Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Chitosan nanoparticles (NPs) serve as useful multidrug delivery carriers in cancer chemotherapy. Chitosan has considerable potential in drug delivery systems (DDSs) for targeting tumor cells. Doxorubicin (DOX) has limited application due to its resistance and lack of specificity. Chitosan NPs have been used for DOX delivery because of their biocompatibility, biodegradability, drug encapsulation efficiency, and target specificity.
- chitosan NPs
- drug delivery
- drug resistance
- stimuli-sensitive
- chemotherapy
1. Active and Passive Drug Delivery
Effective drug delivery to tumor cells via chitosan NPs is divided into two categories (active and passive) (Figure 4). In passive DOX delivery, chitosan NPs accumulate in tumor tissue through leaky or defective vessels using the permeability and retention (EPR) effect [128]. NPs carrying anticancer drugs can easily navigate through the blood vessels in the angiogenic tumor site. This characteristic leads to a higher concentration of these NPs in tumor tissue compared to natural anticancer drugs, a phenomenon referred to as the EPR effect [129]. Once a solid tumor achieves a specific size, the surrounding normal vasculature becomes inadequate to meet the increasing oxygen demands for tumor development. Subsequently, as tumor cells undergo cell death, they release growth factors that stimulate the formation of new blood vessels from nearby capillaries [130]. Angiogenesis denotes the rapid formation of novel and irregular blood vessels, characterized by a disrupted epithelium and the absence of the basal membrane typically present in normal vascular systems. Due to the vascularization needed by rapidly growing tumors, coupled with restricted lymphatic drainage, the resulting irregular vascular architecture gives rise to an amplified EPR effect [131]. However, passive targeted drug delivery exhibited lower therapeutic efficacy and systemic side effects [132].
The targeted delivery of chemotherapeutic drugs presents dual benefits. Firstly, precise delivery to the targeted site reduces the requirement of the dosage, enhancing the efficacy of the therapy method [133]. Secondly, by reducing the overall drug dosage, the manifestation of drug-induced adverse effect is either prevented or significantly minimized. Nanomedicine therapy influences the diverse active and passive targeting capabilities of NPs to deliver drugs to specific target site [134]. Because of these potentials, NPs are used as viable methods for overcoming the drawbacks of traditional cancer therapies, such as nonselective toxicity and drug resistance. Tumor-targeted drug delivery takes use of the differences between malignant and healthy tissues [135]. During tumor progression, the tumor microenvironment changes. Inadequate oxygen supply and glucose to lactate conversion, caused by increased metabolism and growth rates, lead to a fall in the pH of the tumor tissue. This change, in conjunction with hypoxia and glucose deprivation, increases angiogenesis, a mechanism critical for tumor proliferation, migration, and maintenance [136,137]. Many tumors have an overexpression of certain antigens, including on their surfaces, making them potential drug delivery targets. This approach is successful as long as the selected targets for a specific cancer cell type can be recognized with confidence and are not expressed in considerable amounts elsewhere in the body [122,123]. An investigation elaborated on the synthesis of the active targeted water-soluble delivery of DOX [138]. This research involved two biodegradable and biocompatible biopolymers, poly-γ-glutamic acid (PGA) and chitosan. The self-conjugation of these two polymers produced stable and negatively charged NPs with an 80-150 nm diameter. The targeting agent was folic acid and bonded with the NP surface and polyanion. In this study, the stability of NPs, the toxic effect, the active targeting effect, and the DOX release efficiency was examined in in vivo situations. The results showed that the DOX-loaded NPs induced the DOX delivery compared to free drugs without damaging the normal cells. Table 2 shows the DOX deliveries via chitosan NPs with different combinations of NPs for cancer treatment.
