Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Sparse and conflicting evidence exists regarding the localization, expression, and regulation of phase II drug-metabolizing enzymes and drug transporters across gestational stages. To resolve the uncertainties and assumptions in current knowledge, additional pharmacokinetic (PK) data and clinical pharmacology research are required to understand drug metabolism and transport in the pregnant woman and in the placenta.
- pregnancy
- placenta
- gestational change
- phase II enzyme
- drug transporter
1. Introduction
Physiological changes during pregnancy affect drug pharmacokinetics (PK), including absorption, distribution, metabolism, and elimination [1]. PK changes that affect the activity of drug-metabolizing enzymes and drug transporters can differ in each pregnancy trimester [2]. These gestational changes inform the selection of safe and effective drug doses for pregnant patients and guide the decision to conduct appropriate dose monitoring during pregnancy. Approximately 81% of pregnant women take at least one prescription or over-the-counter medication during gestation, excluding vitamins and dietary supplements [3]. Despite the high prevalence of medication use during pregnancy, most medications are administered “off-label” to pregnant patients, with doses based on PK data from nonpregnant individuals [4]. With limited clinical trials conducted in the pregnant population, PK changes during pregnancy are poorly characterized and optimal dose regimens for pregnant patients are insufficiently investigated [5].
Physiologically based pharmacokinetic (PBPK) modeling is an increasingly used method for predicting drug exposure during pregnancy [5]. Utilizing mathematical equations, PBPK models incorporate known physiological changes into a mechanistic model that describes drug PK [6]. Informed pregnancy PBPK models may support the evaluation of PK data in the pregnant population, guide the proposal of safe and effective doses for clinical drug development programs, and supplement clinical pharmacology studies during regulatory approval [7]. One aspect of PBPK model predictions is informed by adequate knowledge of gestational changes in drug-metabolizing enzymes and drug transporters; for instance, knowledge of these gestational changes may inform the robust prediction of drug renal clearance, systemic exposure, and their changes across pregnancy trimesters [6]. However, due to sparse or conflicting data, the gestational changes of only a small number of phase II enzymes and drug transporters have been incorporated into PBPK modeling software. The current gaps in knowledge emphasize the need to study changes in phase II enzymes and drug transporters across gestational trimesters.
Several literature reviews have been published that examine changes in enzyme and/or transporter expression in the pregnant woman and in the placenta. Gestational changes in select phase II enzymes and renal drug transporters have been elucidated through pharmacokinetic analysis of probe drugs administered during pregnancy [8][9]. Additional evidence suggests that drug-metabolizing enzymes and drug transporters in the placenta largely affect fetal drug exposure [10], but the lack of available or consistent information regarding gestational changes in some placental transporters necessitates further research [11]. Transcription factors, steroid hormones, genetic variations, and pregnancy complications have also been observed to change the expression of placental drug transporters [12][13].
2. Placenta
The human placenta links the fetus to the mother, providing nutrients to and removing wastes from the fetal circulation [14]. In addition to its function of supporting fetal metabolism, the placenta may play a fetoprotective role by extruding xenobiotics, such as drugs, from the fetal circulation.
2.1. Placental Anatomy
The human placenta possesses a hemochorial structure, in which the fetal tissue directly contacts maternal blood [15]. The fetal tissue consists of syncytiotrophoblasts, cytotrophoblasts, and vascular endothelial cells, of which the syncytiotrophoblast cells are the main barrier to drug transport [14][15]. The syncytiotrophoblasts comprise a maternal-facing brush border membrane (i.e., apical membrane) and a fetal-facing basal membrane (i.e., basolateral membrane) [14]. The apical membrane constitutes the main site of exchange for drugs, nutrients, and endogenous molecules between the maternal and fetal circulation, while the basolateral membrane provides structural attachment to cytotrophoblasts or fetal connective tissue, which houses the fetal capillaries [14][16]. Molecules are transported from the maternal uterine vasculature, across the apical and basolateral membranes of the syncytiotrophoblasts, through the fetal endothelium, and to the fetal circulation [1].
