Alzheimer’s disease (AD) is an extremely devastating neurodegenerative disease, and there is no cure for it. AD is specified as the misfolding and aggregation of amyloid-β protein (Aβ) and abnormalities in hyperphosphorylated tau protein. Current approaches to treat Alzheimer’s disease have had some success in slowing down the disease’s progression. Chaperone proteins act as molecular caretakers to facilitate cellular homeostasis under standard conditions. Chaperone proteins like heat shock proteins (Hsps) serve a pivotal role in correctly folding amyloid peptides, inhibiting mitochondrial dysfunction, and peptide aggregation. For instance, Hsp90 plays a significant role in maintaining cellular homeostasis through its protein folding mechanisms.
- Alzheimer’s disease
- amyloid-β protein
- Hsp90
- BRICHOS domain chaperone
- chemical chaperone
1. Introduction
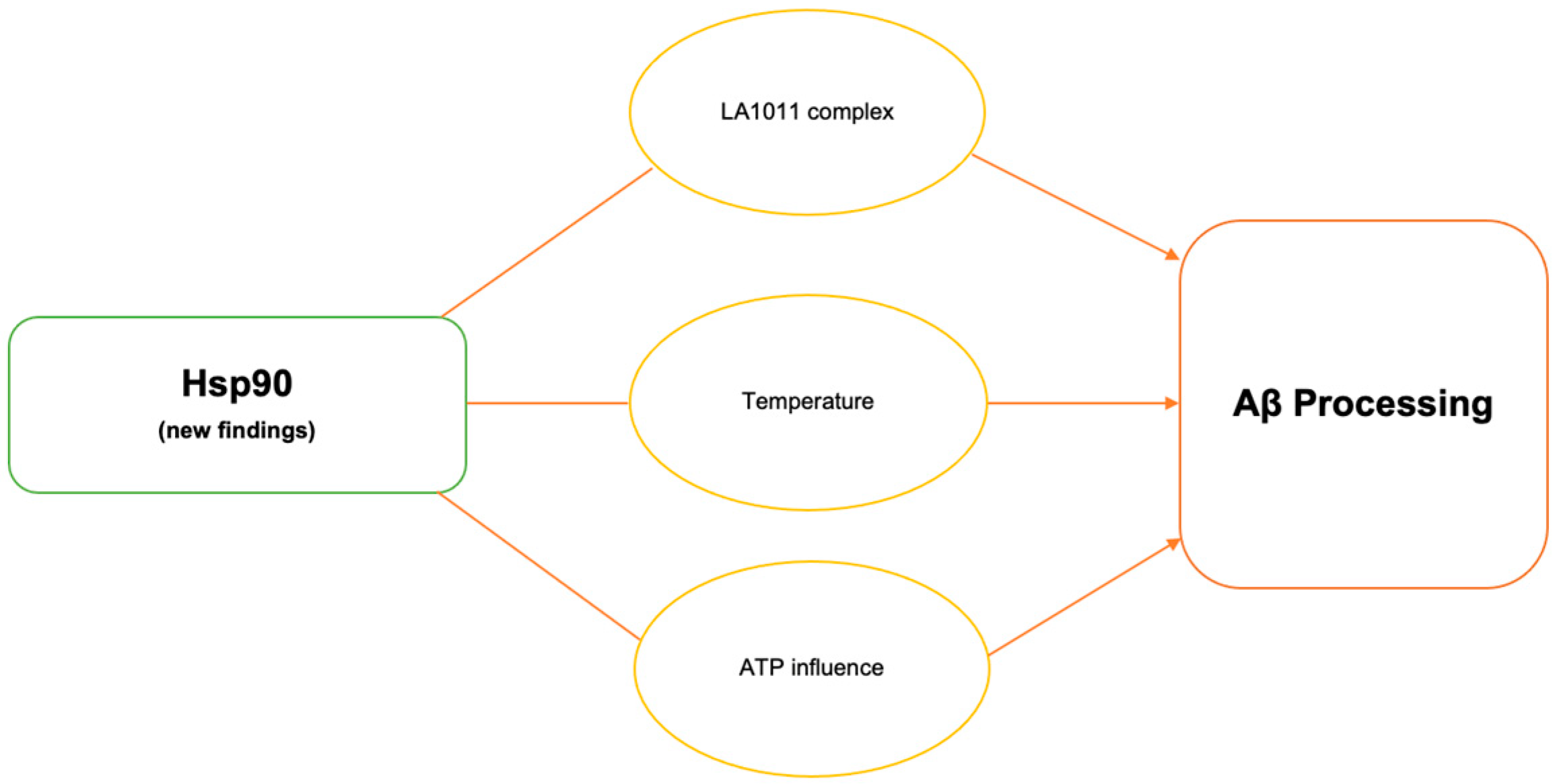
2. Hsp90
2.1. Hsp90–LA1011 Complex
2.2. Temperature on Hsp90 Activity
2.3. Adenosine Triphosphate (ATP)-Mediated Changes in Hsp90 Activity
3. BRICHOS Chaperone Domain
3.1. BRICHOS Interactions
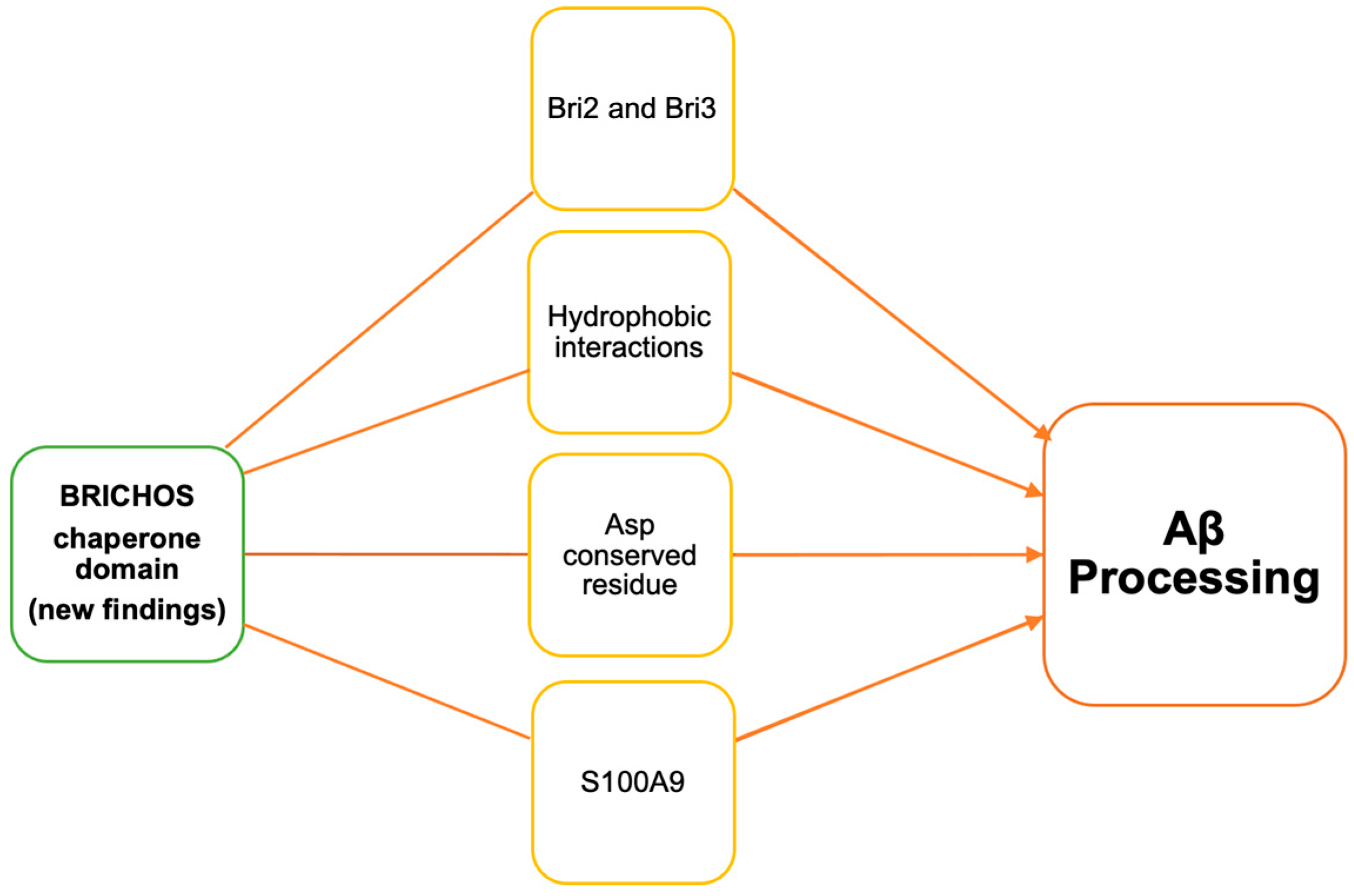
3.2. S100A9 and Bri2 BRICHOS
3.3. BRICHOS Domain Dependent on Conserved Asp Residue
3.4. Bri3 BRICHOS Domain Chaperone’s Efficiency
This entry is adapted from the peer-reviewed paper 10.3390/biom14010016
References
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714.
