are connected with modifications of the C-3 position and include substituted 6-styryl-4-hydroxy-2-pyrones, such as fasciculines A and B, phelligridins B and I, phelligridimer A, phaeolschidins A–E and pinillidine [80,83]. Moreover, the oxidized structures at the benzene ring isolated from micro-organisms as metabolites of the fruiting bodies of Hyrnenochaete mougestii (Poriales) are hymenoquinone and leucohymenoquinone (Figure 2) [7].
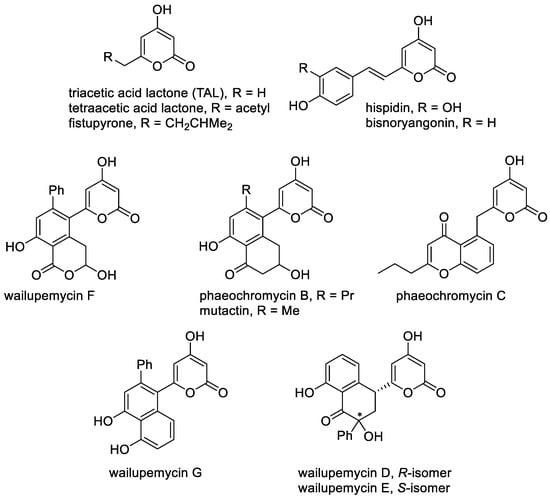
Figure 2. The main representatives of 6-substituted 4-hydroxy-2-pyrones.
An aromatic substituent at the C-6 position occurs in such structures, as wailupemicines, phaeochromycins and mutactin. Wailupemicines are a metabolism product of Streptomyces maritimus, which was found at Wailupe Beach Park along the southeast coast of Oahu (Hawaii), and are of interest as new α-glucosidase inhibitors [84]. Phaeochromycins were isolated from an actinomycete Streptomyces phaeochromogenes and demonstrate anti-inflammatory activity [85].
The most common class of natural 4-hydroxy-2-pyrones includes 3-alkyl-substituted molecules, which contain alkyl and alkenyl substituents at the C-6 position and less often substituents at the C-5 position (Figure 3). A large class includes germicidins A–J as important representatives of 3,6-dialkyl-substituted 4-hydroxy-2-pyrones [86]. These compounds are natural antibiotics that were isolated from Streptomyces bacteria and are responsible for spore germination via inhibition of porcine Na+/K+-activated ATPase [87]. Related molecules are violapyrones A–F, which bear the methyl substituent at the C-3 position and a long aliphatic substituent at the C-6 position [88]. They are secondary metabolites of Streptomyces violascens and demonstrate modest antibacterial activity. Another feature of alkylated 4-pyrones is that they can perform signaling functions [89]. It was reported that photopyrones A–H act as signaling molecules in the cell–cell communication system of the entomopathogenic bacterium Photorhabdus luminescens via the inhibition of quorum sensing. Pseudopyronines A,B bearing long alkyl substituents at the C-3 and C-6 positions isolated from different Pseudomonas strains have antibacterial properties, especially against mycobacteria [9,90]. It is known that 3-methyl-6-alkyl-4-hydroxy-2-pyrones are included in the M. tuberculosis cell wall as permeability regulators. Pseudopyronines, which are similar to these compounds, selectively disrupt the membrane and inhibit the growth of M. tuberculosis by blocking its fatty acid biosynthesis pathway [90].
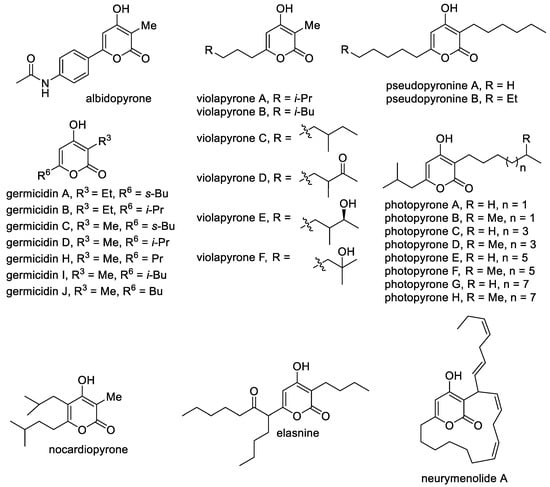
Figure 3. The main representatives of 3-alkyl-substituted 4-hydroxy-2-pyrones.
