Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Macrophage migration inhibitory factor (MIF) and its homolog, D-dopachrome tautomerase (D-DT), are cytokines that play critical roles in the immune response to various infectious diseases. The role of MIF in different types of infections is controversial, as it has either a protective function or a host damage-enhancing function depending on the pathogen. Depending on the specific role of MIF, different therapeutic options for MIF-targeting drugs arise. Human MIF-neutralizing antibodies, anti-parasite MIF antibodies, small molecule MIF inhibitors or MIF-blocking peptides, as well as the administration of exogenous MIF or MIF activity-augmenting small molecules have potential therapeutic applications and need to be further explored in the future. In addition, MIF has been shown to be a potential biomarker and therapeutic target in sepsis. Further research is needed to unravel the complexity of MIF and D-DT in infectious diseases and to develop personalized therapeutic approaches targeting these cytokines. Overall, a comprehensive understanding of the role of MIF and D-DT in infections could lead to new strategies for the diagnosis, treatment, and management of infectious diseases.
- macrophage migration inhibitory factor
- MIF
- infectious disease
- sepsis
- biomarker
- cytokine
- DDT
1. Bacterial Infections
MIF plays a complex role in the host response to bacterial infections. In their 2005 study, Oddo et al. outlined the role of MIF in response to Mycobacterium tuberculosis [1]. An increase in the release of MIF from macrophages in response to Mycobacterium tuberculosis was observed. The neutralization of MIF with anti-MIF antibodies in macrophages resulted in an enhancement in the growth of Mycobacterium tuberculosis. Additional administration of exogenous MIF led to an inhibition of mycobacterial growth. In conclusion, this study demonstrated a protective, bacterial growth inhibitory role of MIF in the immune response against Mycobacterium tuberculosis.
Das et al. showed in 2013 that the −794 CATT5/5 variant, which represents a low-expressor MIF genotype, is more frequently found in patients with a disseminated Mycobacterium tuberculosis infection than in disease-free control patients [2]. MIF-deficient mice also exhibit increased pulmonary pathology and earlier mortality in response to Mycobacterium tuberculosis infection compared to wild-type (WT) mice [2]. Shang et al. further demonstrated a potential use of serum MIF levels as a marker for monitoring treatment efficacy in active pulmonary tuberculosis [3].
In a study by Sumaiya et al. investigating MIF as a diagnostic marker for leptospirosis, elevated serum MIF levels were shown in cases of leptospirosis compared to uninfected control patients [4]. A receiver-operating characteristic curve analysis accordingly showed an area under the curve of >0.9 for differentiation between the two groups. In addition, the study reported a correlation of MIF levels with disease progression and severity.
Zhang et al. investigated the role of MIF in periodontitis in their study [5]. Assessing human immortalized oral epithelial cells, this study found that the secretion of MIF in the supernatant of Porphyromonas gingivalis-infected cells increased compared with that in uninfected control cells. Furthermore, MIF has been shown to be increased in both serum and gingival tissue of patients with periodontitis [5].
In their studies, Savva et al. and Kloek et al. showed the prognostic value of the identification of the genotype of MIF and the determination of MIF plasma levels, whereby high-expression MIF alleles as well as higher-plasma MIF concentrations were associated with a worsened clinical outcome in pneumococcal meningitis [6][7]. Neutralizing antibodies of MIF also improved bacterial clearance and reduced lethality in pneumococcal sepsis in mice [6]. A similar trend was observed in a study by Jose et al. on the role of MIF in Clostridium difficile infections [8]. Here, an improved outcome was seen in mice after the administration of anti-MIF antibodies compared to the use of control antibodies. The severity of symptoms was alleviated (shorter duration and less severe diarrhea) and mortality was lower after administration of anti-MIF antibodies.
These observations contrast with the role of MIF established in a study of Salmonella typhimurium infections [9]. Here, all MIF-deficient mice died within 25 days of per os infection. By comparison, 50% of control mice survived beyond 75 days. There was also increased bacterial growth in the visceral organs of the MIF-deficient mice. The levels of IFN-γ, IL-12, as well as TNF-α, were decreased in the post-infection phase in MIF-deficient mice compared with control mice. Thus, MIF can be considered a protective mediator of the immune response to Salmonella typhimurium.
Adamali et al. investigated the effect of tautomerase null gene MIF knock-in mice in Pseudomonas aeruginosa infections [10]. They found that tautomerase null mice had lower TNF-α and neutrophil levels and a lower bacterial load after infection.
Doroudian et al. investigated the effect of drug-loaded aerosol nanoparticles containing an MIF inhibitor on the inflammatory response against Pseudomonas aeruginosa [11]. The authors used SCD-19, a small molecule inhibitor of the tautomerase enzymatic activity of MIF, to limit MIF activity. MIF-deficient macrophages were found to exhibit enhanced killing of Pseudomonas aeruginosa compared with wild-type bone marrow-derived macrophages. The treatment of bone marrow-derived macrophages with SCD-19 resulted in enhanced bactericidal activity against Pseudomonas aeruginosa. Furthermore, adding recombinant MIF to human airway epithelial cells promoted biofilm formation in a laboratory strain of Pseudomonas aeruginosa, while SCD-19 inhibited attachment to epithelial cells. Thus, the study by Doroudian et al. demonstrates that aerosolized drug delivery systems targeting MIF are a potential treatment option for Pseudomonas aeruginosa infections and cystic fibrosis lung disease.
Consequently, the tautomerase activity of MIF and its ability to promote bacterial biofilm formation appear to be key aspects of Pseudomonas infections [12]. Overall, the role of MIF in bacterial infections is complex and depends on the type of bacteria and the stage of infection. Although they act as a protective factor in a variety pathogens, elevated MIF levels tend to worsen prognosis in pneumococcal pneumonia, for example. MIF variants have also been implicated in the pathogenesis of different bacteria. Other possible uses have been demonstrated beyond their potential therapeutic applications, such as in therapy monitoring and the prediction of clinical course.