- Stelzmann, R.A.; Norman Schnitzlein, H.; Reed Murtagh, F. An english translation of Alzheimer’s 1907 paper, “über eine eigenartige erkankung der hirnrinde”. Clin. Anat. 1995, 8, 429–431.
- Alzheimer’s Association. Facts and Figures. Available online: https://www.alz.org/alzheimer_s_dementia (accessed on 10 November 2023).
- Gupta, A.; Goyal, R. Amyloid beta plaque: A culprit for neurodegeneration. Acta Neurol. Belg. 2016, 116, 445–450.
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778.
- Hazenberg, B.P.C. Amyloidosis. Rheum. Dis. Clin. N. Am. 2013, 39, 323–345.
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235.
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248.
- Wentink, A.; Nussbaum-Krammer, C.; Bukau, B. Modulation of Amyloid States by Molecular Chaperones. Cold Spring Harb. Perspect. Biol. 2019, 11, a033969.
- Bukau, B.; Weissman, J.; Horwich, A. Molecular Chaperones and Protein Quality Control. Cell 2006, 125, 443–451.
- Kampinga, H.H.; Craig, E.A. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 2010, 11, 579–592.
- Li, Z.; Srivastava, P. Heat-shock proteins. Curr. Protoc. Immunol. 2004, 58, A-1T.
- Fink, A.L. Chaperone-mediated protein folding. Physiol. Rev. 1999, 79, 425–449.
- Malik, J.A.; Lone, R. Heat shock proteins with an emphasis on HSP 60. Mol. Biol. Rep. 2021, 48, 6959–6969.
- Yoo, B.C.; Kim, S.H.; Cairns, N.; Fountoulakis, M.; Lubec, G. Deranged Expression of Molecular Chaperones in Brains of Patients with Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2001, 280, 249–258.
- Chen, Y.; Wang, B.; Liu, D.; Li, J.J.; Xue, Y.; Sakata, K.; Zhu, L.-Q.; Heldt, S.A.; Xu, H.; Liao, F.-F. Hsp90 Chaperone Inhibitor 17-AAG Attenuates Aβ-Induced Synaptic Toxicity and Memory Impairment. J. Neurosci. 2014, 34, 2464–2470.
- Kasza, Á.; Hunya, Á.; Frank, Z.; Fülöp, F.; Török, Z.; Balogh, G.; Sántha, M.; Bálind, Á.; Bernáth, S.; Blundell, K.L.; et al. Dihydropyridine Derivatives Modulate Heat Shock Responses and have a Neuroprotective Effect in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 53, 557–571.
- Roe, M.S.; Wahab, B.; Török, Z.; Horváth, I.; Vigh, L.; Prodromou, C. Dihydropyridines Allosterically Modulate Hsp90 Providing a Novel Mechanism for Heat Shock Protein Co-induction and Neuroprotection. Front. Mol. Biosci. 2018, 5, 51.
- Sánchez-Pulido, L.; Devos, D.; Valencia, A. BRICHOS: A conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem. Sci. 2002, 27, 329–332.
- Buxbaum, J.N.; Johansson, J. Transthyretin and BRICHOS: The Paradox of Amyloidogenic Proteins with Anti-Amyloidogenic Activity for Aβ in the Central Nervous System. Front. Neurosci. 2017, 11, 119.
- Willander, H.; Hermansson, E.; Johansson, J.; Presto, J. BRICHOS domain associated with lung fibrosis, dementia and cancer—A chaperone that prevents amyloid fibril formation? FEBS J. 2011, 278, 3893–3904.