2.2. Placental Drug Transport
Both passive diffusion and transporter-mediated transfer are involved in the transport of drug molecules across the placental syncytiotrophoblast [16]. The rate of passive diffusion of a drug can be affected by its molecular weight, pKa, and/or lipophilicity [17]. In general, drugs of high molecular weight demonstrate limited passive transport across the placenta. Drugs that are unionized at physiological pH tend to diffuse across the placental membrane more easily than drugs that are ionized. Passive diffusion is also a common transport mechanism for lipophilic drugs [16].
Drug transporters are membrane proteins that efflux or influx endogenous and exogenous substances [15], offering an alternative mechanism of transport for drug substrates that do not easily diffuse across the placental syncytiotrophoblast [16]. For substrates of multiple drug transporters, the net direction of transport is determined by the relative abundance of each transporter, the affinity of the drug for each transporter, and the mechanisms that regulate transporter activity [14]. The localization of select placental drug transporters and the directionality of their transport are illustrated in Figure 1. A description of each drug transporter family is detailed in subsequent sections.
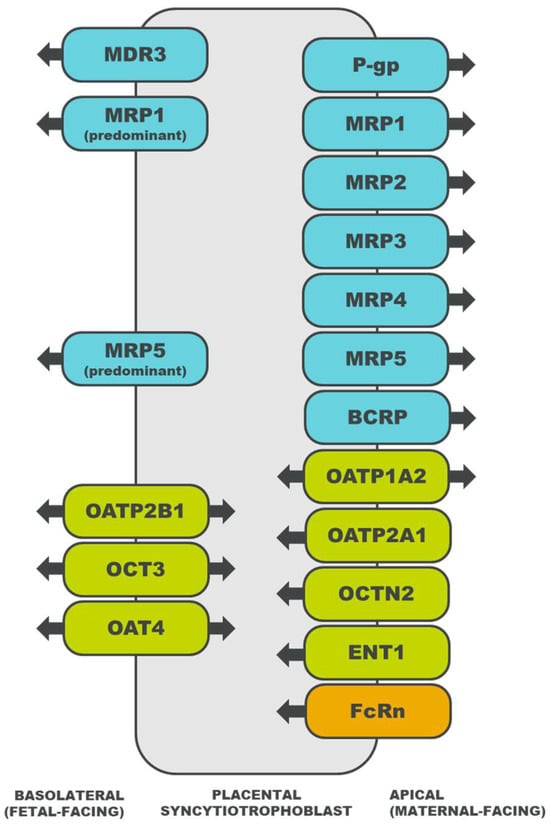
Figure 1. Localization of drug transporters in the placental syncytiotrophoblast and the directionality of their transport [18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36]. ATP-binding cassette (ABC) transporters are depicted in blue, solute carrier (SLC) transporters in green, and an immunoglobulin transporter in orange. MDR, multidrug resistance protein; P-gp, P-glycoprotein; MRP, multidrug resistance associated protein; BCRP, breast cancer resistance protein; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; OCTN, organic cation/carnitine transporter; OAT, organic anion transporter; ENT, equilibrative nucleoside transporter; and FcRn, neonatal Fc receptor.
3. Drug Transporters
3.1. ATP-Binding Cassette Superfamily
Transporters belonging to the ABC superfamily utilize ATP hydrolysis to efflux drugs from cells [15][37]. ABC transporters participate in the final steps of drug PK [38], including drug excretion by the liver or kidney and drug secretion by the placenta.
3.1.1. Multidrug Resistance Protein (MDR) Family
MDR1, alternatively known as P-glycoprotein (P-gp), is the most widely studied transporter within the MDR protein family (ABCB gene family) [10]. Located in the liver, kidney, intestine, and brain, MDR transporters facilitate the secretion of substrates from cells [15]. The placental MDR1, MDR3, and bile salt export pump (BSEP) may limit fetal exposure to toxic and nontoxic compounds [39][40].
3.1.2. Multidrug Resistance-Associated Protein (MRP) Family
Transporters in the MRP protein family (ABCC gene family) are expressed in various tissues, including the liver, kidney, intestine, and brain [41]. The MRP transporters export anionic drugs, as well as their sulfate, glucuronate, and glutathione metabolites. Placental MRP1 [14], MRP2 [22], MRP3 [23], and MRP5 [25] also efflux anionic and/or conjugated substrates, and the direction of transport to or from the fetal circulation depends on the transporter’s localization in the basolateral or apical membrane (Figure 1) [14].
