One of the most prevalent primary malignant brain tumors is glioblastoma (GB). About 6 incidents per 100,000 people are reported annually. Most frequently, these tumors are linked to a poor prognosis and poor quality of life. There has been little advancement in the treatment of GB. In recent years, some innovative medicines have been tested for the treatment of newly diagnosed cases of GB and recurrent cases of GB. Surgery, radiotherapy, and alkylating chemotherapy are all common treatments for GB. A few of the potential alternatives include immunotherapy, tumor-treating fields (TTFs), and medications that target specific cellular receptors. To provide new multimodal therapies that focus on the molecular pathways implicated in tumor initiation and progression in GB, novel medications, delivery technologies, and immunotherapy approaches are being researched. Of these, oncolytic viruses (OVs) are among the most recent. Coupling OVs with certain modern treatment approaches may have significant benefits for GB patients.
1. Introduction
In the general population, glioblastoma (GB) is one of the most prevalent malignant brain tumors. Most glioblastomas (around 80–90%) arise de novo and without any prior clinical or histologic signs, especially in elderly individuals. [
1]. Currently, one of the most aggressive and incurable cancers is GB [
2]. The WHO revised the categorization of malignancies of the central nervous system (CNS) in 2021, incorporating genetic and molecular characteristics into the definition of various glioma subtypes alongside histological ones [
3]. Less than 5% of people with GB survive more than five years after diagnosis, with the median overall survival for those with the disease falling between 15 and 20 months [
4,
5]. Radiation therapy and chemotherapy are usually used after a surgical resection for GB [
6]. GB can be treated with temozolomide, bevacizumab, lomustine, intravenous carmustine, and carmustine wafer plants, among other chemotherapy medications [
7].
Key risk factors for GB include obesity, cytomegalovirus (CMV), high radiation dosages, and a family history of cancer [
8,
9,
10,
11]. GB has been described as having considerable intratumoral heterogeneity, tumor-induced immunosuppression of the microenvironment, and minimal infiltrating immunity [
12,
13,
14]. The blood–brain barrier (BBB) [
15], glioma stem cells (GSC) [
16,
17], and a low tumor mutational load [
18,
19] are also present.
Recent developments in molecular pathogenesis have led to a better understanding of the GB microenvironment, including its interactions with the human immune system and its genetic, epigenomic, transcriptomic, and proteomic characterization [
20]. Among the most promising therapies for GB and brain tumors are novel therapeutic approaches including oncolytic viral therapy [
21].
2. Overview Glioblastoma
2.1. Introduction of Glioblastoma
Initially, it was believed that GB only came from glial cells, but research now reveals that they may also come from other cell types that have characteristics of neural stem cells. These cells are in various phases of development from stem cells to neurons to glia, with molecular changes in signaling pathways acting largely as a determinant of phenotypic diversity rather than diverse cell types of origin [
22].
2.2. Molecular Description
More than 600 genes were sequenced from more than 200 human tumor samples as a result of genomic profiling and the Cancer Genome Atlas project by Parsons et al., 2008, which revealed the complex genetic profile of GB and established a set of three core signaling pathways that are frequently activated (namely, the tumor protein p53 (p53) pathway, the receptor tyrosine kinase/Ras/phosphoinositide 3-kinase signaling pathway, and the retinoblastoma pathway) [
23]. Epidermal growth factor receptor (EGFR) overexpression, mutations in the PTEN gene, and deletion of chromosome 10q are among the genetic changes common to primary GBM. Chromosome 19q deletion, p53 mutations, and isocitrate dehydrogenase 1 (IDH1) mutations are typically observed in secondary GB [
24,
25,
26].
Transcriptome studies have become significant methods for grouping cancers into molecular subgroups that differ in terms of their clinical behavior and reaction to treatment [
28]. However, when taking into account the extremely aggressive isocitrate dehydrogenase (IDH) wild-type group, the transcriptome categorization has not been able to predict prognosis and pharmacologic vulnerability for specific cancers, such as GBM [
29,
30]. Specifically, the absence of correlation between survival and physiologically defined subgroups of IDH wild-type GBM has impeded the quest to identify the distinct mechanisms that maintain tumor development in patient subgroups. The transcriptome subgroups utilized to define GBM are preferentially concentrated in tumor cells displaying unique lineage-specific biological states, according to recent results in single cells. The possibility of using the basic biological processes of individual GBM cells to create a clinically meaningful categorization of bulk tumors is still unproven.