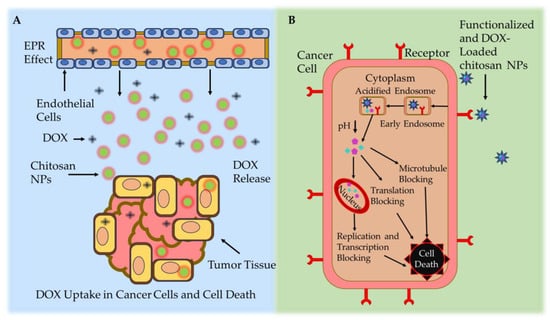
Figure 4. Chitosan-based NP drug delivery mechanisms. (A) Passive targeting mechanism: leaky tumor vessels release the DOX-loaded chitosan NPs at the cancer site via the EPR effect. (B) Active targeting mechanism: DOX-loaded chitosan NPs accumulate in cancerous cells via ligand-mediated endocytosis [139].
Table 2. Active and passive targeted drug deliveries by using chitosan NPs for DOX drug delivery in cancer treatments.
| NP Types | Cancer Type or Cell Line | Drugs | Active or Passive | Remarks | Ref. |
|---|---|---|---|---|---|
| Chitosan and O-HTCC (ammonium-quaternary derivative of chitosan) NPs | Kidney and osteosarcoma cancer/Vero and SaOs-2 cell lines | DOX | Passive | High encapsulation but low releasing capacity. | [140] |
| PEGylated chitosan NPs | Breast cancer/MCF-7 cell line | DOX | Active | Three-times enhanced cytotoxicity. | [141] |
| DSe-CMC (diselenide-cross-linked carboxymethylchitosan) NPs | Liver cancer/HepG2 and H22 cell lines |
DOX | Passive | Releasing capacity enhanced below the acid and redox environment. | [142] |
| L61-OE-CS (acid-labile ortho-ester-modified pluronic and chitosan) NPs |
Liver/HepG2 and H22 cell lines |
DOX | Passive | Drug releasing rate was enhanced at an acidic pH. | [143] |
| Chitosan/alginate NPs | Breast cancer/MCF-7 and MDA-MB-231 cell lines | DOX and HCQ | Passive | Inhibited the autophagic degradation and enhanced the drug delivery. |
[144] |
| Chitosan–MgFe2O4 magnetic NPs | Breast cancer SKBR-3 cell line | DOX | Passive | An 84.28% encapsulation efficiency and an 85.86% releasing capacity. | [145] |
| PP-CS (anti-PD-L1 peptide and chitosan) NPs | Colon cancer/CT26 cell line | DOX | Active | Strong synergetic immunogenic response and induced tumor regression. | [146] |
| CS-PAPBA (chitosan–poly(N-3-acrylamidophenylboronic acid) NPs | Liver cancer/H22 cell line | DOX | Active | Enhanced the deep penetration and accumulation in tumor cells. | [147] |
| LGCC (lactobionic acid–guanidinobenzoic acid–cystamine bismethacrylamide-cross-linked chitosan-poly(methyl methacrylate))NPs | Breast cancer/CXCR 4 cell line |
DOX | Active | Significant suppression of CXCR 4-positive hepatocarcinoma and breast cancer cells. | [148] |
| Chitosan NPs/CMD (carboxymethyl dextran) | Lung cancerA549 cell line | DOX and IGF-1R siRNA | Active | Synergistic result of DOX cytotoxicity and apoptosis in cancerous cells. | [149] |
| Chitosan–SPIO (superparamagnetic iron oxide) magnetic NPs | Ovarian cancer/A2780 and OVCAR-3 cell lines | DOX | Passive | High-tumor-growth inhibition after 96 h of exposure. | [150] |
| HA (hyaluronic acid)-chitosan NPs | Breast cancer/MDA-MB 231 cell line | DOX–miR-34a | Active | Codelivery enhanced the efficiency and reduced the resistance and side effects. | [151] |
| Chitosan–Raloxifene NPs | Breast cancer/MCF-7 cell line | DOX | Active | A 95% encapsulated and 60% DOX-release capacity; inhibited cell growth. | [152] |
| Chitosan NPs | Colorectal cancer/HT-29 cell line | DOX and HMGA2–siRNA | Passive | Combination was effective against tumor cells. | [153] |
| Modified chitosan NPs | MCF-7 and Caco-II cell line | DOX | Passive | Higher loading ability and effectively eliminated tumors. | [154] |
