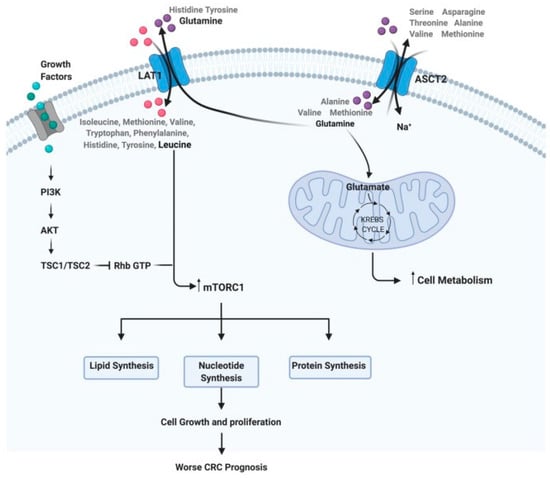
Amino acids are the building blocks of proteins and essential players in pathways such as the citric acid and urea cycle, purine and pyrimidine biosynthesis, and redox cell signaling. Therefore, it is unsurprising that these molecules have a significant role in cancer metabolism and its metabolic plasticity.
1. Introduction
Colorectal cancer can be considered a severe public health problem [
1]. The third most frequent cancer presents considerable mortality, motivated by a diagnosis often made at an advanced stage of the disease. On the other hand, whenever detected early, the probability of controlling the disease is high, with reasonable survival rates at five years [
2]. Thus, combating it depends on effective screening programs, early diagnosis, and individualized therapies with continuous and effective monitoring. These are the only ways to reduce the incidence and improve the outcomes of treated cases regarding survival and quality of life, simultaneously reducing the economic burden.
Currently, control of this type of cancer is based on three aspects: screening, diagnosis, and treatment. In screening programs, fecal occult blood tests and colonoscopies (on a smaller scale) are still the most used methods starting from 50 years of age in asymptomatic individuals. However, implementing molecular tests that measure the immune system’s response to the attack of colorectal cancer in screening programs could prove to be an essential step to achieve. A colonoscopy beyond screening is the main weapon in prevention (a colonoscopy with polypectomy prevents patients with adenomatous polyps from developing colorectal cancer) and tumor diagnosis. When the diagnosis of invasive cancer is made, it is necessary to carry out correct staging to plan an individualized therapy that may include surgery, radiotherapy, and chemotherapy in different modalities. Monitoring the response to treatment, screening for recurrence, and selecting the ideal treatment are also essential aspects of managing this oncological entity.
Biomarkers are substances that can be measured in biological fluids or tissues and predict the incidence or outcome of disease [
3]. A promising biomarker should fulfill five essential requirements: good specificity, good sensitivity, cost effectiveness, ease of implementation, and the ability to detect the disease at its early stages. While sensitivity refers to the ability of the biomarker to detect diseased patients, specificity relates to its capability to reject other pathologies or healthy individuals.
Colorectal cancer-specific biomarkers are still an area in development. Many biomarkers with different characteristics are currently being analyzed, which can be investigated in blood, urine, or tissue using various methods with different sensitivities, specificities, and clinical practice applicability. One example of these markers is the carcinoembryonic antigen (CEA) measured in blood, which is the most common tool used in the clinic for disease monitoring after resection surgery. Others include tumor biomarkers, such as Kirsten rat sarcoma virus (KRAS) and V-Raf murine sarcoma viral oncogene homolog B (BRAF) mutations or microsatellite instability, which have implications for therapeutic selection [
4]. In the case of predictive biomarkers for response to neoadjuvant treatment in Locally Advanced Rectal Cancer (LARC), most biomarkers so far have been imaging variables and genetic parameters, as well as DNA- and RNA-based (TYMS polymorphisms, cell-free DNA, expression studies, microRNAs) [
5,
6,
7]. Some are expensive, require state-of-the-art technology and complex laboratory procedures, and are not fully applied to the clinic yet.
Within this scope, whether in a targeted or untargeted fashion, metabolomics seems to be a tool for developing new biomarkers in cancer. Metabolomics uses body fluids and techniques to study low molecular weight molecules, such as amino acids, lipids, carbohydrates, and intermediates of various metabolic pathways. Some are already established procedures for screening and diagnosing other diseases, such as metabolic disorders. Metabolomics further allows the study of pathways known to be deregulated in cancer cells, providing a more thorough picture of the patient’s metabolic state. Glycolysis, the TriCarboxylic Acid Cycle (TCA), and mitochondrial metabolism have gathered much attention concerning cancer metabolism’s peculiarities. Their vast replicative potential, their metabolic plasticity, and their choice of aerobic glycolysis and concomitant lactate production as a source of energy—the Warburg effect—have made cancer cells an interesting source of possible metabolites for screening [
8].