2.3. Risk Factors
One of the few recognized risk factors that has been proven to increase the likelihood of developing gliomas is exposure to ionizing radiation [
35]. Radiation-induced GB is generally diagnosed years after receiving therapeutic radiation that was prescribed for another tumor or illness. [
36]. Other environmental risk factors for the growth of gliomas include exposure to vinyl chloride, pesticides, smoking, petroleum refining, and the production of synthetic rubber. It has not been demonstrated that exposure to electromagnetic fields, formaldehyde, or nonionizing radiation from cell phones causes GB [
37]. Less than 1% of people with glioma have a recognized hereditary disease; however, some specific genetic diseases, such as neurofibromatosis 1 and 2, tuberous sclerosis, Li–Fraumeni syndrome, retinoblastoma, and Turcot syndrome, are associated with an elevated risk of glioma development [
38].
2.4. Clinical Presentation and Imaging
The anatomical components of the affected brain and the size and location of the tumor can all have a significant impact on how a patient with newly diagnosed GB presents [
39,
40]. Patients frequently have headaches and localized or progressive neurologic impairments as signs of elevated intracranial pressure. Up to 25% of patients have a seizure as their first symptom, and up to 50% of patients can have one later on in the course of the disease [
41,
42]. Antiepileptic medicines (AEDs) are already part of the standard of care for patients who present with seizures, although routine use of AEDs in individuals without seizures is not advised [
43,
44]. At the time of diagnosis, corticosteroids are frequently prescribed to patients to assist in reducing vasogenic edema and relieve related signs and symptoms.
2.5. Current Treatment Options
The current standard of care is for concomitant radiotherapy with temozolomide and the greatest amount of safe surgical resection possible [
46]. Because these tumors are commonly invasive and typically located in expressive regions of the brain, such as regions that regulate speech, motor function, and the senses, extensive and full surgical resection of GBM is challenging. Radical removal of the initial tumor mass is not curative due to the high degree of invasiveness, and infiltrating tumor cells typically stay in the nearby brain, causing the disease to develop or return in the future [
47].
2.6. Role of Immunosuppressive Mechanism in Glioblastoma and Resistance to Immunotherapy
One typical feature of GBM that limits a favorable prognosis is recurrence. Not all patients had access to second-line therapy at this time (about 50% did not receive any treatment while their condition progressed) [
56,
57]. Numerous studies demonstrate that GBM is associated with an immunosuppressive microenvironment as a result of an increase in factors generated by tumor cells, including FASL, PD-1, indolamine 2, 3dioxygenase (IDO), and STAT3. Additionally, microglia cells have the ability to create IL-1 and TGF-B, which in turn regulate local myeloid and lymphatic immune cells and support systemic immunosuppression [
58]. By modifying the expression of several extracellular and intracellular mediators, myeloid cells promote the tumor by ensuring an immunosuppressive microenvironment [
59]. These variables all alter the cytotoxic T lymphocyte (CTL) phenotype, which raises the quantities of immunosuppressive markers like PD-1. Several research aims to boost anticancer immune responses by utilizing these approaches. For example, vaccination therapy or anti-PD-1 and anti-CTLA-4 treatments are used to kill tumor cells that have GBM-associated antigens like EGFRvIII [
60]. On the other hand, a treatment called viral oncolytic therapy applies a virus that can stimulate the immune system against the tumor. Attenuated oncolytic viruses propagate into tumor cells by taking advantage of the absence of a viral defense system [
61].
In recent times, the Hippo pathway has been extensively researched as a molecular mechanism to regulate angiogenesis, invasion, migration, and proliferation of tumors. Numerous investigations demonstrate that YAP may establish contact between immune cells and tumors, especially with TAMs [
66].