2. Viral Infections
In addition to its role in the interactions in bacterial infections, MIF plays a key role in the immune system’s response to viral infections. MIF is known to play a role in several stages of viral pathogenesis, with different viral pathogens showing varying profiles of MIF.
In dengue virus infections, serum MIF levels have been shown to correlate with disease severity, with higher levels in patients who died of dengue hemorrhagic fever (DHF) than in patients who survived DHF and patients with mild dengue fever [13]. MIF-deficient mice presented with lower viremia, reduced proinflammatory cytokine levels, and delayed lethality compared with WT mice [14]. A 2018 study by Chen et al. investigated the role of macrophage migration inhibitory factor (MIF) in nonstructural protein 1 (NS1)-induced glycocalyx degradation during dengue virus infection [15]. The results of this study suggest that MIF is directly involved in NS1-induced glycocalyx degradation, and that targeting MIF may be a potential therapeutic strategy for the prevention of vascular leakage induced by the dengue virus. In a 2020 review, Lai et al. outlined the three major pathogenic roles of MIF in dengue virus infection [16]: the facilitation of virus replication, a contribution to vascular leakage, and the modulation of immune cell function.
In a 2018 study, Trifone et al. presented the effect of MIF in monocyte-derived macrophages (MDMs) infected with human immunodeficiency virus type I (HIV) [17]. The stimulation of HIV-infected MDMs with MIF increased the induction of IL-1β, IL-6, IL-8, TNF-α, and sICAM. This effect was reversed with the application of a CD74-blocking antibody, except for in the case of sICAM. In addition to the increased production of cytokines, the predisposition of unactivated CD4+ T cells to HIV infection was shown. Thus, the interaction between MIF and CD74 could play a role in viral dissemination and reservoir seeding of HIV [17]. In 2022, Trifone et al. further demonstrated that MIF stimulation in HIV infection leads to an increased Th17-like cell profile and thus a permissive cell type toward HIV-1 [18].
In a study of verruca vulgaris, MIF levels of biopsies were compared between patients with common warts caused by human papillomavirus and a healthy control group [19]. One lesional and one perilesional biopsy were taken in the case group, and these were in turn extracted from the same sites in the control group. The MIF levels of the biopsies were increased in the case group compared with the control group. However, no significant difference was found between lesional and perilesional biopsies. Therefore, it can be asserted that MIF plays a role in the development of HPV-infected cells into common warts [19].
Smith et al. showed in their study that MIF-deficient mice have a reduced viral load, less inflammation, and lower mortality from influenza A virus (IAV) compared to WT mice [20]. Treatment with an anti-MIF antibody improved survival after IAV infection. In comparison, de Souza et al. demonstrated in a study that MIF plays an important role in viral clearance in respiratory syncytial virus (RSV) infections and that the inhibition of MIF in mice macrophages is associated with an increase in viral load [21]. Interestingly, according to the current state of the literature, this effect appears to be unique in the field of viral infections. Furthermore, in this study, it could be demonstrated that the induction of MIF expression by RSV is dependent on the generation of reactive oxygen species (ROS) through the activation of NADPH oxidase, similar to the mechanism observed for T. gondii-triggered MIF expression [21][22].
Recently, Dheir et al. presented a study evaluating the prognostic significance of MIF in pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [23]. On average, MIF levels were found to be elevated in patients in an intensive care unit (ICU) compared with patients in a normal unit. However, the discriminatory power of MIF between ICU and normal ward patients in this study was lower than that of other blood biomarkers, such as D-Dimer, Troponin or Ferritin. Aksakal et al. also reported the diagnostic and prognostic value of MIF in SARS-CoV-2 infections [24]. Serum MIF levels were higher in infected patients compared with the control group, and serum MIF levels were higher in severely than moderately ill patients in the study.
3. Fungal Infections
MIF has also been shown to be involved in fungal infections. In their study, Stojanovic et al. demonstrated the role of MIF in the infection with Aspergillus fumigatus [25]. MIF-deficient mice showed a comparatively higher mortality when infected. Neutralization of MIF in WT mice with a specific inhibitor also negatively affected survival. While IFN-γ and IL-17 increased in WT mice during infection, they remained unchanged in MIF-deficient mice post-infection. An opposite tendency was observed in the secretion of IL-4, which was increased in MIF-deficient mice after infection compared to WT mice.
In their study, Mirkov et al. demonstrated that MIF acts as a resistance factor in sublethal infection with Aspergillus fumigatus and promotes the clearance of fungi in visceral organs and the brain [26].
Nicolo et al. demonstrated in their research that in mice infected with Candida albicans, the pro-inflammatory immune response was lower after an injection of anti-MIF IgG than after the administration of control IgG [27]. In addition, the mortality rate was increased in mice receiving anti-MIF IgG.
Xu et al. addressed the role of MIF in fungal keratitis in their study [28]. They showed that MIF as well as TNF-α and IL-6 are increased by Aspergillus fumigatus. Using a specific inhibitor of MIF, TNF-α and IL-6 were reduced, as was the proinflammatory response. MIF deficiency was found to be a protective factor in the study.
Overall, while MIF has been shown to play a complex role in fungal infections, the exact mechanism of action at work and the extent of the MIF contribution to the infection remain under investigation. Overall, additional research is needed to fully understand its role in fungal infections.
4. Parasitic Infections
In a study, Flores et al. investigated the role of MIF in the immune response against Toxoplasma gondii [29]. The authors demonstrated that MIF played a protective role. Conversely, MIF-deficient mice produced fewer proinflammatory cytokines, had more severe organ damage, and succumbed more rapidly to infection than WT mice.