Among the vast array of molecules that might play a primordial role in the pathogenesis of cancer, amino acids have been attracting substantial attention. They have not only their role as building blocks for the synthesis of proteins but also can be considered alternative fuels in cancer due to their involvement in biosynthetic pathways [
9]. For instance, glutamine and branched-chain amino acids provide organic molecules to fuel the TCA cycle; glutamate, glycine, and cysteine are used for glutathione synthesis, an antioxidant molecule essential to counter the production of reactive oxygen species during cell proliferation. In the same way, polyamines resulting from arginine catabolism have been associated with cancer development for decades [
10]. Proteins involved in amino acid metabolism have also been under cancer research. They include transmembrane solute carriers and amino acid transporters, such as L-type amino acid transporter 1 and alanine–serine–cysteine transporter 2, which are usually upregulated in colorectal cancer [
11]. Even though there is substantial biochemical evidence for the relevance of amino acids in cancer, there is still a lack of clinical output focusing exclusively on their use as biomarkers for the diagnosis, prognosis, and monitoring of therapeutic response in CRC (
Figure 1).
Figure 1. Steps of the amino acid system. For example: how glutamine and leucine levels can interfere with cell metabolism, growth, and proliferation, implying a worse prognosis in CRC.
2. Amino Acid Profiling in Colorectal Cancer Patients
2.1. Cancer Tissue Metabolomics
Research has shown that transmembrane amino acid transporters are upregulated in colorectal cancer [
11], facilitating their import to the cell. These transporters are now considered possible therapeutic targets. A larger concentration of amino acids in CRC tissues compared to normal mucosa is a hallmark in the published literature, which is consistent with a higher need for nutrients and the upregulation of protein turnover to support the high proliferation rates of cancer cells [
17,
18,
19,
20,
21]. It has also been shown that this increase in amino acid content is also a distinctive feature between colorectal tumors and advanced adenoma, with satisfying sensitivity and specificity [
18].
Another common trend seems to be an upregulation of glutamate in tumor tissues, which may be related to augmented glutamine degradation by cancer cells [
19,
22,
23,
24,
25,
26]. The prognostic potential of tissue glutamate has further been confirmed with a large sample of 376 surgical specimens from four independent cohorts of patients, using gas chromatography as an analytical platform [
24]. Glycine also presents increased concentration in tumor specimens, with possible prognostic capabilities [
18,
20,
27].
2.2. Serum and Plasma Metabolomics in Colorectal Cancer Patients
Although studies focusing exclusively on plasma-free or serum amino acid levels in colorectal cancer patients are scarce (
Table 1), a common observation among published data is a decrease in the concentration of several amino acids in circulation [
28,
29,
30,
31,
32], such as tyrosine, methionine, and threonine.
2.2.1. Glutamine and Glutamate Metabolism
Glutamine is the most abundant free amino acid and an important player in cancer proliferation due to its role as an alternative fuel and anaplerotic substrate of the TCA cycle [
33]. Even being a non-essential amino acid, “glutamine addiction” is a known hallmark of cancer cells. As such, it is worth investigating if significant disturbances in its metabolism are found in circulation in CRC patients. In serum and plasma, glutamine seems to be consistently downregulated in CRC patients [
6,
31,
34,
35,
36], which may be related to the excessive consumption of this amino acid by the tumors. Only one study verified the opposite trend [
28].
There are inconsistencies in what concerns serum glutamate. While some authors describe an elevation of circulating glutamate in CRC patients compared to controls [
35,
36], others have seen the opposite trend [
6,
28,
37]. It is worth mentioning that the work by Tan et al. is remarkably consistent due to the use of a patient-set validation in a large test population [
6]. Furthermore, using two different chromatography techniques, these authors could distinguish stage I patients from healthy controls.
2.2.2. Glycine and Serine Metabolism
Glycine is a component of glutathione and is one of the main antioxidant molecules of the cell, and, as such, it has a relevant role in redox homeostasis [
38]. An upregulation of glycine and serine biosynthesis is a characteristic of cancer cells, which produce intermediates to synthesize these amino acids through their high rates of aerobic glycolysis. Nonetheless, there is no clear trend regarding circulating levels of these amino acids in CRC, as both upregulations and downregulations have been observed for glycine [
30,
35] and serine [
6,
30,
35].
2.2.3. Tryptophan Metabolism
Tryptophan, an essential amino acid, is known to have a role in intestinal health—it is catabolized by the gut microbiota to produce indoles, compounds involved in numerous aspects of gut homeostasis. The kynurenine pathway, through which most of the ingested tryptophan is catabolized, generates compounds that induce the proliferation arrest of T lymphocytes and, as such, suppress the antitumor immune response [
39]. In this regard, tryptophan has been correlated with immune activation and quality of life in CRC patients [
40], and targeting its catabolism is currently sprouting several lines of investigation. Data obtained using several analytical platforms have shown a clear tendency of reduced circulating tryptophan in CRC patients [
6,
31,
36,
37,
41], including a study using plasma from early-stage volunteers (0/I/II) [
42,
43]. Interestingly, pre-operative serum tryptophan has been singled out as a possible discriminator between colon and rectal cancer [
44].