3. Novel Oncolytic Viral Therapy for Treatment of Glioblastoma
OVs are useful in treating GB because of their ability to replicate virally quickly in rapidly proliferating cells, their absence of distant metastases, and their alignment with the brain environment [
67,
68]. The anticancer immune response begins by converting “cold tumors” that are immunosuppressed by the microenvironment into “hot tumors” that are sensitive to the immune system [
69,
70,
71,
72]. Inducing an immunological response to inadvertently kill cancer cells through several mechanisms, including apoptosis, necrosis, and autophagy, is known as immunogenic cell death (ICD) [
73,
74,
75]. Releases of damage-associated molecular patterns (DAMPs), viral pathogen-associated molecular patterns (PAMPs), tumor-associated antigens (TAAs), and several other cytokines are indicative of this [
76,
77]. Oncolytic viruses enhance the function of antigen-presenting cells (APCs), which reach lymph nodes to recruit cytotoxic CD8+ T lymphocytes (CTLs) and attract them to the infection site, where they destroy tumor-inducing cells [
78,
79,
80].
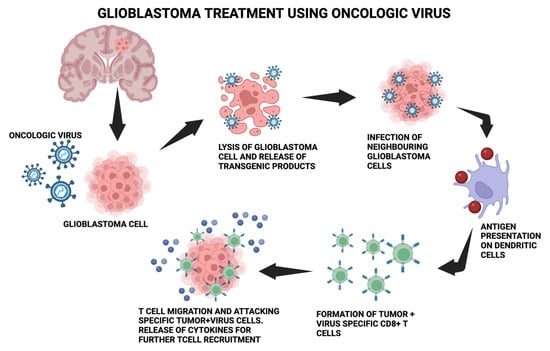
Figure 1 depicts the molecular process mentioned.
Figure 1. The molecular process behind using the oncologic virus for glioblastoma treatment. The oncolytic virus is introduced into glioblastoma cells, which causes lysis and the release of transgenic products. Neighboring glioblastoma cells get infected, leading to antigen presentation on dendritic cells and further leading to the formation of tumor + virus-specific CD8+T cells. T-cell migration and attacking of specific tumor + virus cells occurs.
Virotherapy is currently thought to be a promising immunotherapy for GB. The two types of OVs are replication-competent OVs, which only reproduce in cancer cells, and replication-deficient viral vectors, which are employed as carriers for additional therapeutic genes. Viruses that are produced through genetic engineering and naturally occurring viruses make up the first group [
81,
82]. The first group includes Newcastle disease viruses (NDV), reoviruses, and parvoviruses. The genetic modification of adenoviruses (Ad), herpes simplex virus (HSV), vaccine viruses (VV), vesicular stomatitis viruses (VSV), polioviruses, and measles viruses (MV) can decrease their pathogenicity and increase their tumor selectivity. To facilitate virus propagation and subsequent stimulation of the antitumor immune response, certain OVs use specialized receptors expressed on tumor cells.
In clinical studies for the treatment of GB, more than 20 oncolytic viruses have been examined. HSV-1 [
83,
84,
85], adenovirus [
86], reovirus [
87], MVs [
88,
89], NDVs [
90], and poliovirus [
91] are a few of them. Novel techniques for OV distribution are being developed to overcome the BBB limitation. One such technique is the convection-enhanced distribution (CED) of the recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO) [
92]. CED is a cutting-edge novel technology that transfers therapeutic chemicals in the interstitial regions of the CNS using a pressure gradient in a catheter [
92]. For virotherapy to be successful, oncolytic viruses must be delivered effectively and safely. Intratumoral delivery was selected as the main method due to the difficulty of delivering viruses to the CNS and the immune system’s ability to eliminate them [
93].