An opposite trend was observed in a study on Plasmodium yoelii infections [30]. In this research, MIF-deficient mice showed lower parasitemia and delayed host mortality. Seven days post-Plasmodium yoelii 17XL infection, higher levels of IL-4, IL-10, IL-12, and IL-17 were observed in MIF-deficient mice than WT mice. In contrast, IFN-γ production was higher in WT mice.
A similar tendency was seen in the immune response to Nippostrongylus brasiliensis [31]. Here, MIF-deficient mice exhibited a lower parasite load as well as lower IL-6 induction seven days after infection than WT specimens. The mesenteric lymph nodes of MIF-deficient mice also showed higher GATA3 expression seven days post-infection. GATA3 is a Th2 transcription factor that regulates the development, maintenance, and proliferation of T cells and controls innate lymphoid cells [32]. WT mice treated with an MIF tautomerase inhibitor showed enhanced parasite clearance [31].
Certain protozoan parasites such as Entamoeba, Toxoplasma, and Plasmodium secrete an MIF cytokine homolog as a virulence factor that leads to host damage and exacerbates the disease [33][34][35][36][37][38][39][40][41][42]. In their study, Chen et al. investigated the effect of MIF in Trichomonas vaginalis under nutrient stress. A homologous MIF protein of Trichomonas vaginalis (TvMIF) was shown to be a survival factor during nutrient stress, and the overexpression of MIF as well as addition of recombinant human macrophage migration inhibitory factor increased parasite survival [43]. Furthermore, serum starvation was shown to increase the secretion of TvMIF, and the presence of TvMIF in serum starvation inhibited apoptosis through ROS suppression. Moreover, the gene knockout of TvMIF was shown to negatively affect parasite survival.
Liu et al. demonstrated the protective effect of the recombinant Toxoplasma gondii MIF protein vaccine (rTgMIF) after infection with Toxoplasma gondii in mice [44]. Immunization resulted in an increase in IFN-γ levels and a slight increase in IL-4 levels, indicating a shift toward a Th1-type immune response. Mice immunized with rTgMIF had significantly prolonged survival compared to controls in cases of acute infection with Toxoplasma gondii and significantly fewer brain cysts in instances of chronic infection with Toxoplasma gondii. rTgMIF could therefore be a candidate for vaccination and protection against Toxoplasma gondii [44].
In their study, Ghosh et al. demonstrated that mice treated with metronidazole and anti-Entamoeba histolytica MIF antibodies exhibited reduced tissue damage and intestinal inflammation when infected with Entamoeba histolytica than mice treated with metronidazole alone [33]. The anti-parasite MIF-blocking antibodies did not cross-react with human MIF in the study, and therefore specific anti-parasite MIF-blocking antibodies may offer therapeutic benefits in the future, although additional research is needed [33]. An overview of the possible options of MIF-targeting drugs is presented in Figure 1.
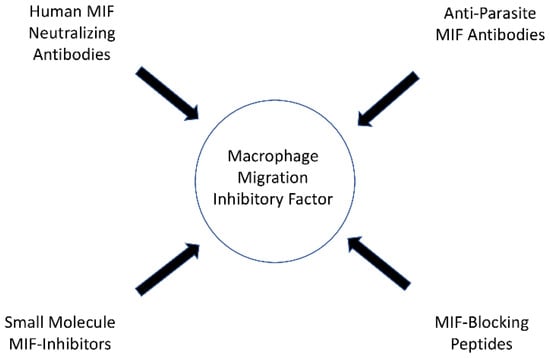
Figure 1. Possible options of MIF targeting drugs.
5. Sepsis
The pathogenesis of sepsis is complex and involves the dysregulation of the immune response, leading to widespread inflammation and tissue damage. Bacterial lipopolysaccharide (LPS) is a component of the outer membrane in Gram-negative bacteria and is the most potent microbial mediator in the pathogenesis of sepsis [45][46]. In 1993, Bernhagen et al. showed that MIF is released by anterior pituitary cells in response to LPS, thereby indicating the possibility of MIF serving as a biomarker in sepsis [47][48]. Cecal ligation and puncture (CLP) is the most commonly used model to study sepsis [49]. Escherichia coli represents the most common Gram-negative pathogen of sepsis [50][51]. Using two models in mice, one triggered after an intraperitoneal injection of Escherichia coli and the other after CLP, Calandra et al. in 2000 reported an increase in MIF. This was found to occur first in the peritoneal cavity, and subsequently in the systemic circulation [50]. A protective effect of anti-MIF antibodies was also found in both models. However, the injection of exogenous MIF with Escherichia coli increased mortality compared to the injection of Escherichia coli alone.
From this point, several studies have been conducted on the role of MIF in sepsis [52][53][54][55][56][57][58][59]. In their study, Tilstam et al. found an increase in MIF levels after CLP [52]. MIF-deficient mice were also found to have delayed lethality and a threefold higher survival compared to WT mice. MIF-deficient mice exhibited reduced hypothermia, lower plasma creatine kinase levels, and lower plasma levels of several inflammatory cytokines (TNF- α, IL1-α, and IL1-β) after CLP treatment, while IL-10 levels were increased in the cases of peritoneal lavage. Furthermore, the study showed an association between a reduced number of small peritoneal macrophages (SPM) and survival after CLP. The number of SPM was in turn decreased in MIF-deficient mice, and the transference of SPM to MIF-deficient mice had a negative effect on survival [52].
In their study, Tohyama et al. showed that protection and thus improved survival in mice against sepsis can be achieved by the use of autoantibodies against MIF induced by a DNA vaccination [60]. The research of Al-Abed et al. showed that an inhibitor of MIF tautomerase improved survival in sepsis in mice [61]. Therefore, it can be concluded that MIF has emerged as a potential biomarker and therapeutic target in sepsis. Research has shown that MIF levels are significantly elevated in patients with sepsis and that higher levels are associated with worse clinical outcomes. Further research is needed to fully understand the role of MIF in sepsis and to develop effective treatments for this life-threatening condition.