3.1.3. Breast Cancer Resistance Protein (BCRP)
BCRP belongs to the ABCG gene family, with protein expression identified in the liver, kidney, intestine, and lung [14]. Though it can transport charged drugs and glucuronated metabolites [14], BCRP demonstrates a preference for the transport of sulfate conjugates [42]. In the placenta, BCRP effluxes xenobiotics [26], sulfated steroids [29], and bile acids [43] from the fetal circulation, which may confer protection to the fetus [42].
3.2. Solute Carrier Superfamily
Transporters belonging to the SLC superfamily influx drugs into cells via secondary active transport (e.g., antiport or symport) and passive transport (e.g., uniport) [37][38]. SLC transporters enable various processes of drug PK [38], such as drug absorption across the luminal membrane of enterocytes and drug distribution across the apical membrane of placental syncytiotrophoblasts.
3.2.1. Organic Anion-Transporting Polypeptide (OATP) Family
The OATP transporters, encoded by the SLC21/SLCO genes, are expressed in the liver, kidney, intestine, and brain [44]. OATP substrates include amphipathic organic compounds, such as drugs, bile acids, steroids, hormones, and peptides. OATP-mediated uptake may work in concert with ABC-mediated efflux [44], an interaction that has also been observed between placental transporters, including OATP2B1 and BCRP [29].
3.2.2. Organic Cation Transporter (OCT) Family
Transporters in the OCT family are encoded by the SLC22 genes [16]. OCT1 is largely expressed in the liver, OCT2 in the kidney, and OCT3 in the central nervous system and placenta; there is minimal overlap in tissue distribution between members of the OCT family [45]. OCTs enable the passive uptake of organic cations, which is coupled with cellular efflux facilitated by a different transporter on the opposite cellular membrane [16]. In addition to transporting cationic compounds, placental OCT3 also exhibits a high affinity for monoamines [30].
3.2.3. Organic Cation/Carnitine Transporter (OCTN) Family
The OCTN transporters, encoded by the SLC22 genes, have been identified in the liver, kidney, and intestine [45]. OCTNs transport cationic substrates and carnitine [44]. As carnitine influx transporters, placental OCTNs may function to supply carnitine for fetal development and placental metabolism [16].
3.2.4. Organic Anion Transporter (OAT) Family
Among the transporters in the OAT or SLC22 family, OAT1 and OAT3 have been widely studied due to their importance in drug transport [45]. Observed in the liver, kidney, intestine, central nervous system, skeletal muscle, heart, lung, pancreas, and adrenal gland, OATs are anion exchangers that pair the uptake of an anionic substrate with the efflux of another anion [44]. Though OAT1-3 are predominantly expressed in excretory organs [16], OAT4 mediates the transport of anions [27] and the uptake of estrogen precursors [46] in the placenta.
3.2.5. Concentrative Nucleoside Transporter (CNT) Family
CNTs are members of the SLC28 gene family and aid in the transport of nucleotides, nucleosides, and nucleoside analogs [47]. CNTs contribute to the biosynthesis, absorption, metabolism, and elimination of nucleotides in the brain, intestine, liver, and kidney, respectively [48]. Though limited expression of CNTs occurs in the placenta [34], CNT1 has been speculated to supply pyrimidine nucleosides for placental development [16].
3.2.6. Equilibrative Nucleoside Transporter (ENT) Family
ENTs are members of the SLC29 gene family, facilitating the transport of nucleosides, nucleobases, and monoamines [47]. Like CNTs, ENTs have been identified in the brain, intestine, liver, and kidney [34][48]. While ENT expression has been observed in the placenta, it is unclear whether these transporters are functional [34].
3.2.7. Multidrug and Toxin Extrusion (MATE) Family
The MATE transporters, encoded by the SLC47 genes, efflux organic cations from cells, often in tandem with the influx activity of OCTs [44]. MATE1 is expressed in the liver, kidney, skeletal muscle, and adrenal gland. MATE2 has two additional protein variants, MATE2-B and MATE2-K, with MATE2-K specifically expressed in the kidney. Based upon the observed interaction between MATE1 and OCT3 in the rat placenta, coupled transport of organic cations is also predicted to occur in the human placenta [49].
