Aquaponics emerges as a beacon of hope, showcasing how humanity can adapt, innovate, and thrive while preserving the delicate balance of our natural resources. Within aquaponic systems, a symbiotic cycle unfolds: fish waste serves as vital nutrients for plants, while these same plants act as natural filters, purifying the water destined to circulate back to the fish tanks. This harmonious relationship between aquatic life and vegetation fosters a closed-loop ecosystem, significantly curbing water wastage and elevating the system’s overall sustainability. This method not only produces high-quality organic vegetables and fruits but also sustainable protein sources, addressing the challenges of both food security and water conservation.
- aquaponics
- automation
- food sustainability
- food sovereignty
1. Introduction
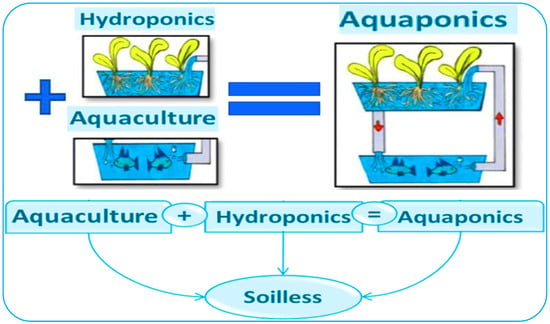
2. Aquaponics
2.1. Nomenclature in Aquaponics and Legislation
2.2. Aquaponic Systems Development
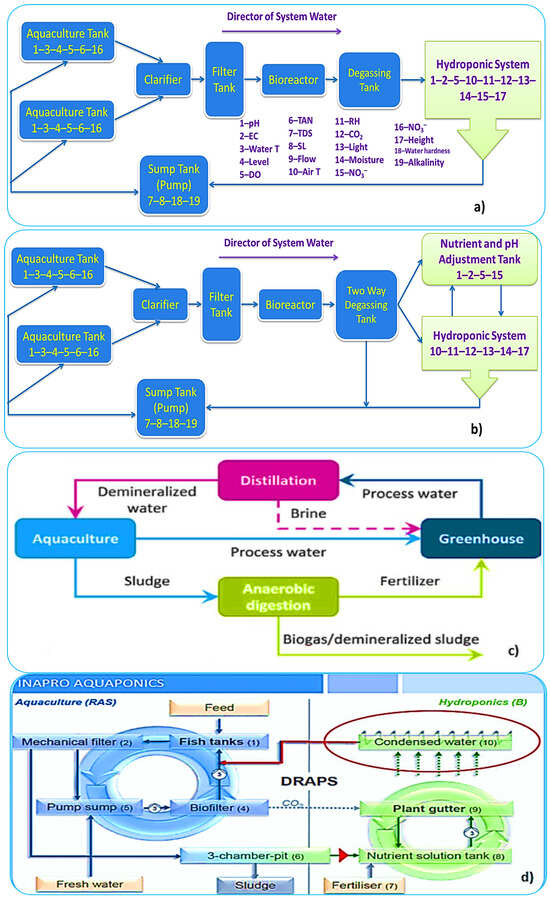
2.3. Recirculating Aquaculture Systems (RASs)
2.4. Hydroponic Components
3. Aquaponics Systems Performance
3.1. Fish Species, Feed, and Growth Indicators
3.2. Plant Species, Nutrients, Growth, and Indexes
3.3. Nitrifying Bacteria and Microflora
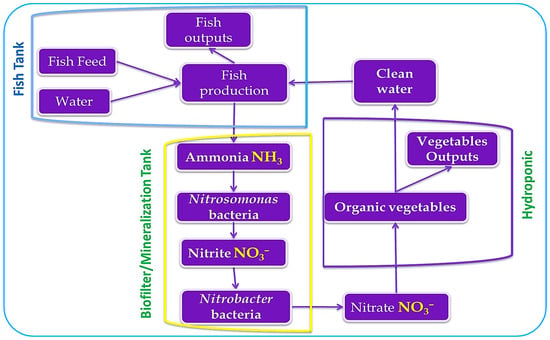
3.4. Water Quality, Consumption, and Use Efficiency in Aquaponics
4. Technology
4.1. Smart Aquaponic Systems
4.2. Internet of Things (IoT) Systems
4.3. Big Data
4.4. Artificial Intelligence (AI)
5. Economic Feasibility, Energy Consumption, and Benefits
5.1. Economic Feasibility
5.2. Energy Consumption
5.3. Social, Economic, and Environmental Benefits for Food Security
This entry is adapted from the peer-reviewed paper 10.3390/w15244310
References
- Ibrahim, L.A.; Abu-Hashim, M.; Shaghaleh, H.; Elsadek, E.; Hamad, A.A.; Alhaj Hamoud, Y. A Comprehensive Review of the Multiple Uses of Water in Aquaculture-Integrated Agriculture Based on International and National Experiences. Water 2023, 15, 367.