6. D-Dopachrome Tautomerase
Besides MIF, D-dopachrome tautomerase also represents a cytokine that plays a key role in the host immune response [62]. D-DT gene is also located on chromosome 22q11.2. D-DT is a homolog of MIF, has a relatively similar overall structure and is also called MIF-2 [62][63][64]. Compared to MIF, D-DT expression was demonstrated in fewer localizations, which included cardiomyocytes, hepatocytes, and dendritic cells as well as the bronchial and intestinal epithelium [65]. D-DT differs structurally from MIF by lacking the pseudo (E)LR domain essential for MIF’s chemokine function and the CXXC redox motif found in MIF [66]. While there is limited research on the regulation of D-DT transcription, one study has shown that its upregulation under hypoxia is dependent on a specific hypoxia-responsive element [65][67]. The amino acid similarity compared to MIF is limited, and D-DT is not as well understood in terms of its function as its more famous family member [63]. In addition to CD74, D-DT can also interact with CXCR7 [63]. MIF and D-DT do have some common catalytic and immunological functions—they trigger pro-inflammatory cascades via ERK-1/2-MAP kinases, and they counteract the anti-inflammatory effect of glucocorticoids [66]. There are also additive effects between MIF and D-DT, such as in the recruitment of neutrophils to the lungs [68]. In contrast, MIF and DDT play a different role in physiological processes such as adipogenesis and correlate differently with obesity [66]. D-DT has so far shown interesting results when applied in studies on spinal cord injury, amyotrophic lateral sclerosis, multiple sclerosis, tumors, skin diseases, heart failure, myocardial infarction, alveolar repair, orthotopic liver transplantation, and sepsis [52][64][69][70][71][72][73][74][75][76][77][78].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines12010002
References
- Oddo, M.; Calandra, T.; Bucala, R.; Meylan, P.R.A. Macrophage Migration Inhibitory Factor Reduces the Growth of Virulent Mycobacterium Tuberculosis in Human Macrophages. Infect. Immun. 2005, 73, 3783–3786.
- Das, R.; Koo, M.-S.; Kim, B.H.; Jacob, S.T.; Subbian, S.; Yao, J.; Leng, L.; Levy, R.; Murchison, C.; Burman, W.J.; et al. Macrophage Migration Inhibitory Factor (MIF) Is a Critical Mediator of the Innate Immune Response to Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, E2997–E3006.
- Shang, Z.B.; Wang, J.; Kuai, S.G.; Zhang, Y.Y.; Ou, Q.F.; Pei, H.; Huang, L.H. Serum Macrophage Migration Inhibitory Factor as a Biomarker of Active Pulmonary Tuberculosis. Ann. Lab. Med. 2018, 38, 9–16.
- Sumaiya, K.; Akino Mercy, C.S.; Muralitharan, G.; Hajinur Hirad, A.; Alarfaj, A.A.; Natarajaseenivasan, K. Assessment of Serum Macrophage Migration Inhibitory Factor (MIF) as an Early Diagnostic Marker of Leptospirosis. Front. Cell Infect. Microbiol. 2021, 11, 781476.
- Zhang, D.; Xu, T.; Xu, Q.; Dong, Q.; Luo, Y.; Gao, L.; Pan, Y. Expression Profile of Macrophage Migration Inhibitory Factor in Periodontitis. Arch. Oral. Biol. 2021, 122, 105003.
- Savva, A.; Brouwer, M.C.; Roger, T.; Valls Serón, M.; Le Roy, D.; Ferwerda, B.; van der Ende, A.; Bochud, P.-Y.; van de Beek, D.; Calandra, T. Functional Polymorphisms of Macrophage Migration Inhibitory Factor as Predictors of Morbidity and Mortality of Pneumococcal Meningitis. Proc. Natl. Acad. Sci. USA 2016, 113, 3597–3602.
- Kloek, A.T.; Seron, M.V.; Schmand, B.; Tanck, M.W.T.; van der Ende, A.; Brouwer, M.C.; van de Beek, D. Individual Responsiveness of Macrophage Migration Inhibitory Factor Predicts Long-Term Cognitive Impairment after Bacterial Meningitis. Acta Neuropathol. Commun. 2021, 9, 4.
- Jose, S.; Mukherjee, A.; Abhyankar, M.M.; Leng, L.; Bucala, R.; Sharma, D.; Madan, R. Neutralization of Macrophage Migration Inhibitory Factor Improves Host Survival after Clostridium Difficile Infection. Anaerobe 2018, 53, 56–63.
- Koebernick, H.; Grode, L.; David, J.R.; Rohde, W.; Rolph, M.S.; Mittrücker, H.-W.; Kaufmann, S.H.E. Macrophage Migration Inhibitory Factor (MIF) Plays a Pivotal Role in Immunity against Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 2002, 99, 13681–13686.
- Adamali, H.; Armstrong, M.E.; McLaughlin, A.M.; Cooke, G.; McKone, E.; Costello, C.M.; Gallagher, C.G.; Leng, L.; Baugh, J.A.; Fingerle-Rowson, G.; et al. Macrophage Migration Inhibitory Factor Enzymatic Activity, Lung Inflammation, and Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 162–169.
- Doroudian, M.; O’Neill, A.; O’Reilly, C.; Tynan, A.; Mawhinney, L.; McElroy, A.; Webster, S.S.; MacLoughlin, R.; Volkov, Y.; E Armstrong, M.; et al. Aerosolized Drug-Loaded Nanoparticles Targeting Migration Inhibitory Factors Inhibit Pseudomonas Aeruginosa-Induced Inflammation and Biofilm Formation. Nanomedicine 2020, 15, 2933–2953.