3.1. DNA Viruses
3.1.1. Herpes Simplex Virus Type I
The HSV-based oncolytic virus was the first modified viral strain assessed for experimental treatment in a murine GB model [
97]. A double-stranded DNA virus belonging to the Herpesviridae family, HSV-1 has double-stranded DNA. Nectin-1, a cell surface protein that is widely expressed in GB, serves as a binding site for HSV-1 [
98]. The US Food and Drug Administration (FDA) approved talimogene laherparepvec (T-VEC) in 2015 to treat advanced, unresectable melanoma that is not amenable to surgery [
98,
99]. T-VEC expresses granulocyte-macrophage colony-stimulating factor (GM-CSF). GB patients are participating in clinical studies with many modified HSV constructs, including G207, HSV-1716, M032, and MVR-C252. Tumor selectivity was improved in all HSV recombinants by removing both copies of the RL1 gene, which codes for the viral protein (ICP34.5 producing neurovirulence) [
100,
101]. HSV G207, a recombinant strain with a malfunctioning viral ribonucleotide reductase (RR), was unable to reproduce in healthy cells. By producing the homologic gene, tumor cells make up for the loss of RR [
102]. Third-generation oncolytic HSV-1 strain G47, sometimes referred to as DELYTACT, has demonstrated encouraging results in phase II clinical studies [
103]. G47 was created by modifying the ICP47 gene with a deletion to improve the immune system’s ability to recognize tumors utilizing MHC class I [
103]. For 19 adult patients with glioblastoma that was either residual or recurrent, a phase II single-arm study using G47Δ was initiated. Up to six intratumoral doses of G47Δ were given. A one-year survival rate of 84.2% indicates that the stated endpoint was achieved. The most frequent side effect was fever in 17 out of 19 patients. The patients’ biopsies revealed CD4+/CD8+ cells that had infiltrated the tumors [
104]。
3.1.2. Adenovirus
Adenoviruses are non-enveloped, double-stranded DNA viruses that have an icosahedral shape [
117]. Conditionally replicative adenoviruses (CRads) have been developed in a number of variations and have demonstrated promising anti-GB activity in clinical settings [
118]. Furthermore, individuals with GB alone or in conjunction with other ICIs are now undergoing clinical studies with a genetically engineered adenovirus (
Table 1). Reportedly, malignant gliomas have been effectively treated with an alternative gene-mediated cytotoxic treatment approach [
119]. The phase II clinical study employed adenovirus glatimagene besadenovec (AdV-tk), which possesses the HSV thymidine kinase gene and kills cancer cells upon interacting with alacyclovir [
120]. Removing the viral replication genes is one way to stop off-targets in normal cells, which can still proliferate in tumor cells. China has approved H101 (Oncorine), which is similar to oncolytic adenovirus ONYX-015, for the treatment of head and neck cancer [
121,
122]. A loss in the E1B-55K gene limited the recombinant adenovirus ONYX-015 oncolytic’s capacity to replicate to tumors with p53 abnormalities [
123]. Two genetic changes were found in DNX-2401, an OV based on serotype 5 Ad (Ad5) [
124]. When the E1A gene is removed and an RGD-4C motif is added to the fiber’s HI loop, the virus switches to replicate in cells with impaired pRB pathways that generate v3- and v5-integrins, both of which are indicators of glioma cells [
125]. Due to this alteration, adenoviruses may infiltrate cells even in the presence of trace levels of their major receptor, the cox-sackie-adenovirus receptor, on brain tumor cells [
126]. The OX40L gene is produced by the second generation of DNX-2401, DNX-2440 (also known as Delta-24-RGDOX), to improve T-cell-mediated immunity by encouraging the proliferation of CD8+ specific-tumor T cells [
127,
128]
3.1.3. Parvoviruses
The Parvoviridae family of single-stranded icosahedral DNA viruses includes parvoviruses. Various animal species can be infected by one of about 134 different parvovirus serotypes [
132]. A small oncolytic virus called H-1 parvovirus has shown anticancer efficacy against GB [
133]. Additionally, H-1PV causes glioma cells to undergo apoptosis and breaks down their resistance to a number of chemotherapeutic drugs [
134]. Human U87-MG glioma models in rats showed tumor shrinkage in preclinical studies with the H-1PV [
135]. As a result, the ParvOryx01 trial for individuals with recurrent GB (NCT01301430) was started. ParvOryx01 proposed that tumor-infiltrating lymphocytes (TILs) were responsible for inducing immune responses in the removed tumor tissues of GB patients [
136]. In high-grade human gliomas, radiation promotes H-1PV viral oncolysis, which may be considered in animal glioma models [
119]. Bevacizumab with H-1PV improved the mean survival to 15.4 months in five patients with recurrent GB and produced remission in three of them [
137]. These results are associated with the synergistic effect of bevacizumab and H-1PV in controlling GB TME and decreasing VEGF [
138]. The first clinical evidence of using H-1PV in combination with bevacizumab and an immune checkpoint inhibitor (nivolumab) was shown in a multimodal clinical study including three patients with recurrent GB. Every participant achieved clinical improvement and confirmed tumor shrinkage, with 78% of cases showing complete or partial remission [
139].