3.3. Neonatal Fc Receptor (FcRn)
FcRn is an immunoglobulin G (IgG) receptor that is distributed in the liver, kidney, lung, and skin [50]. In the placenta, FcRn mediates the transplacental transport of IgG from the mother to the fetus (Figure 1) [36]. As suggested with in vitro experiments, the mechanism of IgG transport involves endocytosis of the IgG–FcRn complex into acidic endosomes, transcytosis of the complex to the opposite membrane, and pH-triggered dissociation of the complex upon membrane fusion [51][52]. In addition to facilitating the transplacental transfer of IgG, the FcRn system may also enable the transplacental transport of monoclonal antibodies, which may increase fetal drug exposure or necessitate therapeutic drug monitoring during pregnancy [53].
4. Phase II Enzymes
4.1. Methyltransferase (MT) Superfamily
MTs transfer a methyl group from a donor molecule to a substrate [54][55] and are located in cells of the liver, kidney, intestine, brain, and blood [56]. In the placenta, MTs may affect the homeostasis of the human chorionic gonadotropin (HCG) hormone [54] and may contribute to placental and embryonic development [55].
4.2. Glutathione S-Transferase (GST) Superfamily
Enzymes in the GST superfamily catalyze the conjugation of glutathione moieties to xenobiotic electrophiles or reactive oxygen species [57]. GSTs are distributed in the liver, kidney, intestine, brain, heart, lung, pancreas, and spleen [58]. Placental GSTs detoxify and bioactivate xenobiotics [57].
4.3. N-Acetyltransferase (NAT) Superfamily
NATs transfer an acetyl group to a nitrogen acceptor of primary arylamines and hydrazines [59]. The NAT superfamily includes N-acetyltransferase 1 (NAT1), which is expressed in the intestine, bladder, and breast, and N-acetyltransferase 2 (NAT2), which is expressed in the liver and intestine [60]. Both NAT1 and NAT2 are expressed in the placenta, with NAT1 providing a greater contribution to placental acetylation capacity [59].
4.4. Sulfotransferase (SULT) Superfamily
Enzymes in the SULT superfamily catalyze the sulfonation of endogenous substrates and xenobiotics [56]. SULTs have been identified in cells of the liver, kidney, intestine, brain, blood, lung, adrenal gland, breast, endometrium, and ovary, though tissue distribution varies between SULT subfamilies. Placental SULTs catalyze the biotransformation of estrogens to regulate intracellular steroid concentrations [10][61].
4.5. UDP-Glucuronosyltransferase (UGT) Superfamily
UGTs catalyze the addition of glucuronic acid to endogenous substrates and hydrophobic drug molecules, forming β-D-glucuronide metabolites [62]. Though mostly distributed in the liver, UGTs have also been observed in the gastrointestinal tract, kidney, brain, lung, pancreas, breast, and nasal epithelium. In the placenta, UGTs may participate in the metabolism of steroid substrates, thyroid hormones, and bile acids [63].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15112624
References
- Feghali, M.; Venkataramanan, R.; Caritis, S. Pharmacokinetics of Drugs in Pregnancy. Semin. Perinatol. 2015, 39, 512–519.
- Isoherranen, N.; Thummel, K.E. Drug Metabolism and Transport during Pregnancy: How Does Drug Disposition Change during Pregnancy and What Are the Mechanisms that Cause Such Changes? Drug Metab. Dispos. 2013, 41, 256–262.
- Lupattelli, A.; Spigset, O.; Twigg, M.J.; Zagorodnikova, K.; Mårdby, A.C.; Moretti, M.E.; Drozd, M.; Panchaud, A.; Hämeen-Anttila, K.; Rieutord, A.; et al. Medication Use in Pregnancy: A Cross-Sectional, Multinational Web-Based Study. BMJ Open 2014, 4, e004365.
- Caritis, S.N.; Venkataramanan, R. Obstetrical, Fetal, and Lactation Pharmacology—A Crisis That Can No Longer Be Ignored. Am. J. Obstet. Gynecol. 2021, 225, 10–20.