- Poska, H.; Leppert, A.; Tigro, H.; Zhong, X.; Kaldmäe, M.; Nilsson, H.E.; Hebert, H.; Chen, G.; Johansson, J. Recombinant Bri3 BRICHOS domain is a molecular chaperone with effect against amyloid formation and non-fibrillar protein aggregation. Sci. Rep. 2020, 10, 9817.
- Leppert, A.; Poska, H.; Landreh, M.; Abelein, A.; Chen, G.; Johansson, J. A new kid in the folding funnel: Molecular chaperone activities of the BRICHOS domain. Protein Sci. 2023, 32, e4645.
- Nishimura, T.; Akiyoshi, K. Artificial Molecular Chaperone Systems for Proteins, Nucleic Acids, and Synthetic Molecules. Bioconj. Chem. 2020, 31, 1259–1267.
- Roe, S.M.; Török, Z.; McGown, A.; Horváth, I.; Spencer, J.; Pázmány, T.; Vigh, L.; Prodromou, C. The Crystal Structure of the Hsp90-LA1011 Complex and the Mechanism by Which LA1011 May Improve the Prognosis of Alzheimer’s Disease. Biomolecules 2023, 13, 1051.
- Smedlund, K.B.; Sanchez, E.R.; Hinds, T.D., Jr. FKBP51 and the molecular chaperoning of metabolism. Trends Endocrinol. Metab. 2021, 32, 862–874.
- Rowles, J.E.; Keane, K.N.; Gomes Heck, T.; Cruzat, V.; Verdile, G.; Newsholme, P. Are Heat Shock Proteins an Important Link between Type 2 Diabetes and Alzheimer Disease? Int. J. Mol. Sci. 2020, 21, 8204.
- Noorani, A.A.; Yamashita, H.; Gao, Y.; Islam, S.; Sun, Y.; Nakamura, T.; Enomoto, H.; Zou, K.; Michikawa, M. High temperature promotes amyloid β-protein production and γ-secretase complex formation via Hsp90. J. Biol. Chem. 2020, 295, 18010–18022.
- Priyanka; Seth, P. Insights into the Role of Mortalin in Alzheimer’s Disease, Parkinson’s Disease, and HIV-1-Associated Neurocognitive Disorders. Front. Cell Dev. Biol. 2022, 10, 903031.
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-deMiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488.
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583.
- Hoye, A.T.; Davoren, J.E.; Wipf, P.; Fink, M.P.; Kagan, V.E. Targeting mitochondria. Acc. Chem. Res. 2008, 41, 87–97.
- Picone, P.; Nuzzo, D.; Giacomazza, D.; Di Carlo, M. β-Amyloid Peptide: The Cell Compartment Multi-faceted Interaction in Alzheimer’s Disease. Neurotox. Res. 2020, 37, 250–263.
- Wang, H.; Lallemang, M.; Hermann, B.; Wallin, C.; Loch, R.; Blanc, A.; Balzer, B.N.; Hugel, T.; Luo, J. ATP Impedes the Inhibitory Effect of Hsp90 on Aβ(40) Fibrillation. J. Mol. Biol. 2021, 433, 166717.
- Ali, M.M.U.; Roe, S.M.; Vaughan, C.K.; Meyer, P.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. Crystal structure of an Hsp90–nucleotide–p23/Sba1 closed chaperone complex. Nature 2006, 440, 1013–1017.
- Picard, D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol. Life Sci. 2002, 59, 1640–1648.
- McClellan, A.J.; Xia, Y.; Deutschbauer, A.M.; Davis, R.W.; Gerstein, M.; Frydman, J. Diverse Cellular Functions of the Hsp90 Molecular Chaperone Uncovered Using Systems Approaches. Cell 2007, 131, 121–135.
- Österlund, N.; Vosselman, T.; Leppert, A.; Gräslund, A.; Jörnvall, H.; Ilag, L.L.; Marklund, E.G.; Elofsson, A.; Johansson, J.; Sahin, C.; et al. Mass Spectrometry and Machine Learning Reveal Determinants of Client Recognition by Antiamyloid Chaperones. Mol. Cell. Proteom. 2022, 21, 100413.