Elasnine contains branched alkyl substituents that are modified by a carbonyl group. It is interesting as an effective and selective inhibitor of human sputum (leukocyte) elastase, which is implicated in many inflammatory disease states [7]. Also, elasnine can be used in controlling marine biofilms and displays feasibility and advantages when used as a signal molecule to develop eco-friendly technologies. Elasnine’s action is connected with disturbing the regulation of the ATP-binding cassette transport system and the bacterial secretion system [91]. The marine-pyrone macrolide neurymenolide A contains the 4-hydroxy-2-pyrone moiety, which was previously isolated from the Fijian red macroalga, namely, Neurymenia fraxinifolia, and characterized as an antibacterial agent against antibiotic-resistant strains [92]. It was observed that neurymenolide A significantly delays the in vivo polymerization of tubulin to form microtubules and bipolar mitotic spindles at the prophase–metaphase transition. A feature of albidopyrone is the presence of an aromatic substituent at the C-6 position, and it demonstrates a moderate inhibitory activity against protein-tyrosin phosphatase B, which is the major negative regulator of insulin signaling [93]. Nocardiopyrone contains a completely alkyl-substituted pyrone ring and was isolated from the marine microorganism Nocardiopsis [94].
Although natural 4-hydroxy-2-pyrones are usually polyacetates, some polypropionates were found in mollusks, fungi and bacteria [95]. Fusaripyrone A and exiguapyrone were isolated from the mollusks Haminoea and contain an unusually long chain and form via a regular condensation process starting with propionyl-CoA and continuing with elongation of C3 units up to the linear C30-polypropionates after cyclization [96]. These compounds can play the role of chemical markers for these marine organisms [95]. Nipyrones A and B were isolated from a marine sponge-derived fungus Aspergillus niger and exhibit moderate antibacterial efficacy against four pathogenic bacteria [97]. Salinipyrones A and B were isolated from the marine-derived bacterium Salinispora pacifica. These metabolites are by-products of the PKS system, which is associated with the rosamicin macrolide antibiotics [32,98]. A related molecule, namely, capsulactone, was isolated from an endophytic fungus Penicillium capsulatum obtained from the leaves of Panax notoginseng and demonstrated weak antibacterial activity (Figure 4) [99].
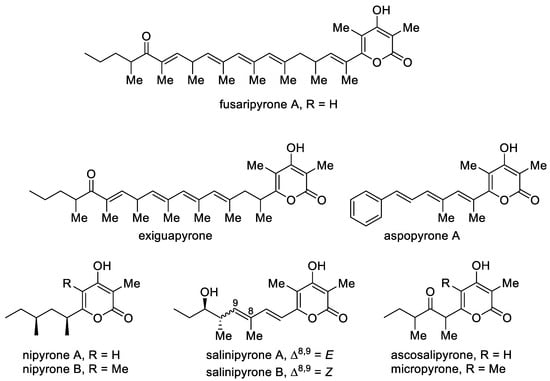
Figure 4. The major representatives of 3-alkyl-substituted 4-hydroxy-2-pyrones as polypropionate polyketides.
The structurally similar micropyrone and ascosalipyrone [100] contain the carbonyl group and were isolated from Helichrysum italicum ssp. microphyllum and the endophytic and obligate marine fungus Ascochyta salicorniae of the green alga Ulva sp., respectively. Bioassay-guided investigation of Okinawan plant-associated fungus Aspergillus sp. led to the isolation of aspopyrone A [101], which exhibited significant protein tyrosine phosphatase 1B (PTP1B) and T-cell PTP inhibitory activities.
A separate group includes 3-acyl-4-hydroxy-2-pyrones (Figure 5). This class of compounds stands out due to its biological properties. The simplest of this series, namely, dehydroacetic acid, is used as a food preservative (E265) and in cosmetics due to its antibacterial and fungicidal properties [68]. A structurally similar 6-methyl-4-hydroxy-2-pyrone, namely, pogostone, which was isolated from patchouli oil, inhibits both Gram-negative and Gram-positive bacteria and demonstrates anti-cancer activities [102]. Furthermore, pogostone can be used as a repellent and insecticide [103]. Csypyrones B are 3-acetyl-α-pyrone compounds bearing the carboxylic acid side chain as the result of oxidation of the corresponding alkyl substituent and were isolated from the fungus Aspergillus oryzae [104].
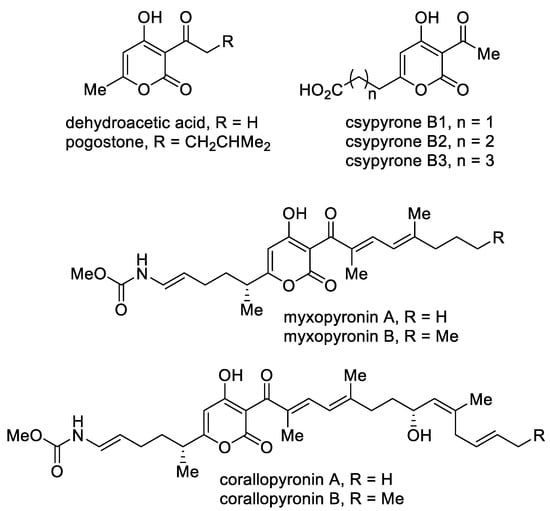
Figure 5. The major representatives of 3-acyl-4-hydroxy-2-pyrones.
