Green extraction techniques represent a paradigm shift in the field of NP extraction, emphasizing sustainability, environmental consciousness, and efficiency. These methods prioritize minimizing the environmental impact associated with traditional extraction processes. Their importance lies in addressing the limitations of conventional techniques, such as low yields, substantial solvent usage, and the risk of degrading heat-sensitive compounds. The benefits of green extraction include enhanced efficiency, reduced solvent consumption, and the preservation of valuable bioactive compounds. Examples of green extraction techniques encompass “supercritical fluid extraction (SFE), subcritical water extraction (SWE), ultrasound-assisted extraction (UAE), and microwave-assisted extraction (MAE)”, among others. The significance and advantages of each method contribute to a more sustainable and eco-friendly approach to NP extraction. Detailed discussions on each green extraction technique are provided below, highlighting their unique principles, applications, and advantages in obtaining bioactive natural products.
- bioactive natural products
- natural products extraction
- green extraction
- extraction techniques
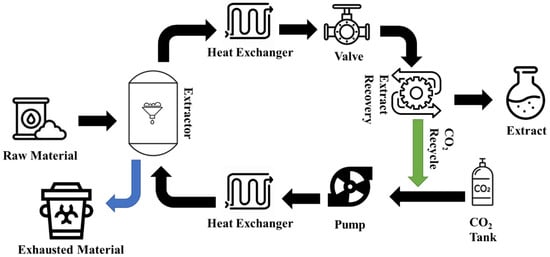
1. Supercritical Fluid Extraction (SFE)
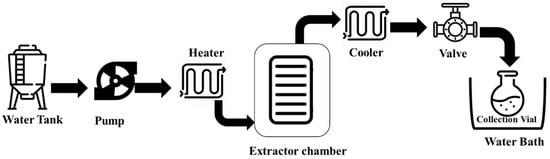
2. Subcritical Water Extraction (SWE)
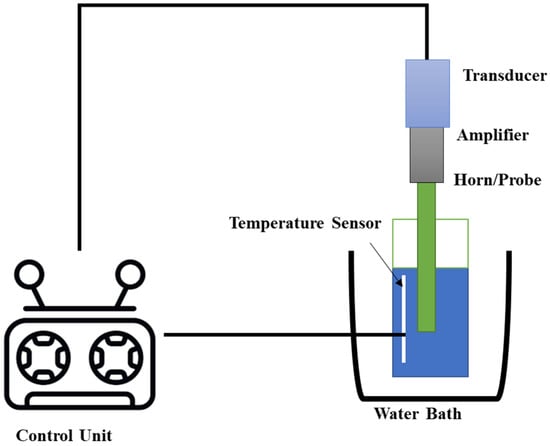
3. Ultrasound-Assisted Extraction (UAE)
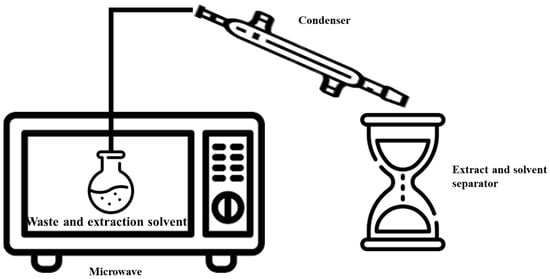
4. Microwave-Assisted Extraction (MAE)
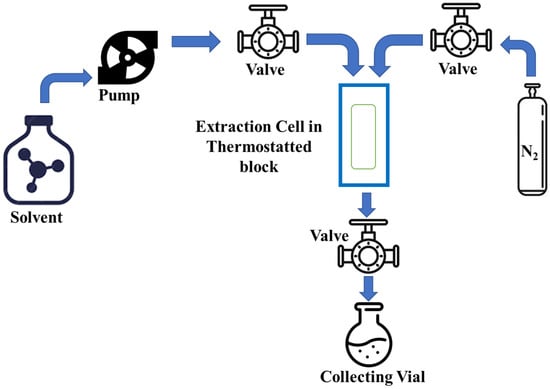
5. Pressurized Liquid Extraction (PLE)
6. Enzyme-Assisted Extraction (EAE)
This entry is adapted from the peer-reviewed paper 10.3390/pr11123444
References
- Easmin, S.; Sarker, Z.I.; Ferdosh, S.; Shamsudin, S.H.; Yunus, K.; Uddin, A.; Sarker, M.R.; Jahurul, M.H.A.; Hossain, S.; Khalil, H.A. Bioactive Compounds and Advanced Processing Technology: Phaleria macrocarpa (Sheff.) Boerl, a Review. J. Chem. Technol. Biotechnol. 2014, 90, 981–991.