- Elsadek, E. Use of Automatic Control to Improve the Performance of Field Irrigation Systems; Damietta University: Damietta, Egypt, 2018.
- Elnemr, M.K.; El-Sheikha, A.M.; Elsadek, E.A. Determination of Optimal Location of Soil Moisture Sensing Devices for Trickle Irrigation Systems. Misr. J. Agric. Eng. 2019, 36, 157–174.
- Franz Gouertoumbo, W.; Alhaj Hamoud, Y.; Guo, X.; Shaghaleh, H.; Ali Adam Hamad, A.; Elsadek, E. Wheat Straw Burial Enhances the Root Physiology, Productivity, and Water Utilization Efficiency of Rice under Alternative Wetting and Drying Irrigation. Sustainability 2022, 14, 16394.
- Elsadek, E.; Zhang, K.; Mousa, A.; Ezaz, G.T.; Tola, T.L.; Shaghaleh, H.; Hamad, A.A.A.; Alhaj Hamoud, Y. Study on the In-Field Water Balance of Direct-Seeded Rice with Various Irrigation Regimes under Arid Climatic Conditions in Egypt Using the AquaCrop Model. Agronomy 2023, 13, 609.
- Abu-hashim, M.; Shaban, K. Deficit irrigation management as strategy to adapt water scarcity-potential application on Mediterranean saline soils. Egypt Soil Sci. 2017, 5, 261–271.
- Ali, H.; Ahmed, N.; Abu-hashim, M. Potential effect of irrigation intervals and potassium phthalate on fennel plants grown in semi-arid regions. Egypt. J. Soil Sci. 2020, 60, 83–98.
- Smit, B.; Pilifosova, O. Adaptation to Climate Change in the Context of Sustainable Development and Equity. Clim. Chang. Impacts Adapt. Vulnerability 2001, 8, 877.
- Zhang, L.; Dabipi, I.K.; Brown, W.L., Jr. Internet of Things Applications for Agriculture. In Internet of Things A to Z; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 507–528. ISBN 9781119456735.
- Tschirner, M.; Kloas, W. Increasing the Sustainability of Aquaculture Systems: Insects as Alternative Protein Source for Fish Diets. GAIA—Ecol. Perspect. Sci. Soc. 2017, 26, 332–340.
- Lee, C.; Wang, Y.-J. Development of a Cloud-Based IoT Monitoring System for Fish Metabolism and Activity in Aquaponics. Aquac. Eng. 2020, 90, 102067.
- Lova Raju, K.; Vijayaraghavan, V. IoT Technologies in Agricultural Environment: A Survey. Wirel. Pers. Commun. 2020, 113, 2415–2446.
- Yanes, A.R.; Martinez, P.; Ahmad, R. Towards Automated Aquaponics: A Review on Monitoring, IoT, and Smart Systems. J. Cleaner Prod. 2020, 263, 121571.
- Kish, Z. Food Sovereignty BT—Encyclopedia of Global Justice; Chatterjee, D.K., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 352–358. ISBN 978-1-4020-9160-5.