- Panstruga, R.; Donnelly, S.C.; Bernhagen, J. A Cross-Kingdom View on the Immunomodulatory Role of MIF/D-DT Proteins in Mammalian and Plant Pseudomonas Infections. Immunology 2022, 166, 287–298.
- Chen, L.-C.; Lei, H.-Y.; Liu, C.-C.; Shiesh, S.-C.; Chen, S.-H.; Liu, H.-S.; Lin, Y.-S.; Wang, S.-T.; Shyu, H.-W.; Yeh, T.-M. Correlation of Serum Levels of Macrophage Migration Inhibitory Factor with Disease Severity and Clinical Outcome in Dengue Patients. Am. J. Trop. Med. Hyg. 2006, 74, 142–147.
- Assunção-Miranda, I.; Amaral, F.A.; Bozza, F.A.; Fagundes, C.T.; Sousa, L.P.; Souza, D.G.; Pacheco, P.; Barbosa-Lima, G.; Gomes, R.N.; Bozza, P.T.; et al. Contribution of Macrophage Migration Inhibitory Factor to the Pathogenesis of Dengue Virus Infection. FASEB J. 2010, 24, 218–228.
- Chen, H.-R.; Chao, C.-H.; Liu, C.-C.; Ho, T.-S.; Tsai, H.-P.; Perng, G.-C.; Lin, Y.-S.; Wang, J.-R.; Yeh, T.-M. Macrophage Migration Inhibitory Factor Is Critical for Dengue NS1-Induced Endothelial Glycocalyx Degradation and Hyperpermeability. PLoS Pathog. 2018, 14, e1007033.
- Lai, Y.-C.; Chao, C.-H.; Yeh, T.-M. Roles of Macrophage Migration Inhibitory Factor in Dengue Pathogenesis: From Pathogenic Factor to Therapeutic Target. Microorganisms 2020, 8, 891.
- Trifone, C.; Salido, J.; Ruiz, M.J.; Leng, L.; Quiroga, M.F.; Salomón, H.; Bucala, R.; Ghiglione, Y.; Turk, G. Interaction Between Macrophage Migration Inhibitory Factor and CD74 in Human Immunodeficiency Virus Type I Infected Primary Monocyte-Derived Macrophages Triggers the Production of Proinflammatory Mediators and Enhances Infection of Unactivated CD4+ T Cells. Front. Immunol. 2018, 9, 1494.
- Trifone, C.; Baquero, L.; Czernikier, A.; Benencio, P.; Leng, L.; Laufer, N.; Quiroga, M.F.; Bucala, R.; Ghiglione, Y.; Turk, G. Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection. Viruses 2022, 14, 2218.
- Sorour, N.E.; Hamed, A.M.; Tabl, H.A.-E.M.; Ahmed, A.A.-E.A. Assessment of Macrophage Migration Inhibitory Factor in Patients with Verruca Vulgaris. Clin. Cosmet. Investig. Dermatol. 2019, 12, 591–595.
- Smith, C.A.; Tyrell, D.J.; Kulkarni, U.A.; Wood, S.; Leng, L.; Zemans, R.L.; Bucala, R.; Goldstein, D.R. Macrophage Migration Inhibitory Factor Enhances Influenza-Associated Mortality in Mice. JCI Insight 2019, 4, e128034.
- de Souza, G.F.; Muraro, S.P.; Santos, L.D.; Monteiro, A.P.T.; da Silva, A.G.; de Souza, A.P.D.; Stein, R.T.; Bozza, P.T.; Porto, B.N. Macrophage Migration Inhibitory Factor (MIF) Controls Cytokine Release during Respiratory Syncytial Virus Infection in Macrophages. Inflamm. Res. 2019, 68, 481–491.
- Kim, J.H.; Lee, J.; Bae, S.-J.; Kim, Y.; Park, B.-J.; Choi, J.-W.; Kwon, J.; Cha, G.-H.; Yoo, H.J.; Jo, E.-K.; et al. NADPH Oxidase 4 Is Required for the Generation of Macrophage Migration Inhibitory Factor and Host Defense against Toxoplasma Gondii Infection. Sci. Rep. 2017, 7, 6361.
- Dheir, H.; Yaylaci, S.; Sipahi, S.; Genc, A.C.; Cekic, D.; Tuncer, F.B.; Cokluk, E.; Kocayigit, H.; Genc, A.B.; Salihi, S.; et al. Does Macrophage Migration Inhibitory Factor Predict the Prognosis of COVID-19 Disease? J. Infect. Dev. Ctries. 2021, 15, 398–403.
- Aksakal, A.; Kerget, B.; Kerget, F.; Aşkın, S. Evaluation of the Relationship between Macrophage Migration Inhibitory Factor Level and Clinical Course in Patients with COVID-19 Pneumonia. J. Med. Virol. 2021, 93, 6519–6524.
- Stojanovic, I.; Mirkov, I.; Kataranovski, M.; Glamoclija, J.; Stosic-Grujicic, S. A Role for Macrophage Migration Inhibitory Factor in Protective Immunity against Aspergillus Fumigatus. Immunobiology 2011, 216, 1018–1027.
- Mirkov, I.; Belij, S.; Kataranovski, M.; Zolotarevski, L.; Glamoclija, J.; Stojanovic, I.; Stosic-Grujicic, S. The Relevance of the Migration Inhibitory Factor (MIF) for Peripheral Tissue Response in Murine Sublethal Systemic Aspergillus Fumigatus Infection. Med. Mycol. 2012, 50, 476–487.