3.1.4. Myxoma Virus
A part of the poxvirus family with double-stranded DNA is the myxoma virus (MYXV) [
140,
141]. MYXV can cause an oncolytic impact when it replicates in cells like GB that lack an interferon system [
142]. The deletion of the viral antiapoptotic protein M011L in the M011L-deficient MYXV virus boosted apoptosis in malignant glioma cells [
143]. A prospective candidate OV that has shown promise in several preclinical cancer models is MYXV. Furthermore, MYXV is an appealing OV platform due to its remarkable safety profile outside of rabbits, its extremely selective tropism for a wide variety of cancer cell types, and the limitation of viral multiplication in original non-transformed human cells.
3.1.5. Vaccinia Virus (VV)
The Poxviridae family includes the double-stranded DNA virus known as a vaccine. Smallpox was eradicated with the aid of VV. Because VV may infect any kind of cell by membrane fusion with a non-integrative replication cycle, it is a suitable platform for oncolytic viral engineering against GB [
144]. The only recombinant VV that has shown therapeutic benefits in brain tumor patients is TG6002 [
145]. The TG6002 genome contains two additional gene deletions for the RR and thymidine kinase (TK) genes. By introducing the FCU1 gene, the chemotherapy prodrug 5-flucytosine (5-FC) was also converted into 5-fluorouracil (5-FU) [
146]. Prior research has demonstrated that systemic PD-1 blockade medication and local injection of oncolytic VV together are more effective than either treatment alone.
3.2. RNA Viruses
3.2.1. Measles Virus
The measles virus (MV) is a single-stranded RNA virus with a negative sense that belongs to the Paramyxoviridae family [
147]. The MV enters cells via engaging with the overexpressed CD46 cell receptor on tumor cells as well as the viral hemagglutinin (H) protein [
148]. Recombinant MVs entered clinical trials after glioma xenografts showed strong anticancer efficacy in them [
149,
150]. To monitor viral expression in cells, such recombinants express the human sodium iodide symporter (NIS) or the human carcinoembryonic antigen (CEA) [
151]. NIS allows for the monitoring of viruses using various isotopes and has the potential to enhance viral cytopathogenic effects [
152,
153]. The present studies deliver the maximum practicable dosages as a result of the observed dose–response correlations.
3.2.2. Vesicular Stomatitis Virus (VSV)
The VSV is a single-stranded, negative-sense RNA virus that belongs to the Rhabdoviridae family. The spike glycoprotein (G) of the VSV is linked to the low-density lipoprotein receptor (LDL-R), a cell receptor that is broadly dispersed [
137]. The VSV is employed as an oncolytic drug against various malignancies by replicating in tumor cells via the abnormalities in their interferon system [
138,
139]. rVSV (GP) and VSV-EBOV are terms for engineered VSVs that have had the envelope glycoprotein (GP) substituted with GP from the Ebola virus and the non-neurotropic lymphocytic choriomeningitis virus, respectively [
140,
141]. Despite entering a phase I clinical trial, an oncolytic VSV is suppressed by viral-mediated production of interferon (IFN)β, which has been demonstrated to increase the virus’s safety.
3.2.3. Reoviruses
When the Ras-signaling pathway is triggered in glioma cells, reoviruses—double-stranded RNA non-enveloped viruses—can multiply in the cells [
139]. Reovirus RNA genome mutations occur quite quickly. This offers a degree of flexibility that may be used to choose reovirus variants with higher levels of oncolytic activity. The reovirus genome may also be genetically altered, providing further possibilities for boosting the oncolytic activity. One such method is the insertion of tiny therapeutic transgenes [
139]. Reoviruses having the benefit of not being linked to any severe human diseases, and there are now more and more clinical trials including the use of reovirotherapy to treat cancer. With the modest effectiveness of reovirus as a monotherapy, the emphasis has shifted to combination regimens thus far. Apart from genetic alteration, conventional bioselection is an additional mechanism that may be employed to augment the oncolytic capabilities of reoviruses.