- Rakocy, J.E. Aquaponics—Integrating Fish and Plant Culture. In Aquaculture Production Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 344–386. ISBN 9781118250105.
- Lennard, W.A. Aquaponics: A Nutrient Dynamic Process and the Relationship to Fish Feeds. World Aquac. 2015, 46, 20–23.
- Wittman, H. Food Sovereignty: A New Rights Framework for Food and Nature? Environ. Soc. 2011, 2, 87–105.
- Goddek, S.; Joyce, A.; Wuertz, S.; Körner, O.; Bläser, I.; Reuter, M.; Keesman, K.J. Decoupled Aquaponics Systems BT—Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 201–229. ISBN 978-3-030-15943-6.
- European Parliament & Council of the European Union (EU) 2018/848 Regulation. Organic production and labeling of organic products and repealing Council Regulation (EC) No 834/2007 (30 May). Off. J. Eur. Union OJEU 2018, OJL 150, 1–92.
- Joly, A.; Junge, R.; Bardocz, T. Aquaponics Business in Europe: Some Legal Obstacles and Solutions. Ecocycles 2015, 1, 3–5.
- Mchunu, N.; Lagerwall, G.; Senzanje, A. Aquaponics in South Africa: Results of a National Survey. Aquac. Reports 2018, 12, 12–19.
- BBC. Good Food What Does Organic Mean. Available online: https://www.bbcgoodfood.com/howto/guide/o (accessed on 15 September 2022).
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Haïssam Jijakli, M.; Kotzen, B. Towards Commercial Aquaponics: A Review of Systems, Designs, Scales and Nomenclature. Aquac. Int. 2018, 26, 813–842.
- Kloas, W.; Groß, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Staaks, G.; Suhl, J.; Tschirner, M.; Wittstock, B.; et al. A New Concept for Aquaponic Systems to Improve Sustainability, Increase Productivity, and Reduce Environmental Impacts. Aquac. Environ. Interact. 2015, 7, 179–192.
- Thorarinsdottir, R. Aquaponics Guidelines; University of Iceland: Haskolaprent, Iceland, 2015; p. 68.
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of Plant Species on Nitrogen Recovery in Aquaponics. Bioresour. Technol. 2015, 188, 92–98.
- Delaide, B.; Delhaye, G.; Dermience, M.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Plant and Fish Production Performance, Nutrient Mass Balances, Energy and Water Use of the PAFF Box, a Small-Scale Aquaponic System. Aquac. Eng. 2017, 78, 130–139.
- Monsees, H.; Kloas, W.; Wuertz, S. Decoupled Systems on Trial: Eliminating Bottlenecks to Improve Aquaponic Processes. PLoS ONE 2017, 12, e0183056.
- de Graaf, F.; Goddek, S. Smarthoods: Aquaponics Integrated Microgrids. In Aquaponics Food Production Systems; Springer International Publishing: Cham, Switzerland, 2019; pp. 379–392.
- Lekang, O. (Ed.) Aquaculture Engineering, 1st ed.; Blackwell Publishing Ltd.: Hong Kong, China, 2007.
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen Transformations in Aquaponic Systems: A Review. Aquac. Eng. 2017, 76, 9–19.
- Ngo Thuy Diem, T.; Konnerup, D.; Brix, H. Effects of Recirculation Rates on Water Quality and Oreochromis Niloticus Growth in Aquaponic Systems. Aquac. Eng. 2017, 78, 95–104.
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Liang, S.; Wang, J.; Yan, R. Attempts to Improve Nitrogen Utilization Efficiency of Aquaponics through Nitrifies Addition and Filler Gradation. Environ. Sci. Pollut. Res. 2016, 23, 6671–6679.
- Maucieri, C.; Nicoletto, C.; van Os, E.; Anseeuw, D.; van Havermaet, R.; Junge, R. Hydroponic Technologies BT—Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 77–110. ISBN 978-3-030-15943-6.