- Nicolo, C.; Le Roy, D.; Reymond, M.K.; Roger, T.; Calandra, T. Macrophage Migration Inhibitory Factor Plays an Important Role in the Host Innate Immune Defenses against Candida Infection. Int. J. Infect. Dis. 2006, 10, S46–S47.
- Xu, Q.; Hu, L.-T.; Wang, Q.; Lin, J.; Jiang, N.; Li, C.; Zhao, G.-Q. Expression of Macrophage Migration Inhibitory Factor in Aspergillus Fumigatus Keratitis. Int. J. Ophthalmol. 2019, 12, 711–716.
- Flores, M.; Saavedra, R.; Bautista, R.; Viedma, R.; Tenorio, E.P.; Leng, L.; Sánchez, Y.; Juárez, I.; Satoskar, A.A.; Shenoy, A.S.; et al. Macrophage Migration Inhibitory Factor (MIF) Is Critical for the Host Resistance against Toxoplasma Gondii. FASEB J. 2008, 22, 3661–3671.
- Salazar-Castañón, V.H.; Juárez-Avelar, I.; Legorreta-Herrera, M.; Rodriguez-Sosa, M. Macrophage Migration Inhibitory Factor Contributes to Immunopathogenesis during Plasmodium Yoelii 17XL Infection. Front. Cell Infect. Microbiol. 2022, 12, 968422.
- Damle, S.R.; Martin, R.K.; Cross, J.V.; Conrad, D.H. Macrophage Migration Inhibitory Factor Deficiency Enhances Immune Response to Nippostrongylus Brasiliensis. Mucosal Immunol. 2017, 10, 205–214.
- Wan, Y.Y. GATA3: A Master of Many Trades in Immune Regulation. Trends Immunol. 2014, 35, 233–242.
- Ghosh, S.; Padalia, J.; Ngobeni, R.; Abendroth, J.; Farr, L.; Shirley, D.-A.; Edwards, T.; Moonah, S. Targeting Parasite-Produced Macrophage Migration Inhibitory Factor as an Antivirulence Strategy With Antibiotic-Antibody Combination to Reduce Tissue Damage. J. Infect. Dis. 2020, 221, 1185–1193.
- Sun, T.; Holowka, T.; Song, Y.; Zierow, S.; Leng, L.; Chen, Y.; Xiong, H.; Griffith, J.; Nouraie, M.; Thuma, P.E.; et al. A Plasmodium-Encoded Cytokine Suppresses T-Cell Immunity during Malaria. Proc. Natl. Acad. Sci. USA 2012, 109, E2117–E2126.
- Baeza Garcia, A.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.J.; Ulmer, J.B.; et al. Neutralization of the Plasmodium-Encoded MIF Ortholog Confers Protective Immunity against Malaria Infection. Nat. Commun. 2018, 9, 2714.
- Moonah, S.N.; Abhyankar, M.M.; Haque, R.; Petri, W.A. The Macrophage Migration Inhibitory Factor Homolog of Entamoeba Histolytica Binds to and Immunomodulates Host Macrophages. Infect. Immun. 2014, 82, 3523–3530.
- Ngobeni, R.; Abhyankar, M.M.; Jiang, N.M.; Farr, L.A.; Samie, A.; Haque, R.; Moonah, S.N. Entamoeba Histolytica-Encoded Homolog of Macrophage Migration Inhibitory Factor Contributes to Mucosal Inflammation during Amebic Colitis. J. Infect. Dis. 2017, 215, 1294–1302.
- Ghosh, S.; Leaton, L.A.; Farr, L.; Barfield, A.; Moonah, S. Interaction between Parasite-Encoded JAB1/CSN5 and Macrophage Migration Inhibitory Factor Proteins Attenuates Its Proinflammatory Function. Sci. Rep. 2018, 8, 10241.
- Twu, O.; Dessí, D.; Vu, A.; Mercer, F.; Stevens, G.C.; de Miguel, N.; Rappelli, P.; Cocco, A.R.; Clubb, R.T.; Fiori, P.L.; et al. Trichomonas Vaginalis Homolog of Macrophage Migration Inhibitory Factor Induces Prostate Cell Growth, Invasiveness, and Inflammatory Responses. Proc. Natl. Acad. Sci. USA 2014, 111, 8179–8184.
- Sommerville, C.; Richardson, J.M.; Williams, R.A.M.; Mottram, J.C.; Roberts, C.W.; Alexander, J.; Henriquez, F.L. Biochemical and Immunological Characterization of Toxoplasma Gondii Macrophage Migration Inhibitory Factor. J. Biol. Chem. 2013, 288, 12733–12741.
- Holowka, T.; Castilho, T.M.; Garcia, A.B.; Sun, T.; McMahon-Pratt, D.; Bucala, R. Leishmania-Encoded Orthologs of Macrophage Migration Inhibitory Factor Regulate Host Immunity to Promote Parasite Persistence. FASEB J. 2016, 30, 2249–2265.
- Buchko, G.W.; Abendroth, J.; Robinson, H.; Zhang, Y.; Hewitt, S.N.; Edwards, T.E.; Van Voorhis, W.C.; Myler, P.J. Crystal Structure of a Macrophage Migration Inhibitory Factor from Giardia Lamblia. J. Struct. Funct. Genomics 2013, 14, 47–57.
- Chen, Y.-P.; Twu, O.; Johnson, P.J. Trichomonas Vaginalis Macrophage Migration Inhibitory Factor Mediates Parasite Survival during Nutrient Stress. mBio 2018, 9, e00910-18.
- Liu, K.; Wen, H.; Cai, H.; Wu, M.; An, R.; Chu, D.; Yu, L.; Shen, J.; Chen, L.; Du, J. Protective Effect Against Toxoplasmosis in BALB/c Mice Vaccinated With Toxoplasma Gondii Macrophage Migration Inhibitory Factor. Front. Microbiol. 2019, 10, 813.