- Cole, S.; Coppola, P.; Kerwash, E.; Nooney, J.; Lam, S.P. Pharmacokinetic Characterization to Enable Medicine Use in Pregnancy, the Potential Role of Physiologically-Based Pharmacokinetic Modeling: A Regulatory Perspective. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 547–549.
- Coppola, P.; Kerwash, E.; Cole, S. The Use of Pregnancy Physiologically Based Pharmacokinetic Modeling for Renally Cleared Drugs. J. Clin. Pharmacol. 2022, 62 (Suppl. S1), S129–S139.
- Coppola, P.; Kerwash, E.; Cole, S. Physiologically Based Pharmacokinetics Model in Pregnancy: A Regulatory Perspective on Model Evaluation. Front. Pediatr. 2021, 9, 687978.
- Tasnif, Y.; Morado, J.; Hebert, M.F. Pregnancy-Related Pharmacokinetic Changes. Clin. Pharmacol. Ther. 2016, 100, 53–62.
- Anderson, G.D. Pregnancy-Induced Changes in Pharmacokinetics: A Mechanistic-Based Approach. Clin. Pharmacokinet. 2005, 44, 989–1008.
- Myllynen, P.; Immonen, E.; Kummu, M.; Vähäkangas, K. Developmental Expression of Drug Metabolizing Enzymes and Transporter Proteins in Human Placenta and Fetal Tissues. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1483–1499.
- Dallmann, A.; Liu, X.I.; Burckart, G.J.; van den Anker, J. Drug Transporters Expressed in the Human Placenta and Models for Studying Maternal-Fetal Drug Transfer. J. Clin. Pharmacol. 2019, 59 (Suppl. S1), S70–S81.
- Staud, F.; Ceckova, M. Regulation of Drug Transporter Expression and Function in the Placenta. Expert Opin. Drug Metab. Toxicol. 2015, 11, 533–555.
- Kozlosky, D.; Barrett, E.; Aleksunes, L.M. Regulation of Placental Efflux Transporters during Pregnancy Complications. Drug Metab. Dispos. 2022, 50, 1364–1375.
- Joshi, A.A.; Vaidya, S.S.; St-Pierre, M.V.; Mikheev, A.M.; Desino, K.E.; Nyandege, A.N.; Audus, K.L.; Unadkat, J.D.; Gerk, P.M. Placental ABC Transporters: Biological Impact and Pharmaceutical Significance. Pharm. Res. 2016, 33, 2847–2878.
- Yamashita, M.; Markert, U.R. Overview of Drug Transporters in Human Placenta. Int. J. Mol. Sci. 2021, 22, 13149.
- Staud, F.; Cerveny, L.; Ceckova, M. Pharmacotherapy in Pregnancy; Effect of ABC and SLC Transporters on Drug Transport across the Placenta and Fetal Drug Exposure. J. Drug Target. 2012, 20, 736–763.
- Myllynen, P.; Pasanen, M.; Vähäkangas, K. The Fate and Effects of Xenobiotics in Human Placenta. Expert Opin. Drug Metab. Toxicol. 2007, 3, 331–346.
- Ushigome, F.; Takanaga, H.; Matsuo, H.; Yanai, S.; Tsukimori, K.; Nakano, H.; Uchiumi, T.; Nakamura, T.; Kuwano, M.; Ohtani, H.; et al. Human Placental Transport of Vinblastine, Vincristine, Digoxin and Progesterone: Contribution of P-Glycoprotein. Eur. J. Pharmacol. 2000, 408, 1–10.
- Kozłowska-Rup, D.; Czekaj, P.; Plewka, D.; Sikora, J. Immunolocalization of ABC Drug Transporters in Human Placenta from Normal and Gestational Diabetic Pregnancies. Ginekol. Pol. 2014, 85, 410–419.
- Nagashige, M.; Ushigome, F.; Koyabu, N.; Hirata, K.; Kawabuchi, M.; Hirakawa, T.; Satoh, S.; Tsukimori, K.; Nakano, H.; Uchiumi, T.; et al. Basal Membrane Localization of MRP1 in Human Placental Trophoblast. Placenta 2003, 24, 951–958.
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. Independent Regulation of Apical and Basolateral Drug Transporter Expression and Function in Placental Trophoblasts by Cytokines, Steroids, and Growth Factors. Drug Metab. Dispos. 2007, 35, 595–601.