- Lennard, W.A.; Leonard, B. V A Comparison of Three Different Hydroponic Sub-Systems (Gravel Bed, Floating and Nutrient Film Technique) in an Aquaponic Test System. Aquac. Int. 2006, 14, 539–550.
- Maucieri, C.; Forchino, A.A.; Nicoletto, C.; Junge, R.; Pastres, R.; Sambo, P.; Borin, M. Life Cycle Assessment of a Micro Aquaponic System for Educational Purposes Built Using Recovered Material. J. Cleaner Prod. 2018, 172, 3119–3127.
- Pattillo, A. An Overview of Aquaponic Systems: Aquaculture Components; NCRAC Technical Bulletins; IOWA State University: Ames, IA, USA, 2017; pp. 1–18.
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture; Cayuga Aqua Ventures: Ithaca, NY, USA, 2010; ISBN 0971264627.
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics-Integrating Fish and Plant Culture; Oklahoma Cooperative Extension Service. Division of Agricultural Sciences and Natural Resources; Oklahoma State University: Stillwater, OK, USA, 2016.
- Love, D.C.; Uhl, M.S.; Genello, L. Energy and Water Use of a Small-Scale Raft Aquaponics System in Baltimore, Maryland, United States. Aquac. Eng. 2015, 68, 19–27.
- Oladimeji, A.S.; Olufeagba, S.O.; Ayuba, V.O.; Sololmon, S.G.; Okomoda, V.T. Effects of Different Growth Media on Water Quality and Plant Yield in a Catfish-Pumpkin Aquaponics System. J. King Saud Univ. Sci. 2020, 32, 60–66.
- Ru, D.; Liu, J.; Hu, Z.; Zou, Y.; Jiang, L.; Cheng, X.; Lv, Z. Improvement of Aquaponic Performance through Micro- and Macro-Nutrient Addition. Environ. Sci. Pollut. Res. 2017, 24, 16328–16335.
- Obirikorang, K.A.; Sekey, W.; Gyampoh, B.A.; Ashiagbor, G.; Asante, W. Aquaponics for Improved Food Security in Africa: A Review. Front. Sustain. Food Syst. 2021, 5, 705549.
- Saha, S.; Monroe, A.; Day, M.R. Growth, Yield, Plant Quality and Nutrition of Basil (Ocimum Basilicum L.) under Soilless Agricultural Systems. Ann. Agric. Sci. 2016, 61, 181–186.
- International Aquaculture Feed Formulation Database (IAFFD). Feed Ingredient Composition Database. 2018. Available online: https://www.iaffd.com/home.html?v=4.01 (accessed on 3 March 2022).
- Li, C.; Zhang, B.; Luo, P.; Shi, H.; Li, L.; Gao, Y.; Lee, C.T.; Zhang, Z.; Wu, W.-M. Performance of a Pilot-Scale Aquaponics System Using Hydroponics and Immobilized Biofilm Treatment for Water Quality Control. J. Clean. Prod. 2019, 208, 274–284.
- Ibrahim, L.A.; Shaghaleh, H.; Abu-Hashim, M.; Elsadek, E.A.; Hamoud, Y.A. Exploring the Integration of Rice and Aquatic Species: Insights from Global and National Experiences. Water 2023, 15, 2750.
- Bailey, D.S.; Ferrarezi, R.S. Valuation of Vegetable Crops Produced in the UVI Commercial Aquaponic System. Aquac. Rep. 2017, 7, 77–82.
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial Aquaponics Production and Profitability: Findings from an International Survey. Aquaculture 2015, 435, 67–74.
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M. Lettuce (Lactuca sativa L. Var. Sucrine) Growth Performance in Complemented Aquaponic Solution Outperforms Hydroponics. Water 2016, 8, 467.
- Rakocy, J.; Shultz, R.C.; Bailey, D.S.; Thoman, E.S. Aquaponic Production of Tilapia and Basil: Comparing a Batch and Staggered Cropping System. Acta Hortic. 2004, 648, 63–69.