| CS-FA (chitosan–folic acid)/CS-SA-MNPs (succinic anhydride magnetic nanoparticles) | Lung cancer/MG-63 and A549 cell lines | DOX | Active | NPs deliberated as an effective pH-dependent nano-DDS. | [155] |
| FA (folic acid)–chitosan NPs | Liver cancer/HepG2 cell line | DOX | Active | Inhibited the cell cycle at the G2/M phase. | [156] |
| COOH–chitosan MSNs (mesoporous silica nanoparticle) | Breast cancer/TNBC and HER2 cell lines | DOX | Active | Enhanced drug release; increased the efficiency of DDS. | [157] |
| AAP-CS-NPs (Auricularia auricular polysaccharide–chitosan NPs) | Breast cancer/MCF-7 | DOX –HCl | Passive | Enhanced cellular uptake compared to free DOX. | [158] |
| Chitosan–TPP NPs | Lung cancer/A549 cell line | DOX | Passive | Encapsulation efficiency of approximately 95% and a good cytotoxic effect. | [159] |
| Ce6–chitosan–TPP NPs | Breast cancer/MCF-7 cell line | DOX | Passive | Significant enhancement observed in the drug release rate. | [160] |
| Chitosan magnetic NPs | Breast cancer/MCF-7 cell line | DOX | Passive | Higher drug released at a pH of 4.2 as compared to a pH of 5. | [161] |
| CPCN NPs (collagen peptide chitosan nanoparticles) | Cervical cancer/HeLa cell line | DOX–HCl | Passive | Enhanced drug release and increased apoptotic cell rate. | [162] |
2. Modified Chitosan–DOX Drug Deliveries
2.1. Amino Acid-Modified Chitosan NPs
Amino acids are crucial molecules for all living cells; essential and nonessential amino acids play a key role in cell growth and proliferation. In the tumor microenvironment, the rapid growth and proliferation of the tumor cells require more amino acids to synthesize protein [163]. Traditional drug delivery approaches have focused on “starving cancer cells to death” by blocking nutrient intake [164]. Due to the hydrophilic nature of amino acids, they cannot cross animal cell membranes [165]. The transfer of amino acids into the cell requires a specific transporter on the animal cell membrane [166]. Several amino acid transporters have been discovered to cross the animal cell membrane, categorized according to substrate specificity and coupling ions [166]. Consequently, amino acid transporters have been considered an emerging goal for cancer chemotherapy. In cancer cells, the blockage of amino acid transporters is more specific and avoids unwanted nontarget effects [164,165]. On the other hand, chitosan NPs modified with amino acids can serve various purposes. Amino acids have the potential to improve the transport of therapeutic drugs, stabilize the nanoparticles, and allow specific interactions with biological targets. This specificity offers an opportunity for targeted tumor therapy, such as application in boron neutron capture therapy (BNCT), positron emission tomography (PET), and chemotherapeutic DDS [167,168]. Nowadays, therapy with amino acids (TAAI) for cancer treatment has gained more attention. A new method has been designed using amino acids and polymers for cancer treatment [169]. DOX-loaded chitosan glutamic acid (CS-Ga–DOX) NPs were prepared through the ionic gelation method. CS-Ga–DOX NPs exhibited a positive zeta potential and spherical structure. At a pH of 5.5 and 7.4, DOX was continuously released with a mutual burst. CS-Ga–DOX NPs have great potential as a pH-responsive nanocarrier for anticancer chemotherapy.