- Gabarin, R.S.; Li, M.; Zimmel, P.A.; Marshall, J.C.; Li, Y.; Zhang, H. Intracellular and Extracellular Lipopolysaccharide Signaling in Sepsis: Avenues for Novel Therapeutic Strategies. J. Innate Immun. 2021, 13, 323–332.
- Opal, S.M. Endotoxins and Other Sepsis Triggers. Contrib. Nephrol. 2010, 167, 14–24.
- Bernhagen, J.; Calandra, T.; Mitchell, R.A.; Martin, S.B.; Tracey, K.J.; Voelter, W.; Manogue, K.R.; Cerami, A.; Bucala, R. MIF Is a Pituitary-Derived Cytokine That Potentiates Lethal Endotoxaemia. Nature 1993, 365, 756–759.
- Grieb, G.; Merk, M.; Bernhagen, J.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF): A Promising Biomarker. Drug News Perspect. 2010, 23, 257–264.
- Toscano, M.G.; Ganea, D.; Gamero, A.M. Cecal Ligation Puncture Procedure. J. Vis. Exp. 2011, 51, 2860.
- Calandra, T.; Echtenacher, B.; Roy, D.L.; Pugin, J.; Metz, C.N.; Hültner, L.; Heumann, D.; Männel, D.; Bucala, R.; Glauser, M.P. Protection from Septic Shock by Neutralization of Macrophage Migration Inhibitory Factor. Nat. Med. 2000, 6, 164–170.
- Cohen, J.; Abraham, E. Microbiologic Findings and Correlations with Serum Tumor Necrosis Factor-Alpha in Patients with Severe Sepsis and Septic Shock. J. Infect. Dis. 1999, 180, 116–121.
- Tilstam, P.V.; Schulte, W.; Holowka, T.; Kim, B.-S.; Nouws, J.; Sauler, M.; Piecychna, M.; Pantouris, G.; Lolis, E.; Leng, L.; et al. MIF but Not MIF-2 Recruits Inflammatory Macrophages in an Experimental Polymicrobial Sepsis Model. J. Clin. Investig. 2021, 131, e127171.
- Beishuizen, A.; Thijs, L.G.; Haanen, C.; Vermes, I. Macrophage Migration Inhibitory Factor and Hypothalamo-Pituitary-Adrenal Function during Critical Illness. J. Clin. Endocrinol. Metab. 2001, 86, 2811–2816.
- Bozza, F.A.; Gomes, R.N.; Japiassú, A.M.; Soares, M.; Castro-Faria-Neto, H.C.; Bozza, P.T.; Bozza, M.T. Macrophage Migration Inhibitory Factor Levels Correlate with Fatal Outcome in Sepsis. Shock. 2004, 22, 309–313.
- Emonts, M.; Sweep, F.C.G.J.; Grebenchtchikov, N.; Geurts-Moespot, A.; Knaup, M.; Chanson, A.L.; Erard, V.; Renner, P.; Hermans, P.W.M.; Hazelzet, J.A.; et al. Association between High Levels of Blood Macrophage Migration Inhibitory Factor, Inappropriate Adrenal Response, and Early Death in Patients with Severe Sepsis. Clin. Infect. Dis. 2007, 44, 1321–1328.
- Gao, L.; Flores, C.; Fan-Ma, S.; Miller, E.J.; Moitra, J.; Moreno, L.; Wadgaonkar, R.; Simon, B.; Brower, R.; Sevransky, J.; et al. Macrophage Migration Inhibitory Factor in Acute Lung Injury: Expression, Biomarker, and Associations. Transl. Res. 2007, 150, 18–29.
- Payen, D.; Lukaszewicz, A.-C.; Legrand, M.; Gayat, E.; Faivre, V.; Megarbane, B.; Azoulay, E.; Fieux, F.; Charron, D.; Loiseau, P.; et al. A Multicentre Study of Acute Kidney Injury in Severe Sepsis and Septic Shock: Association with Inflammatory Phenotype and HLA Genotype. PLoS ONE 2012, 7, e35838.
- Miyauchi, T.; Tsuruta, R.; Fujita, M.; Kaneko, T.; Kasaoka, S.; Maekawa, T. Serum Macrophage Migration Inhibitory Factor Reflects Adrenal Function in the Hypothalamo-Pituitary-Adrenal Axis of Septic Patients: An Observational Study. BMC Infect. Dis. 2009, 9, 209.
- Lehmann, L.E.; Novender, U.; Schroeder, S.; Pietsch, T.; von Spiegel, T.; Putensen, C.; Hoeft, A.; Stüber, F. Plasma Levels of Macrophage Migration Inhibitory Factor Are Elevated in Patients with Severe Sepsis. Intensive Care Med. 2001, 27, 1412–1415.
- Tohyama, S.; Onodera, S.; Tohyama, H.; Yasuda, K.; Nishihira, J.; Mizue, Y.; Hamasaka, A.; Abe, R.; Koyama, Y. A Novel DNA Vaccine-Targeting Macrophage Migration Inhibitory Factor Improves the Survival of Mice with Sepsis. Gene Ther. 2008, 15, 1513–1522.
- Al-Abed, Y.; Dabideen, D.; Aljabari, B.; Valster, A.; Messmer, D.; Ochani, M.; Tanovic, M.; Ochani, K.; Bacher, M.; Nicoletti, F.; et al. ISO-1 Binding to the Tautomerase Active Site of MIF Inhibits Its pro-Inflammatory Activity and Increases Survival in Severe Sepsis. J. Biol. Chem. 2005, 280, 36541–36544.
- Zan, C.; Yang, B.; Brandhofer, M.; El Bounkari, O.; Bernhagen, J. D-Dopachrome Tautomerase in Cardiovascular and Inflammatory Diseases-A New Kid on the Block or Just Another MIF? FASEB J. 2022, 36, e22601.