- Adler, P.R.; Harper, J.K.; Wade, E.M.; Takeda, F.; Summerfelt, S.T. Economic Analysis of an Aquaponic System for the Integrated Production of Rainbow Trout and Plants. Int. J. Recirc. Aquac. 2000, 1, 15–34.
- Knaus, U.; Palm, H.W. Effects of the Fish Species Choice on Vegetables in Aquaponics under Spring-Summer Conditions in Northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 473, 62–73.
- Estrada-Perez, N.; Hernandez-Llamas, A.; Ruiz-Velazco, M.J.; Zavala-Leal, I.; Romero-Bañuelos, C.A.; Cruz-Crespo, E.; Juárez-Rossete, C.; Domínguez-Ojeda, D.; Campos-Mendoza, A. Stochastic Modelling of Aquaponic Production of Tilapia (Oreochromis niloticus) with Lettuce (Lactuca sativa) and Cucumber (Cucumis sativus). Aquac. Res. 2018, 49, 3723–3734.
- Goddek, S.; Vermeulen, T. Comparison of Lactuca Sativa Growth Performance in Conventional and RAS-Based Hydroponic Systems. Aquac. Int. 2018, 26, 1377–1386.
- Buzby, K.M.; Lin, L.-S. Scaling Aquaponic Systems: Balancing Plant Uptake with Fish Output. Aquac. Eng. 2014, 63, 39–44.
- Pinho, S.M.; Molinari, D.; de Mello, G.L.; Fitzsimmons, K.M.; Coelho Emerenciano, M.G. Effluent from a Biofloc Technology (BFT) Tilapia Culture on the Aquaponics Production of Different Lettuce Varieties. Ecol. Eng. 2017, 103, 146–153.
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic Systems and Water Management in Aquaponics: A Review. Ital. J. Agron. 2017, 11, 1–11.
- Pinho, S.M.; de Mello, G.L.; Fitzsimmons, K.M.; Emerenciano, M.G.C. Integrated Production of Fish (Pacu Piaractus Mesopotamicus and Red Tilapia Oreochromis Sp.) with Two Varieties of Garnish (Scallion and Parsley) in Aquaponics System. Aquac. Int. 2018, 26, 99–112.
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming. FAO Fish. Aquac. Tech. Pap. 2014, 589, 1–288.
- Schmautz, Z.; Graber, A.; Jaenicke, S.; Goesmann, A.; Junge, R.; Smits, T.H.M. Microbial Diversity in Different Compartments of an Aquaponics System. Arch. Microbiol. 2017, 199, 613–620.
- Bartelme, R.P.; McLellan, S.L.; Newton, R.J. Freshwater Recirculating Aquaculture System Operations Drive Biofilter Bacterial Community Shifts around a Stable Nitrifying Consortium of Ammonia-Oxidizing Archaea and Comammox Nitrospira. Front. Microbiol. 2017, 8, 101.
- Gilles, S.; Ismiño, R.; Sánchez, H.; David, F.; Núñez, J.; Dugué, R.; Darias, M.J.; Römer, U. An Integrated Closed System for Fish-Plankton Aquaculture in Amazonian Fresh Water. Animal 2014, 8, 1319–1328.
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of PH on Nitrogen Transformations in Media-Based Aquaponics. Bioresour. Technol. 2016, 210, 81–87.
- da Silva Cerozi, B.; Fitzsimmons, K. Use of Bacillus Spp. to Enhance Phosphorus Availability and Serve as a Plant Growth Promoter in Aquaponics Systems. Sci. Hortic. 2016, 211, 277–282.
- Odema, M.; Adly, I.; Wahba, A.; Ragai, H. Smart Aquaponics System for Industrial Internet of Things (IIoT). In Proceedings of the International Conference on Advanced Intelligent Systems and Informatics 2017, Cairo, Egypt, 9–11 September 2017; Hassanien, A., Shaalan, K., Gaber, T., Tolba, M., Eds.; Springer: Cham, Switzerland, 2018; Volume 639, pp. 844–854.