2.2.4. Branched-Chain Amino Acid Metabolism
These essential amino acids, which include valine, leucine, and isoleucine, are used by tumors for biosynthetic purposes. By activating the mammalian Target Of Rapamycin (mTOR) pathway, they can stimulate protein translation and are a nitrogen source for nucleotide synthesis. They are also incorporated into the TCA cycle through the production of Acetyl-CoA (acetyl coenzyme A). Interestingly, blood tests for the detection and recurrence of CRC based on their metabolism have been reported [
38]. Diminished circulating levels of branched-chain amino acids in CRC patients seem quite common [
28,
29,
31,
45,
46,
47]. Geijsen and colleagues verified this phenomenon for valine and leucine in CRC plasma using 268 patients and 353 controls [
45]. Serum isoleucine has also been reported as a possible discriminator between colon and rectal cancer [
44].
2.2.5. Proline Metabolism
Proline is used in collagen synthesis, the main component of the extracellular matrix (ECM), and has roles in cell adhesion and migration and the development of tissues. Both depleted [
30,
35,
37] and raised [
20,
36] levels of serum proline have been found in CRC patients compared to healthy individuals.
Table 1. Studies that related amino acid levels in serum/plasma in patients with colorectal tumors compared with healthy individuals (amino acids with diagnosis/screening potential).
| Reference |
Year |
Type of Cancer |
TNM Stage |
Patients (n) |
Healthy Controls (n) |
Biological Sample |
Laboratory Techniques |
| Tan et al. [6] |
2013 |
CRC |
I/II (68%) |
101 |
102 |
Serum |
GC-TOFMS/UPLC-QTOFMS |
| Chan et al. [20] |
2009 |
CRC |
Various stages |
31 |
31 |
Serum |
HR-MAS NMR/GC-MS |
| Barberini et al. [29] |
2019 |
CRC |
Various stages |
15 |
9 |
Plasma |
GC-MS |
| Leichtle et al. [30] |
2012 |
CRC |
Various stages |
59 |
58 |
Serum |
ET-MS |
| Troisi et al. [34] |
2022 |
Adenomas, CRC |
I/II |
150 |
50 |
Serum |
GC-MS |
| Gu et al. [35] |
2019 |
Adenomas, CRC |
Various stages |
62 |
38 |
Serum |
GC-MS |
| Zhu et al. [37] |
2014 |
Adenomas, CRC |
Various stages |
142 |
92 |
Serum |
LC-TMS |
| Nishiumi et al. [42] |
2017 |
Adenomas, CRC |
I/II |
282 |
291 |
Plasma |
GC-T/QMS |
| Nishiumi et al. [43] |
2012 |
CRC |
I/II |
59 |
63 |
Serum |
GC-MS |
| Geijsen et al. [45] |
2019 |
CRC |
Various stages |
268 |
353 |
Plasma |
UHPLC-QTOF-MS |
| Farshidfar et al. [46] |
2016 |
Adenomas, CRC |
Various stages |
320 |
254 |
Serum |
GC-MS |
| Ma et al. [47] |
2012 |
CRC |
Various stages |
30 |
20 |
Serum |
GC-MS |
| Wang et al. [48] |
2017 |
CRC |
I/II |
55 |
40 |
Urine |
H-NMR |
| Reference |
Glutamate |
Glutamate |
Glycine |
Serine |
Threonine |
Tryptophane |
Proline |
Valine |
Leucine |
Isoleucine |
| Tan et al. [6] |
D |
D |
|
I |
|
D |
|
|
|
|
| Chan et al. [20] |
|
|
|
|
|
|
I |
|
|
|
| Barberini et al. [29] |
|
|
|
|
|
|
|
D |
D |
D |
| Leichtle et al. [30] |
|
|
I |
|
|
|
D |
|
|
|
| Troisi et al. [34] |
D |
|
|
|
|
|
|
|
|
|
| Gu et al. [35] |
D |
I |
D |
D |
|
|
D |
D |
D |
D |
| Zhu et al. [37] |
D |
I |
|
|
|
D |
I |
|
|
|
| Nishiumi et al. [42] |
|
|
|
|
|
D |
|
|
|
|
| Nishiumi et al. [43] |
D |
I |
|
|
|
|
|
|
|
|
| Geijsen et al. [45] |
|
|
|
|
|
|
|
D |
D |
|
| Farshidfar et al. [46] |
|
|
|
|
|
|
|
D |
D |
D |
| Ma et al. [47] |
|
|
|
|
|
|
|
D |
D |
D |
| Wang et al. [48] |
|
|
D |
D |
D |
D |
Legend: I—increase; D—decrease; GC-MS—gas chromatography–mass spectrometry; UHPLC-QTOF-MSa—ultrahigh performance liquid chromatography–quadrupole time-of-flight mass spectrometry; H-NMR—proton nuclear magnetic resonance spectroscopy; ET-MS—electrospray tandem–mass spectrometry; HR-MAS NMR—high-resolution magic angle spinning nuclear magnetic resonance; GC-TOFMS—gas chromatography–time-of-flight mass spectrometry; UPLC-QTOFMS—ultraperformance liquid chromatography–quadrupole time-of-flight mass spectrometry; GC-T/QMS—gas chromatography/triple–quadrupole mass spectrometry.
This entry is adapted from the peer-reviewed paper 10.3390/cancers16010069