Mixopyronins and corallopyronins are functionalized N-alkenylcarbamate 3-acyl-4-hydroxy-2-pyrones bearing chiral centers. These molecules are promising natural antibiotics produced by the terrestrial bacterium Myxococcus fulvus Mx f50 and possess antibacterial activity against Gram-positive and Gram-negative pathogens [105]. The pyrones are rare inhibitors of the bacterial RNA polymerase (RNAP) “switch region” as non-competitive inhibitors with rifampicin. RNAP is a highly conserved protein, which makes the possible application of these molecules in medical practice important. From the point of view of biological activity and the possibility of biotechnological production, special attention is focused on corallopyronin A as an antibiotic undergoing preclinical studies [106].
A few 4-hydroxy-2-pyrone conjugates are known to have carbohydrates and terpenes, which are attracted to the C-3 position of the pyrone ring (Figure 6). Such structures exhibit a wide spectrum of biological activity and are an attractive target for total synthesis. Carbohydrate derivatives, namely, fusapyrone and deoxyfusapyrone, were isolated from Fusarium semitectum [8,107,108,109]. These compounds show considerable antifungal activity (Botrytis cinerea, Aspergillus parasiticus and Penicillium brevi-compactum) [109]. Epipyrone A (Orevactaene) is a polyene pigment isolated from Epicoccum nigrum with broad-spectrum antifungal activity [110,111]. Moreover, this molecule interferes with the RNA binding activity of the regulatory protein Rev in human immunodeficiency virus type I; demonstrates anti-microbial activity; and displays inhibitory activities against cytopathic effect of influenza A virus (H1N1) and NF-κB-dependent gene expression, cysteine and serine proteases [110].
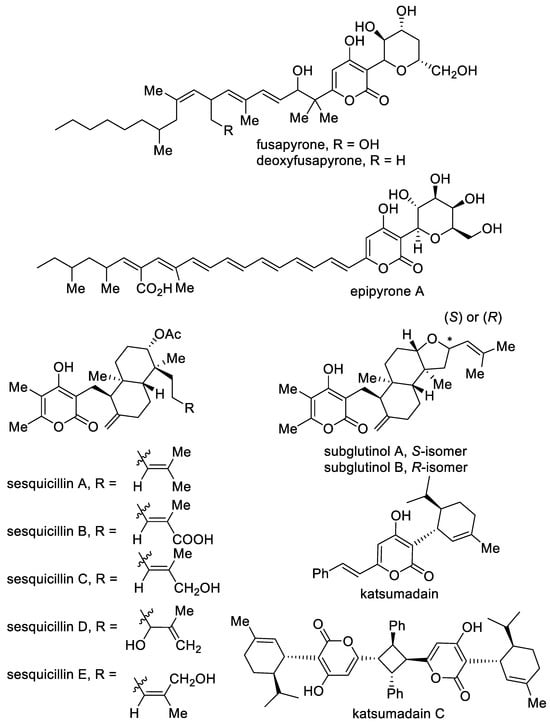
Figure 6. The major representatives of conjugates based on 4-hydroxy-2-pyrones.
A large class of pyrano-diterpene conjugates includes sesquicillins A–E [112], which were isolated from the fungi Albophoma. Sesquicillins are insecticides and cytotoxic molecules showing moderate inhibitory activity against the growth of Artemia salina (brine shrimps) and Jurkat cells.
Another meroterpenoid, namely, subglutinol A, is a natural product isolated from Fusarium subglutinans, which is an endophytic fungus from the vine Tripterygium wilfordii [113]. This compound demonstrated multimodal immune-suppressive effects on activated T cells in vitro. These results suggest the potential of subglutinol as a novel therapeutic for inflammatory diseases. Katsumadain C bears a four-membered cycle and is isolated from Alpinia katsumadain. This molecule is a product of katsumadain dimerization and is used as an anti-emetic and stomachic agent [74]. Various pyrones bearing a terpene fragment, such as sartorypyrones [114,115], aszonapyrones [114,116] and metarhizin A [117], are also isolated and of interest as biologically important compounds.
This entry is adapted from the peer-reviewed paper 10.3390/org4040037
This entry is offline, you can click here to edit this entry!