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced Aquaponics: Evaluation of Intensive Tomato Production in Aquaponics vs. Conventional Hydroponics. Agric. Water Manage. 2016, 178, 335–344.
- Sace, C.F.; Fitzsimmons, K.M. Vegetable Production in a Recirculating Aquaponic System Using Nile Tilapia (Oreochromis Niloticus) with and without Freshwater Prawn (Macrobrachium Rosenbergii). Acad. J. Agric. Res. 2013, 1, 236–250.
- Wada, T. Theory and Technology to Control the Nutrient Solution of Hydroponics. In Plant Factory Using Artificial Light; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–14. ISBN 978-0-12-813973-8.
- Stone, N.M.; Thomforde, H.K. Understanding Your Fish Pond Water Analysis Report. Available online: https://www.uaex.uada.edu/publications/PDF/FSA-9090.pdf (accessed on 14 June 2022).
- Bhatnagar, A.; Devi, P. Water Quality Guidelines for the Management of Pond Fish Culture. Int. J. Environ. Sci. 2013, 3, 1980–2009.
- Goddek, S.; Körner, O. A Fully Integrated Simulation Model of Multi-Loop Aquaponics: A Case Study for System Sizing in Different Environments. Agric. Syst. 2019, 171, 143–154.
- Shaalan, M.; El-Mahdy, M.; Saleh, M.; El-Matbouli, M. Aquaculture in Egypt: Insights on the Current Trends and Future Perspectives for Sustainable Development. Rev. Fish. Sci. Aquac. 2018, 26, 99–110.
- Wang, D.; Zhao, J.; Huang, L.; Xu, D. Design of A Smart Monitoring and Control System for Aquaponics Based on OpenWrt BT. In Proceedings of the 5th International Conference on Information Engineering for Mechanics and Materials, Huhhot, China, 25–26 July 2015; Atlantis Press: Amsterdam, Netherlands; 2015; pp. 937–942.
- Pasha, A.K.; Mulyana, E.; Hidayat, C.; Ramdhani, M.A.; Kurahman, O.T.; Adhipradana, M. System Design of Controlling and Monitoring on Aquaponic Based on Internet of Things. In Proceedings of the 2018 4th International Conference on Wireless and Telematics (ICWT), Nusa Dua, Indonesia, 12–13 July 2018; IEEE: New York, NY, USA, 2018; pp. 1–5.
- Aishwarya, K.S.; Harish, M.; Prathibhashree, S.; Panimozhi, K. Survey on IoT Based Automated Aquaponics Gardening Approaches. In Proceedings of the 2018 Second International Conference on Inventive Communication and Computational Technologies (ICICCT), Coimbatore, India, 20–21 April 2018; IEEE: New York, NY, USA, 2018; pp. 1495–1500.
- Zaini, A.; Kurniawan, A.; Herdhiyanto, A.D. Internet of Things for Monitoring and Controlling Nutrient Film Technique (NFT) Aquaponic. In Proceedings of the 2018 International Conference on Computer Engineering, Network and Intelligent Multimedia (CENIM), Surabaya, Indonesia, 26–27 November 2018; IEEE: New York, NY, USA, 2018; pp. 167–171.
- Shaout, A.; Scott, S.G. IoT Fuzzy Logic Aquaponics Monitoring and Control Hardware Real-Time System. In Proceedings of the Proceedings of the International Arab Conference on Information Technology, Yassmine Hammamet, Tunisia, 22–24 December 2017; pp. 22–24.
- Saaid, M.F.; Fadhil, N.S.M.; Ali, M.S.A.M.; Noor, M.Z.H. Automated Indoor Aquaponic Cultivation Technique. In Proceedings of the 2013 IEEE 3rd International Conference on System Engineering and Technology, Shah Alam, Malaysia, 19–20 August 2013; IEEE: New York, NY, USA, 2013; pp. 285–289.