- Aziz, A.H.A.; Idrus, N.F.M.; Putra, N.R.; Awang, M.A.; Idham, Z.; Mamat, H.; Yunus, M.A.C. Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves. ChemEngineering 2022, 6, 59.
- Argun, M.E.; Argun, M.Ş.; Arslan, F.N.; Nas, B.; Ateş, H.; Tongur, S.; Çakmakcı, Ö. Recovery of Valuable Compounds from Orange Processing Wastes Using Supercritical Carbon Dioxide Extraction. J. Clean. Prod. 2022, 375, 134169.
- Putra, N.R.; Yustisia, Y.; Heryanto, B.; Asmaliyah, A.; Miswarti, M.; Rizkiyah, D.N.; Yunus, M.A.C.; Irianto, I.; Qomariyah, L.; Rohman, G.A.N. Advancements and Challenges in Green Extraction Techniques for Indonesian Natural Products: A Review. S. Afr. J. Chem. Eng. 2023, 46, 88–98.
- King, J.W. Modern Supercritical Fluid Technology for Food Applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Khan, M.S.; Mohamed, A.A.A.; Ferdosh, S.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436.
- Vági, E.; Balázs, M.; Komóczi, A.; Mihalovits, M.; Székely, E. Fractionation of Phytocannabinoids from Industrial Hemp Residues with High-Pressure Technologies. J. Supercrit. Fluids 2020, 164, 104898.
- Goyeneche, R.; Fanovich, A.; Rodrígues, C.R.; Nicolao, M.C.; Di Scala, K. Supercritical CO2 Extraction of Bioactive Compounds from Radish Leaves: Yield, Antioxidant Capacity and Cytotoxicity. J. Supercrit. Fluids 2018, 135, 78–83.
- Uquiche, E.; Campos, C.J.R.; Marillán, C. Assessment of the Bioactive Capacity of Extracts from Leptocarpha Rivularis Stalks Using Ethanol-Modified Supercritical CO2. J. Supercrit. Fluids 2019, 147, 1–8.
- Hassim, N.; Markom, M.; Rosli, M.I.; Harun, S. Scale-up Approach for Supercritical Fluid Extraction with Ethanol–Water Modified Carbon Dioxide on Phyllanthus Niruri for Safe Enriched Herbal Extracts. Sci. Rep. 2021, 11, 15818.
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; Da Silva, C. Extraction of Macauba Kernel Oil Using Supercritical Carbon Dioxide and Compressed Propane. Can. J. Chem. Eng. 2018, 97, 785–792.
- Bitwell, C.; Sen, S.I.; Chimuka, L.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585.
- Todd, R.I.; Baroutian, S. A Techno-Economic Comparison of Subcritical Water, Supercritical CO2 and Organic Solvent Extraction of Bioactives from Grape Marc. J. Clean. Prod. 2017, 158, 349–358.
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant Capacity of Bioactives Extracted from Canola Meal by Subcritical Water, Ethanolic and Hot Water Extraction. Food Chem. 2009, 114, 717–726.
- Kim, W.J.; Kim, J.-H.; Veriansyah, B.; Kim, J.D.; Lee, Y.W.; Oh, S.; Tjandrawinata, R.R. Extraction of Bioactive Components from Centella asiatica Using Subcritical Water. J. Supercrit. Fluids 2009, 48, 211–216.
- Zaibunnisa, A.H.; Saim, N.; Said, M.; Osman, H. An Experimental Design Approach for the Extraction of Volatile Compounds from Turmeric Leaves (Curcuma domestica) Using Pressurised Liquid Extraction (PLE). LWT 2009, 42, 233–238.