3.2.4. Newcastle Disease Virus (NDV)
The Paramyxoviridae family includes the negative-sense, single-stranded RNA-enveloped NDV virus [
137]. Interferon-stimulated genes (ISGs) are expressed by the NDV, which is largely an avian virus that preferentially replicates in tumor cells and triggers the type I interferon response in humans [
148,
149].
3.2.5. Seneca Valley Virus Isolate 001 (SVV-001)
A member of the Picornaviridae family of positive-sense single-stranded RNA, the SVV-001 [
151] has shown oncolytic activity against solid tumors, with a particular affinity for cells expressing the endothelium receptor TEM8/ANTXR1 [
152]. The transmembrane glycoprotein adhesion molecule TEM8/ANTXR1 is more prevalent in some cancer types and mediates cell motility and its interactions with the extracellular matrix (ECM) [
153]. TEM8/ANTXR1 is the first biomarker for SVV-based oncolytic viral treatment [
154]. SVV-001 given intravenously has anticancer properties and is able to pass the BBB [
155].
3.2.6. Polioviruses
The Picornaviridae family of positive-sense single-strand RNA viruses includes polioviruses [
156]. The CD155/PVR receptor, which is typically overexpressed on cancerous cells, is used by polioviruses to infect cells [
156].
The internal ribosome entry site (IRES) of an attenuated poliovirus type 1 (Sabin) vaccination strain was substituted with an IRES from a human rhinovirus type 2 in order to reduce the potential neurovirulence [
157,
158]. In the phase I study (NCT01491893) looking at the intratumoral CED of PVSSRIPO in patients with recurrent GB, the safety and absence of neurovirulence were established. Consequently, the PVSRIPO was granted a breakthrough therapeutic classification by the FDA in 2016 [
159].
3.2.7. Sindbis Virus
The Togaviridae family of positive-sense single-stranded RNA viruses includes the Sindbis virus [
163]. Via binding to the laminin receptor (LAMR), Sindbis infects cancer cells and causes death in glioma cells [
164,
165] via tyrosine phosphorylating protein kinase C delta. The Semliki forest virus (SFV4miRT) contains target sequences for miR124, miR125, and miR134 inserted into it; it is expressed more in healthy CNS cells than in glioma cells [
166]. As a result, this virus has a decreased neurotropism, oncolytic effectiveness, and safer profile [
167,
168].
4. Conclusions
The efficacy of oncolytic virus therapy is anticipated to increase when coupled with immunotherapy since the common feature that plays a significant role in showing anticancer effects during oncolytic activities is the formation of specific antitumor immunity. Functional transgenes would enable oncolytic viruses to be equipped with a wide range of anticancer capabilities in the future. Based on the kind and stage of cancer, a combination of suitable viruses may then be selected from this panel. Oncolytic viral therapy appears to be the beginning of a new age in cancer treatment, where patients have the freedom to choose this treatment option. For the purpose of creating oncolytic viruses, a reiterative feedback loop—in which the outcomes of clinical trials inform and influence the design of succeeding generations of viruses—is preferred over a unidirectional method. Particularly with regard to virotherapy in the brain, preclinical laboratory research can only partially address the unique challenges this field faces. The biological effects of viruses vary greatly depending on the species under investigation, in contrast to small molecule therapies. Human viruses including poliovirus, AdV, and HSV are greatly attenuated in tumor models found in rodents, but they may be less so when administered to people. On the other hand, non-human infections including PRV, SIN, and VSV can be harmful to mice, which makes preclinical survival research extremely difficult. Oncolytic viruses are distinct from conventional medications in a number of ways. Since they are live viruses, their effective dosages may vary depending on how quickly they multiply in a therapeutic setting. Little information is currently known on the relationship between viral dosage, in vivo replicative capability, and treatment response. To create safe and effective dose guidelines, more research on viral replication and clinical response in pertinent preclinical models and clinical trials is necessary. In several clinical studies carried out across a wide spectrum of malignancies, oncolytic viruses have so far been linked to a generally acceptable safety profile. However, given these agents’ capacity for replication, infection control measures—such as proper handling, storage, preparation, and delivery of the virus—must be taken seriously. The actual risk of infection is contingent upon the type of virus, co-occurring medical problems in patients, close household contacts, and healthcare personnel who may come into touch with the virus.
This entry is adapted from the peer-reviewed paper 10.3390/medsci12010001