- Ahmad, R.; Tichadou, S.; Hascoet, J.-Y. A Knowledge-Based Intelligent Decision System for Production Planning. Int. J. Adv. Manuf. Technol. 2017, 89, 1717–1729.
- Kumar, N.H.; Baskaran, S.; Hariraj, S.; Krishnan, V. An Autonomous Aquaponics System Using 6LoWPAN Based WSN. In Proceedings of the 2016 IEEE 4th International Conference on Future Internet of Things and Cloud Workshops (FiCloudW), Vienna, Austria, 22–24 August 2016; IEEE: New York, NY, USA, 2016; pp. 125–132.
- Aceto, G.; Persico, V.; Pescapé, A. A survey on information and communication technologies for Industry 4.0: State-of-the-art, taxonomies, perspectives, and challenges. IEEE Commun Surv Tutor. 2019, 21, 3467–3501.
- Tyson, R.V.; Danyluk, M.D.; Simonne, E.H.; Treadwell, D.D. Sustainable aquaponic vegetable and fish co-production. Proc Fla. State Hortic. Soc. 2012, 125, 381–385.
- Lu, J.-Y.; Chang, C.-L.; Kuo, Y.-F. Monitoring Growth Rate of Lettuce Using Deep Convolutional Neural Networks. In Proceedings of the Agricultural and Food Sciences, Computer Science, Environmental Science, Boston, MA, USA, 7–10 July 2019; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2019; p. 1.
- Manju, M.; Karthik, V.; Hariharan, S.; Sreekar, B. Real Time Monitoring of the Environmental Parameters of an Aquaponic System Based on Internet of Things. In Proceedings of the 2017 Third International Conference on Science Technology Engineering & Management (ICONSTEM), Chennai, India, 23–24 March 2017; IEEE: New York, NY, USA, 2017; pp. 943–948.
- Dutta, A.; Dahal, P.; Prajapati, R.; Tamang, P.; Kumar, E.S. IoT Based Aquaponics Monitoring System. In Proceedings of the 1st KEC Conference Proceedings, Lalitpur, Nepal, 27 September 2018; Volume 1, pp. 75–80.
- Elsokah, M.M.; Sakah, M. Next Generation of Smart Aquaponics with Internet of Things Solutions. In Proceedings of the 2019 19th International Conference on Sciences and Techniques of Automatic Control and Computer Engineering (STA), Sousse, Tunisia, 24–26 March 2019; IEEE: New York, NY, USA, 2019; pp. 106–111.
- Sreelekshmi, B.; Madhusoodanan, K.N. Automated Aquaponics System. In Emerging Trends in Engineering, Science and Technology for Society, Energy and Environment; CRC Press: Boca Raton, FL, USA, 2018; pp. 719–724.
- Eneh, A.H.; Udanor, C.N.; Ossai, N.I.; Aneke, S.O.; Ugwoke, P.O.; Obayi, A.A.; Ugwuishiwu, C.H.; Okereke, G.E. Towards an improved internet of things sensors data quality for a smart aquaponics system yield prediction. MethodsX 2023, 11, 102436.
- Karimanzira, D.; Rauschenbach, T. An Intelligent Management System for Aquaponics. Automatisierungstechnik 2021, 69, 345–350.
- Abbasi, R.; Martinez, P.; Ahmad, R. An ontology model to represent aquaponics 4.0 system’s knowledge. Inf. Process Agric. 2022, 9, 514–532.
- Wei, Y.; Li, W.; An, D.; Li, D.; Jiao, Y.; Wei, Q. Equipment and Intelligent Control System in Aquaponics: A Review. IEEE Access 2019, 7, 169306.
- Abbasi, R.; Martinez, P.; Ahmad, R. Crop diagnostic system: A robust disease detection and management system for leafy green crops grown in an aquaponics facility. Artif. Intell. Agric. 2023, 10, 1–12.