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34.
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of Polyphenols from Grape Skins and Defatted Grape Seeds Using Subcritical Water: Experiments and Modeling. Food Bioprod. Process. 2015, 94, 29–38.
- Ong, E.S.; Cheong, J.S.H.; Goh, D. Pressurized Hot Water Extraction of Bioactive or Marker Compounds in Botanicals and Medicinal Plant Materials. J. Chromatogr. A 2006, 1112, 92–102.
- Kovačević, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović–Uzelac, V. Pressurized Hot Water Extraction (PHWE) for the Green Recovery of Bioactive Compounds and Steviol Glycosides from Stevia Rebaudiana Bertoni Leaves. Food Chem. 2018, 254, 150–157.
- Essien, S.; Young, B.R.; Baroutian, S. Subcritical Water Extraction for Selective Recovery of Phenolic Bioactives from Kānuka Leaves. J. Supercrit. Fluids 2020, 158, 104721.
- Nkurunziza, D.; Pendleton, P.; Chun, B.S. Optimization and Kinetics Modeling of Okara Isoflavones Extraction Using Subcritical Water. Food Chem. 2019, 295, 613–621.
- Liu, J.; Li, L.; Wu, W.; Zhang, G.; Zheng, Y.; Ma, C.; Li, W.; Yan, Y.; Xu, Z. Green Extraction of Active Ingredients from Finger Citron Using Subcritical Water and Assessment of Antioxidant Activity. Ind. Crops Prod. 2023, 200, 116821.
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.Ž.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and Biological Screening of Stinging Nettle Leaves Extracts Obtained by Modern Extraction Techniques. Ind. Crops Prod. 2017, 108, 423–430.
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004.
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical Water Extraction of Wild Garlic (Allium ursinum L.) and Process Optimization by Response Surface Methodology. J. Supercrit. Fluids 2017, 128, 79–88.
- Vladić, J.; Nastić, N.; Stanojkoivć, T.; Žižak, Ž.; Čakarević, J.; Popović, L.; Vidović, S. Subcritical Water for Recovery of Polyphenols from Comfrey Root and Biological Activities of Extracts. Acta Chim. Slov. 2019, 66, 473–783.
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560.
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of Subcritical Water Scale-up from Laboratory to Pilot System for Brewer’s Spent Grain Valorization. Ind. Crops Prod. 2023, 191, 115927.
- Khoza, B.S.; Dubery, I.A.; Byth-Illing, H.-A.; Steenkamp, P.A.; Chimuka, L.; Madala, N.E. Optimization of Pressurized Hot Water Extraction of Flavonoids from Momordica Foetida Using UHPLC-qTOF-MS and Multivariate Chemometric Approaches. Food Anal. Methods 2015, 9, 1480–1489.
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive Compounds of Sweet and Sour Cherry Stems Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635.
- Chémat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Périno, S.; Fabiano-Tixier, A.; Vian, M.A. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377.
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Li, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549.
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109.
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325.
- De Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT 2016, 71, 110–115.
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of Different Ultrasound Assisted Extraction Techniques for Pectin from Tomato Processing Waste. Ultrason. Sonochem. 2020, 61, 104812.
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Karuppiah, P.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound Assisted Citric Acid Mediated Pectin Extraction from Industrial Waste of Musa Balbisiana. Ultrason. Sonochem. 2017, 35, 204–209.
- Patience, N.A.; Schieppati, D.; Boffito, D.C. Continuous and Pulsed Ultrasound Pectin Extraction from Navel Orange Peels. Ultrason. Sonochem. 2021, 73, 105480.
- Al-Dhabi, N.A.; Karuppiah, P.; Maran, J.P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochem. 2017, 34, 206–213.
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of Ultrasound-Assisted Extraction of Oil from Papaya Seed by Response Surface Methodology: Oil Recovery, Radical Scavenging Antioxidant Activity, and Oxidation Stability. Food Chem. 2015, 172, 7–17.
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and Ultrasound-assisted Extractions of Polyphenols from Citrus sinensis (Cv. Malta) Peel: A Comparative Study. J. Food Process. Preserv. 2019, 43, e14046.