2.2. Vitamin-Modified Chitosan NPs
Metastasis and stemness are the two main challenges in cancer treatment. Both have a solid connection with drug resistance and a low prognosis, finally leading to the failure of the cancer treatment. It has been described that cancer chemotherapy, particularly with the DOX in breast cancer treatment, can enhance metastasis and stemness. A combination therapy is an efficient method to suppress tumor cells with an enhanced synergistic effect. An advanced therapeutic system was developed by the combination of all-trans retinoic acid (ATRA) with DOX to resolve metastasis and stemness [170]. ATRA is a newly identified Pin1 (specific isomerase highly expressed within various tumor cells). It can effectively stop numerous cancerous pathways by successfully inhibiting and degrading Pin1. In this approach, researchers successfully synthesized a folic acid–chitosan-based polymer with both DOX (FA-CSOSA/DOX) and ATRA (FA-CSOSA/ATRA) NPs for cancer treatment. Due to the presence of FA, the uptake of these NPs was enhanced in cancer cells through folate receptors. This approach has a better synergistic effect as compared to DOX alone in the 4T1 cell line. In vivo, these NPs exhibited an 85.5% tumor inhibition, 2.5-fold higher than that of DOX–HCl alone. Another study was designed to synthesize vitamin E succinate–chitosan–histidine (VCH) multiprogram DOX carriers [171]. The π-π stacking bond between VCH and DOX was confirmed using a UV–vis spectrum. Drug release experimentations showed better pH sensitivity and sustained release results. DOX/VCH NPs were effectively taken up by HepG2 tumor cells, with the suppression rate reaching up to 56.27%. These NPs could combine with histidine and chitosan to attain pH sensitivity and P-gp inhibition, effectively suppressing the tumor cell growth and improving solubility. These multiprogram NPs show promise as an effective drug carrier in cancer chemotherapy without causing damage the healthy cells.
2.3. Antibody-Modified Chitosan NPs
Antibody–drug conjugates (ADCs) constitute a class of cancer cell-targeting drugs that have gained consideration. Monoclonal antibodies (mAbs) were synthesized to target antigens, and this approach was developed with the advent of hybridoma technology in 1975 [172]. Several mAbs have since gained approval, with herceptin being an example used in breast cancer treatment by targeting the HER2 receptor. However, mAbs alone are not enough for cancer treatment due to their low cytotoxicity in cancer cells [173]. Therefore, a new approach was designed to increase the cytotoxic effect in cancer cells. According to this strategy, mAbs were conjugated with biopolymers to enhance the drug efficiency. Similarly, chitosan NPs combined with two types of mAbs (anti-hMAM and anti-HER2) were synthesized for the treatment of breast cancer [141]. These PEGylated DOX-loaded CSNPs with mAbs exhibited enhanced cytotoxicity against MCF-7 cancer cells as compared to DOX-loaded CSNPs without mAbs. The synergetic therapy of immunogenic cell death (ICD) and the immune checkpoint blockade (ICB) has revealed extraordinary results against various types of cancers. For a safe and effective synergetic immunotherapy, researchers proposed all-in-one glycol chitosan NPs (CNPs) that administered anti-PD-L1 peptide (PP) and DOX to target tumor cells [146]. Briefly, a hydrophobic 5-cholanic acid was conjugated to the hydrophilic glycol chitosan backbones to create CNPs. Furthermore, the CNPs’ free amine groups were modified with tumor-targeting antibodies and peptides for targeted tumor drug delivery [174]. PP–CNPs thus avoided subcellular PD-L1 recycling, eventually abolishing the immunological escape mechanism in CT26 colon tumor. When the DOX–PP–CNPs were intravenously injected into mice with CT26 colon tumors, PP and DOX were successfully delivered to the tumor tissues via NP-derived passive and active targeting. This enhanced both lysosomal PD-L1 degradation and substantial ICD, ultimately leading to tumor regression through an antitumor immune response.