- Meyer zu Schwabedissen, H.E.; Jedlitschky, G.; Gratz, M.; Haenisch, S.; Linnemann, K.; Fusch, C.; Cascorbi, I.; Kroemer, H.K. Variable Expression of MRP2 (ABCC2) in Human Placenta: Influence of Gestational Age and Cellular Differentiation. Drug Metab. Dispos. 2005, 33, 896–904.
- Gedeon, C.; Behravan, J.; Koren, G.; Piquette-Miller, M. Transport of Glyburide by Placental ABC Transporters: Implications in Fetal Drug Exposure. Placenta 2006, 27, 1096–1102.
- Kojovic, D.; Ghoneim, R.H.; Serghides, L.; Piquette-Miller, M. Role of HIV and Antiretroviral Therapy on the Expression of Placental Transporters in Women with HIV. AAPS J. 2020, 22, 138.
- Meyer Zu Schwabedissen, H.E.; Grube, M.; Heydrich, B.; Linnemann, K.; Fusch, C.; Kroemer, H.K.; Jedlitschky, G. Expression, Localization, and Function of MRP5 (ABCC5), a Transporter for Cyclic Nucleotides, in Human Placenta and Cultured Human Trophoblasts: Effects of Gestational Age and Cellular Differentiation. Am. J. Pathol. 2005, 166, 39–48.
- Gedeon, C.; Anger, G.; Piquette-Miller, M.; Koren, G. Breast Cancer Resistance Protein: Mediating the Trans-placental Transfer of Glyburide across the Human Placenta. Placenta 2008, 29, 39–43.
- Fokina, V.M.; Patrikeeva, S.; Wang, X.M.; Noguchi, S.; Tomi, M.; König, J.; Ahmed, M.S.; Nanovskaya, T. Role of Uptake Transporters OAT4, OATP2A1, and OATP1A2 in Human Placental Bio-disposition of Pravastatin. J. Pharm. Sci. 2022, 111, 505–516.
- Wang, H.; Yan, Z.; Dong, M.; Zhu, X.; Wang, H.; Wang, Z. Alteration in Placental Expression of Bile Acids Transporters OATP1A2, OATP1B1, OATP1B3 in Intrahepatic Cholestasis of Pregnancy. Arch. Gynecol. Obstet. 2012, 285, 1535–1540.
- Grube, M.; Reuther, S.; Meyer Zu Schwabedissen, H.; Köck, K.; Draber, K.; Ritter, C.A.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Organic Anion Transporting Polypeptide 2B1 and Breast Cancer Resistance Protein Interact in the Transepithelial Transport of Steroid Sulfates in Human Placenta. Drug Metab. Dispos. 2007, 35, 30–35.
- Sata, R.; Ohtani, H.; Tsujimoto, M.; Murakami, H.; Koyabu, N.; Nakamura, T.; Uchiumi, T.; Kuwano, M.; Nagata, H.; Tsukimori, K.; et al. Functional Analysis of Organic Cation Transporter 3 Expressed in Human Placenta. J. Pharmacol. Exp. Ther. 2005, 315, 888–895.
- Grube, M.; Meyer Zu Schwabedissen, H.; Draber, K.; Präger, D.; Möritz, K.U.; Linnemann, K.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Expression, Localization, and Function of the Carnitine Transporter Octn2 (Slc22a5) in Human Placenta. Drug Metab. Dispos. 2005, 33, 31–37.
- Rytting, E.; Audus, K.L. Novel Organic Cation Transporter 2-Mediated Carnitine Uptake in Placental Choriocarcinoma (BeWo) Cells. J. Pharmacol. Exp. Ther. 2005, 312, 192–198.
- Noguchi, S.; Nishimura, T.; Fujibayashi, A.; Maruyama, T.; Tomi, M.; Nakashima, E. Organic Anion Transporter 4-Mediated Transport of Olmesartan at Basal Plasma Membrane of Human Placental Barrier. J. Pharm. Sci. 2015, 104, 3128–3135.