- Tokunaga, K.; Tamaru, C.; Ako, H.; Leung, P. Economics of Small-Scale Commercial Aquaponics in Hawai‘I. J. World Aquac. Soc. 2015, 46, 20–32.
- Engle, C.R. Economics of Aquaponics; No. 5006; Southern Regional Aquaculture Center Publication: Stoneville, MS, USA, 2015.
- Morgenstern, R.; Lorleberg, W.; Biernatzki, R.; Boelhauve, M.; Braun, J.; Haberlah-Korr, V. Pilotstudie “Nachhaltige Aquaponik-Erzeugung Für Nordrhein-Westfalen.” Fachhochschule Südwestfalen, Soest; Fachhochschule Südwestfalen: Iserlohn, Germany, 2017; ISBN 978-3-940956-66-8.
- Lobillo-Eguíbar, J.; Fernández-Cabanás, V.M.; Bermejo, L.A.; Pérez-Urrestarazu, L. Economic Sustainability of Small-Scale Aquaponic Systems for Food Self-Production. Agronomy 2020, 10, 1468.
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.; Jijakli, H.; Thorarinsdottir, R. Challenges of Sustainable and Commercial Aquaponics. Sustainability 2015, 7, 4199–4224.
- Asciuto, A.; Schimmenti, E.; Cottone, C.; Borsellino, V. A Financial Feasibility Study of an Aquaponic System in a Mediterranean Urban Context. Urban For. Urban Green. 2019, 38, 397–402.
- Joyce, A.; Goddek, S.; Kotzen, B.; Wuertz, S. Aquaponics: Closing the Cycle on Limited Water, Land and Nutrient Resources. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–34. ISBN 978-3-030-15943-6.
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED lighting systems for horticulture: Business growth and global distribution. Sustainability 2020, 12, 7516.
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, D. LEDs for energy efficient greenhouse lighting Renew. Sustain. Energy Rev. 2015, 49, 139–147.
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency in two lettuce cultivars as influenced by white plus red versus red plus blue leds. Int. J. Agric. Biol. Eng. 2020, 13, 33–40.
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082.
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant Sci. 2021, 11, 609977.
- Kong, Y.; Nemali, A.; Mitchell, C.; Nemali, K. Spectral quality of light can affect energy consumption and energy-use efficiency of electrical lighting in indoor lettuce farming. HortScience 2019, 54, 865–872.
- Huber, B.M.; Louws, F.J.; Hernández, R. Impact of Different Daily Light Integrals and Carbon Dioxide Concentrations on the Growth, Morphology, and Production Efficiency of Tomato Seedlings. Front. Plant Sci. 2021, 12, 615853.
- Yan, Z.; He, D.; Niu, G.; Zhou, Q.; Qu, Y. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744.
- Zheng, J.F.; He, D.X.; Ji, F. Effects of light intensity and photoperiod on runner plant propagation of hydroponic strawberry transplants under LED lighting. Int. J. Agric. Biol. Eng. 2019, 12, 26–31.
- Olvera-Gonzalez, E.; Escalante-Garcia, N.; Myers, D.; Ampim, P.; Obeng, E.; Alaniz-Lumbreras, D.; Castaño, V. Pulsed led-lighting as an alternative energy savings technique for vertical farms and plant factories. Energies 2012, 14, 1603.
- Gillani, S.A.; Abbasi, R.; Martinez, P.; Ahmad, R. Review on Energy Efficient Artificial Illumination in Aquaponics. Cleaner Circ. Bioecon. 2022, 2, 100015.
- Lund, H. The implementation of renewable energy systems. Lessons learned from the Danish case. Energy 2010, 35, 4003–4009.
- Halim, J. Akuaponik Pekarangan; Penebar Swadaya: Jakarta, Indonesia, 2018.
- Faber, M.; Witten, C.; Drimie, S. Community-Based Agricultural Interventions in the Context of Food and Nutrition Security in South Africa. S. Afr. J. Clin. Nutr. 2011, 24, 21–30.