- Knight, S.D.; Presto, J.; Linse, S.; Johansson, J. The BRICHOS Domain, Amyloid Fibril Formation, and Their Relationship. Biochemistry 2013, 52, 7523–7531.
- Shimozawa, M.; Tigro, H.; Biverstål, H.; Shevchenko, G.; Bergquist, J.; Moaddel, R.; Johansson, J.; Nilsson, P. Identification of cytoskeletal proteins as binding partners of Bri2 BRICHOS domain. Mol. Cell Neurosci. 2023, 125, 103843.
- Manchanda, S.; Galan-Acosta, L.; Abelein, A.; Tambaro, S.; Chen, G.; Nilsson, P.; Johansson, J. Intravenous treatment with a molecular chaperone designed against β-amyloid toxicity improves Alzheimer’s disease pathology in mouse models. Mol. Ther. 2023, 31, 487–502.
- Andrade-Talavera, Y.; Chen, G.; Pansieri, J.; Arroyo-García, L.E.; Toleikis, Z.; Smirnovas, V.; Johansson, J.; Morozova-Roche, L.; Fisahn, A. S100A9 amyloid growth and S100A9 fibril-induced impairment of gamma oscillations in area CA3 of mouse hippocampus ex vivo is prevented by Bri2 BRICHOS. Prog. Neurobiol. 2022, 219, 102366.
- Wang, C.; Iashchishyn, I.A.; Pansieri, J.; Nyström, S.; Klementieva, O.; Kara, J.; Horvath, I.; Moskalenko, R.; Rofougaran, R.; Gouras, G.; et al. S100A9-Driven Amyloid-Neuroinflammatory Cascade in Traumatic Brain Injury as a Precursor State for Alzheimer’s Disease. Sci. Rep. 2018, 8, 12836.
- Wang, C.; Klechikov, A.G.; Gharibyan, A.L.; Wärmländer, S.K.T.S.; Jarvet, J.; Zhao, L.; Jia, X.; Shankar, S.K.; Olofsson, A.; Brännström, T.; et al. The role of pro-inflammatory S100A9 in Alzheimer’s disease amyloid-neuroinflammatory cascade. Acta Neuropathol. 2014, 127, 507–522.
- Horvath, I.; Iashchishyn, I.A.; Moskalenko, R.A.; Wang, C.; Wärmländer, S.K.T.S.; Wallin, C.; Gräslund, A.; Kovacs, G.G.; Morozova-Roche, L.A. Co-aggregation of pro-inflammatory S100A9 with α-synuclein in Parkinson’s disease: Ex vivo and in vitro studies. J. Neuroinflamm. 2018, 15, 172.
- Chen, G.; Andrade-Talavera, Y.; Zhong, X.; Hassan, S.; Biverstål, H.; Poska, H.; Abelein, A.; Leppert, A.; Kronqvist, N.; Rising, A.; et al. Abilities of the BRICHOS domain to prevent neurotoxicity and fibril formation are dependent on a highly conserved Asp residue. RSC Chem. Biol. 2022, 3, 1342–1358.
- Matsuda, S.; Matsuda, Y.; D’Adamio, L. BRI3 inhibits amyloid precursor protein processing in a mechanistically distinct manner from its homologue dementia gene BRI2. J. Biol. Chem. 2009, 284, 15815–15825.
- Matsuda, S.; Matsuda, Y.; Snapp, E.L.; D’Adamio, L. Maturation of BRI2 generates a specific inhibitor that reduces APP processing at the plasma membrane and in endocytic vesicles. Neurobiol. Aging 2011, 32, 1400–1408.
- Martin, L.; Fluhrer, R.; Haass, C. Substrate requirements for SPPL2b-dependent regulated intramembrane proteolysis. J. Biol. Chem 2009, 284, 5662–5670.
- Dolfe, L.; Tambaro, S.; Tigro, H.; Del Campo, M.; Hoozemans, J.J.M.; Wiehager, B.; Graff, C.; Winblad, B.; Ankarcrona, M.; Kaldmäe, M.; et al. The Bri2 and Bri3 BRICHOS Domains Interact Differently with Aβ(42) and Alzheimer Amyloid Plaques. J. Alzheimer’s Dis. Rep. 2018, 2, 27–39.