- Govindarajan, R.; Bakken, A.H.; Hudkins, K.L.; Lai, Y.; Casado, F.J.; Pastor-Anglada, M.; Tse, C.M.; Hayashi, J.; Unadkat, J.D. In Situ Hybridization and Immunolocalization of Concentrative and Equilibrative Nucleoside Transporters in the Human Intestine, Liver, Kidneys, and Placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1809–R1822.
- Griffiths, M.; Yao, S.Y.; Abidi, F.; Phillips, S.E.; Cass, C.E.; Young, J.D.; Baldwin, S.A. Molecular Cloning and Characterization of a Nitrobenzylthioinosine-Insensitive (Ei) Equilibrative Nucleoside Transporter from Human Placenta. Biochem. J. 1997, 328 Pt 3, 739–743.
- Landor, M.; Rubinstein, A.; Kim, A.; Calvelli, T.; Mizrachi, Y. Receptor-Mediated Maternofetal Transfer of Immunoglobulins. Inhibition of Transport of Anti-HIV-1 Immunoglobulin by Generic Immunoglobulins in the In Vitro Perfused Placenta. Int. Arch. Allergy Immunol. 1998, 115, 203–209.
- Ho, R.H.; Kim, R.B. Transporters and Drug Therapy: Implications for Drug Disposition and Disease. Clin. Pharmacol. Ther. 2005, 78, 260–277.
- Petzinger, E.; Geyer, J. Drug Transporters in Pharmacokinetics. Naunyn Schmiedeberg’s Arch. Pharmacol. 2006, 372, 465–475.
- Mölsä, M.; Heikkinen, T.; Hakkola, J.; Hakala, K.; Wallerman, O.; Wadelius, M.; Wadelius, C.; Laine, K. Functional Role of P-Glycoprotein in the Human Blood-Placental Barrier. Clin. Pharmacol. Ther. 2005, 78, 123–131.
- Imperio, G.E.; Javam, M.; Lye, P.; Constantinof, A.; Dunk, C.E.; Reis, F.M.; Lye, S.J.; Gibb, W.; Matthews, S.G.; Ortiga-Carvalho, T.M.; et al. Gestational Age-Dependent Gene Expression Profiling of ATP-Binding Cassette Transporters in the Healthy Human Placenta. J. Cell. Mol. Med. 2019, 23, 610–618.
- Gu, X.; Manautou, J.E. Regulation of Hepatic ABCC Transporters by Xenobiotics and in Disease States. Drug Metab. Rev. 2010, 42, 482–538.
- Suzuki, M.; Suzuki, H.; Sugimoto, Y.; Sugiyama, Y. ABCG2 Transports Sulfated Conjugates of Steroids and Xenobiotics. J. Biol. Chem. 2003, 278, 22644–22649.
- Blazquez, A.G.; Briz, O.; Romero, M.R.; Rosales, R.; Monte, M.J.; Vaquero, J.; Macias, R.I.; Cassio, D.; Marin, J.J. Characterization of the Role of ABCG2 as a Bile Acid Transporter in Liver and Placenta. Mol. Pharmacol. 2012, 81, 273–283.
- Liu, X. SLC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 101–202.
- Nigam, S.K. The SLC22 Transporter Family: A Paradigm for the Impact of Drug Transporters on Metabolic Pathways, Signaling, and Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 663–687.
- Zhang, Y.; Chen, Y.; Dai, B.; Bai, M.; Lu, S.; Lin, N.; Zhou, H.; Jiang, H. Bilirubin Reduces the Uptake of Estrogen Precursors and the Followed Synthesis of Estradiol in Human Placental Syncytiotrophoblasts via Inhibition and Downregulation of Organic Anion Transporter 4. Drug Metab. Dispos. 2022, 50, 341–350.
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A Guide to Plasma Membrane Solute Carrier Proteins. FEBS J. 2021, 288, 2784–2835.
- Young, J.D.; Yao, S.Y.; Baldwin, J.M.; Cass, C.E.; Baldwin, S.A. The Human Concentrative and Equilibrative Nucleoside Transporter Families, SLC28 and SLC29. Mol. Asp. Med. 2013, 34, 529–547.