- Petigny, L.; Périno-Issartier, S.; Wajsman, J.; Chémat, F. Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.). Int. J. Mol. Sci. 2013, 14, 5750–5764.
- Del Hierro, J.N.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martín, D. Ultrasound-Assisted Extraction and Bioaccessibility of Saponins from Edible Seeds: Quinoa, Lentil, Fenugreek, Soybean and Lupin. Food Res. Int. 2018, 109, 440–447.
- Oniszczuk, A.; Podgórski, R. Influence of Different Extraction Methods on the Quantification of Selected Flavonoids and Phenolic Acids from Tilia Cordata Inflorescence. Ind. Crops Prod. 2015, 76, 509–514.
- Da Fonseca Machado, A.P.; Sumere, B.R.; Mekaru, C.; Martínez, J.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of Polyphenols and Antioxidants from Pomegranate Peel Using Ultrasound: Influence of Temperature, Frequency and Operation Mode. Int. J. Food Sci. Technol. 2019, 54, 2792–2801.
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of Acoustic Frequency and Power Density on the Aqueous Ultrasonic-Assisted Extraction of Grape Pomace (Vitis vinifera L.)—A Response Surface Approach. Ultrason. Sonochem. 2014, 21, 2176–2184.
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51, 138–149.
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 719–752.
- Tram, N.N. Optimizing the Extraction Conditions of Phenolic Compounds from Fresh Tea Shoot. J. Food Nutr. Sci. 2015, 3, 106.
- Feng, T.; Zhang, M.; Sun, Q.; Mujumdar, A.S.; Yu, D. Extraction of Functional Extracts from Berries and Their High Quality Processing: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 63, 7108–7125.
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-Assisted Extraction (MAE) Conditions Using Polynomial Design for Improving Antioxidant Phytochemicals in Berberis asiatica Roxb. Ex DC. Leaves. Ind. Crops Prod. 2017, 95, 393–403.
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.E.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of Microwave-Assisted Extraction of Ergosterol from Agaricus bisporus L. by-Products Using Response Surface Methodology. Food Bioprod. Process. 2016, 100, 25–35.
- Ismail-Suhaimy, N.W.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Bawon, P. Optimizing Conditions for Microwave-Assisted Extraction of Polyphenolic Content and Antioxidant Activity of Barleria lupulina Lindl. Plants 2021, 10, 682.
- Mir, S.A.; Rizwan, D.; Bakshi, R.A.; Wani, S.M.; Masoodi, F.A. Extraction of Carotenoids from Agro-Industrial Waste. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2023; pp. 157–178.
- Vînătoru, M.; Mason, T.J.; Călinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178.
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2011, 5, 409–424.
- Álvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A. Pressurized Liquid Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398.
- Fraguela-Meissimilly, H.; Bastías-Montes, J.M.; Vergara, C.E.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.R.; Alcázar-Alay, S.C.; Bedoya, M.G. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421.
- Khanum, F. Therapeutic Foods: An Overview; CRC Press: London, UK, 2021; pp. 31–70.
- Picot-Allain, M.C.N.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156.
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gándara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612.
- Oliveira, A.M.B.; Viganó, J.; Sanches, V.L.; Rostagno, M.A. Extraction of Potential Bioactive Compounds from Industrial Tahiti Lime (Citrus latifólia Tan.) by-Product Using Pressurized Liquids and Ultrasound-Assisted Extraction. Food Res. Int. 2022, 157, 111381.
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869.
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated Strategies for Enzyme Assisted Extraction of Bioactive Molecules: A Review. Int. J. Biol. Macromol. 2021, 191, 899–917.
- Mir-Cerdà, A.; Núñez, Ó.; Granados, M.; Sentellas, S.; Saurina, J. An Overview of the Extraction and Characterization of Bioactive Phenolic Compounds from Agri-Food Waste within the Framework of Circular Bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994.
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic Activity of Armillaria Mellea Polysaccharides: Joint Ultrasonic and Enzyme Assisted Extraction. Ultrason. Sonochem. 2023, 95, 106370.