- Song, S.; Xiao, Z.; Dekker, F.J.; Poelarends, G.J.; Melgert, B.N. Macrophage Migration Inhibitory Factor Family Proteins Are Multitasking Cytokines in Tissue Injury. Cell Mol. Life Sci. 2022, 79, 105.
- Li, H.; He, B.; Zhang, X.; Hao, H.; Yang, T.; Sun, C.; Song, H.; Wang, Y.; Zhou, Y.; Zhu, Z.; et al. D-Dopachrome Tautomerase Drives Astroglial Inflammation via NF-κB Signaling Following Spinal Cord Injury. Cell Biosci. 2022, 12, 128.
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving Complexity of MIF Signaling. Cell Signal 2019, 57, 76–88.
- Illescas, O.; Pacheco-Fernández, T.; Laclette, J.P.; Rodriguez, T.; Rodriguez-Sosa, M. Immune Modulation by the Macrophage Migration Inhibitory Factor (MIF) Family: D-Dopachrome Tautomerase (DDT) Is Not (Always) a Backup System. Cytokine 2020, 133, 155121.
- Pasupuleti, V.; Du, W.; Gupta, Y.; Yeh, I.-J.; Montano, M.; Magi-Galuzzi, C.; Welford, S.M. Dysregulated D-Dopachrome Tautomerase, a Hypoxia-Inducible Factor-Dependent Gene, Cooperates with Macrophage Migration Inhibitory Factor in Renal Tumorigenesis. J. Biol. Chem. 2014, 289, 3713–3723.
- Rajasekaran, D.; Zierow, S.; Syed, M.; Bucala, R.; Bhandari, V.; Lolis, E.J. Targeting Distinct Tautomerase Sites of D-DT and MIF with a Single Molecule for Inhibition of Neutrophil Lung Recruitment. FASEB J. 2014, 28, 4961–4971.
- Xiao, Z.; Osipyan, A.; Song, S.; Chen, D.; Schut, R.A.; van Merkerk, R.; van der Wouden, P.E.; Cool, R.H.; Quax, W.J.; Melgert, B.N.; et al. ThienoPyrimidine-2,4(1H,3H)-Dione Derivative Inhibits d-Dopachrome Tautomerase Activity and Suppresses the Proliferation of Non-Small Cell Lung Cancer Cells. J. Med. Chem. 2022, 65, 2059–2077.
- Caltabiano, R.; De Pasquale, R.; Piombino, E.; Campo, G.; Nicoletti, F.; Cavalli, E.; Mangano, K.; Fagone, P. Macrophage Migration Inhibitory Factor (MIF) and Its Homologue d-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus. Molecules 2021, 26, 184.
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mangano, K.; Di Marco, R.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Upregulated Expression of Macrophage Migration Inhibitory Factor, Its Analogue D-Dopachrome Tautomerase, and the CD44 Receptor in Peripheral CD4 T Cells from Clinically Isolated Syndrome Patients with Rapid Conversion to Clinical Defined Multiple Sclerosis. Medicina 2019, 55, 667.
- Yoshihisa, Y.; Rehman, M.U.; Andoh, T.; Tabuchi, Y.; Makino, T.; Shimizu, T. Overexpression of D-Dopachrome Tautomerase Increases Ultraviolet B Irradiation-Induced Skin Tumorigenesis in Mice. FASEB J. 2021, 35, e21671.
- Alaskarov, A.; Barel, S.; Bakavayev, S.; Kahn, J.; Israelson, A. MIF Homolog D-Dopachrome Tautomerase (D-DT/MIF-2) Does Not Inhibit Accumulation and Toxicity of Misfolded SOD1. Sci. Rep. 2022, 12, 9570.
- Ji, H.; Zhang, Y.; Chen, C.; Li, H.; He, B.; Yang, T.; Sun, C.; Hao, H.; Zhang, X.; Wang, Y.; et al. D-Dopachrome Tautomerase Activates COX2/PGE2 Pathway of Astrocytes to Mediate Inflammation Following Spinal Cord Injury. J. Neuroinflammation 2021, 18, 130.
- Ma, Y.; Su, K.N.; Pfau, D.; Rao, V.S.; Wu, X.; Hu, X.; Leng, L.; Du, X.; Piecychna, M.; Bedi, K.; et al. Cardiomyocyte D-Dopachrome Tautomerase Protects against Heart Failure. JCI Insight 2019, 4, e128900.
- Song, S.; Liu, B.; Habibie, H.; van den Bor, J.; Smit, M.J.; Gosens, R.; Wu, X.; Brandsma, C.-A.; Cool, R.H.; Haisma, H.J.; et al. D-Dopachrome Tautomerase Contributes to Lung Epithelial Repair via Atypical Chemokine Receptor 3-Dependent Akt Signaling. EBioMedicine 2021, 68, 103412.
- Baron-Stefaniak, J.; Schiefer, J.; Lichtenegger, P.; Miller, E.J.; Berlakovich, G.A.; Faybik, P.; Baron, D.M. D-Dopachrome Tautomerase Predicts Outcome but Not the Development of Acute Kidney Injury after Orthotopic Liver Transplantation. HPB 2019, 21, 465–472.
- Voss, S.; Krüger, S.; Scherschel, K.; Warnke, S.; Schwarzl, M.; Schrage, B.; Girdauskas, E.; Meyer, C.; Blankenberg, S.; Westermann, D.; et al. Macrophage Migration Inhibitory Factor (MIF) Expression Increases during Myocardial Infarction and Supports Pro-Inflammatory Signaling in Cardiac Fibroblasts. Biomolecules 2019, 9, 38.
This entry is offline, you can click here to edit this entry!