- Ahmadimoghaddam, D.; Zemankova, L.; Nachtigal, P.; Dolezelova, E.; Neumanova, Z.; Cerveny, L.; Ceckova, M.; Kacerovský, M.; Micuda, S.; Staud, F. Organic Cation Transporter 3 (OCT3/SLC22A3) and Multidrug and Toxin Extrusion 1 (MATE1/SLC47A1) Transporter in the Placenta and Fetal Tissues: Expression Profile and Fetus Protective Role at Different Stages of Gestation. Biol. Reprod. 2013, 88, 55.
- Tegenge, M.A.; Mahmood, I.; Struble, E.B.; Sauna, Z. Pharmacokinetics of Antibodies during Pregnancy: General Pharmacokinetics and Pregnancy Related Physiological Changes (Part 1). Int. Immunopharmacol. 2023, 117, 109914.
- Ellinger, I.; Schwab, M.; Stefanescu, A.; Hunziker, W.; Fuchs, R. IgG Transport across Trophoblast-Derived BeWo Cells: A Model System to Study IgG Transport in the Placenta. Eur. J. Immunol. 1999, 29, 733–744.
- Einarsdottir, H.K.; Stapleton, N.M.; Scherjon, S.; Andersen, J.T.; Rispens, T.; van der Schoot, C.E.; Vidarsson, G. On the Perplexingly Low Rate of Transport of IgG2 across the Human Placenta. PLoS ONE 2014, 9, e108319.
- Seow, C.H.; Leung, Y.; Vande Casteele, N.; Ehteshami Afshar, E.; Tanyingoh, D.; Bindra, G.; Stewart, M.J.; Beck, P.L.; Kaplan, G.G.; Ghosh, S.; et al. The Effects of Pregnancy on the Pharmacokinetics of Infliximab and Adalimumab in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 45, 1329–1338.
- Paik, M.K.; Hwang, B.D.; Lim, K. Human Placenta S-Adenosylmethionine: Protein Carboxyl O-Methyltransferase (Protein Methylase II). Purification and Characterization. Int. J. Biochem. 1988, 20, 1107–1112.
- Zhu, B.T.; Wu, K.Y.; Wang, P.; Cai, M.X.; Conney, A.H. O-methylation of Catechol Estrogens by Human Placental Catechol-O-Methyltransferase: Interindividual Differences in Sensitivity to Heat Inactivation and to Inhibition by Dietary Polyphenols. Drug Metab. Dispos. 2010, 38, 1892–1899.
- Jancova, P.; Anzenbacher, P.; Anzenbacherova, E. Phase II Drug Metabolizing Enzymes. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 103–116.
- Datta, K.; Roy, S.K.; Mitra, A.K.; Kulkarni, A.P. Glutathione S-Transferase Mediated Detoxification and Bioactivation of Xenobiotics during Early Human Pregnancy. Early Hum. Dev. 1994, 37, 167–174.
- Hayes, J.D.; Strange, R.C. Glutathione S-Transferase Polymorphisms and Their Biological Consequences. Pharmacology 2000, 61, 154–166.
- Derewlany, L.O.; Knie, B.; Koren, G. Arylamine N-Acetyltransferase Activity of the Human Placenta. J. Pharmacol. Exp. Ther. 1994, 269, 756–760.
- Minchin, R.F.; Hanna, P.E.; Dupret, J.M.; Wagner, C.R.; Rodrigues-Lima, F.; Butcher, N.J. Arylamine N-Acetyltransferase I. Int. J. Biochem. Cell Biol. 2007, 39, 1999–2005.
- Bernier, F.; Lopez Solache, I.; Labrie, F.; Luu-The, V. Cloning and Expression of cDNA Encoding Human Placental Estrogen Sulfotransferase. Mol. Cell. Endocrinol. 1994, 99, R11–R15.
- Kiang, T.K.; Ensom, M.H.; Chang, T.K. UDP-Glucuronosyltransferases and Clinical Drug-Drug Interactions. Pharmacol. Ther. 2005, 106, 97–132.
- Collier, A.C.; Ganley, N.A.; Tingle, M.D.; Blumenstein, M.; Marvin, K.W.; Paxton, J.W.; Mitchell, M.D.; Keelan, J.A. UDP-Glucuronosyltransferase Activity, Expression and Cellular Localization in Human Placenta at Term. Biochem. Pharmacol. 2002, 63, 409–419.
This entry is offline, you can click here to edit this entry!