2.4. Hyaluronic Acid-Modified Chitosan NPs
Hyaluronic acid (HA) is a polysaccharide found in the extracellular matrix of connective tissues. Structurally, HA has repeating units of N-acetyl-D-glucosamine and D-glucuronic acid connected with the β-1,3 and β-1,4 glycosidic linkage [175]. HA has been extensively used in actively targeted deliveries due to the high attraction for CD44 receptors (overexpressed in stem cells of cancer). The active targeted anticancer drug delivery with HA can enhance the solubility and effectiveness. In this regard, catechol (Cat)-modified chitosan@HA NPs were synthesized to deliver the DOX [176]. The Cat moiety enabled the carriers with good adherence and a constant local distribution of DOX. The ionic gelation method was used to prepare Cat-NPs from Cat-functionalized chitosan and HA. These prepared NPs have a negative charge and spherical shape. As compared to unmodified NPs, these NPs showed better mucoadhesive properties in oral mucosal tissues. In this method, DOX was loaded onto the modified NPs with a high loading capacity of 250 μg/mg, and sustained release was achieved. DOX-loaded Cat-NPs (DOX-NPs) inhibited the expansion of the HN22 carcinoma cell line. DOX-NPs were taken up, accumulated, and caused apoptosis in cells more rapidly as compared to free DOX. The findings showed that the prepared Cat-NPs have great potential and could be used as a novel carrier for the local delivery of DOX to oral cancer cells. Another study explained the HA-modified chitosan NPs for DOX delivery for effective cancer chemotherapy. Magnetic NPs containing chitosan/HA complexed with κ-carrageenan were prepared by using the solution method (hydrothermal method) [177]. An MTT assay was performed to check the effect of these magnetic NPs on MCF-7 and MDA-MB-237 cells. These MNPs have a spherical shape and a 100–150 nm diameter with a 74.1% DOX encapsulation capacity. However, the drug encapsulation capacity was enhanced by increasing the κ-carrageenan amount. Subsequently, the pH-stimulus-responsive drug was released in a sustained manner without side effects.
2.5. PLGA- and PEG-Modified Chitosan NPs
PLGA and PEG are biocompatible and biodegradable synthetic polymers with the ability to increase stability and minimizing side effects. For example, pH-sensitive PEGylated chitosan NPs coated with PLGA were synthesized using the coassembly method to achieve effective DOX delivery for cancer chemotherapy [178]. The obtained DOX-loaded PEGylated chitosan/PLGA NPs (DOX–PCPNs) have a spherical structure, while the chitosan/PLGA formed a solid central core surrounded by hydrophilic PEG. DOX–PCPNs exhibited excellent stability and enhanced drug release in a serum-holding environment. Cytotoxic studies elaborated that the DOX–PCPNs were endocytosed, enhancing the DOX release in an in vitro environment and increasing DOX accumulation in cancer cells to improve antitumor efficiency. The hydrated shells of PEG protected against uptake by macrophage cells. In vivo results exhibited the ability of DOX–PCPNs to efficiently deliver DOX, reducing TRAMP-C1 tumor growth compared to free DOX. DOX–PCPNs exhibit great potential in drug delivery against tumor cells.
2.6. Genetic-Material-Modified Chitosan NPs
Sometimes, monotherapy against cancerous cells is insufficient for treatment. It has disadvantages, such as extreme toxicity in healthy cells and resistance [179,180,181]. Compared to conventional chemotherapy, gene therapy offers a safer route through nonviral vectors, but it exhibits limited efficiency and needs improvement [182,183]. Multidrug resistance (MDR) is also attributed to the malfunction of genes causing chromosomal changes in cancerous cells [184]. Many researchers have investigated gene delivery to treat cancerous cells with drug combinations [185]. The combined chemo- and gene therapy has provided an effective way for cancer treatment to overcome MDR due to its high synergetic effect [186,187,188]. Nucleic acids and genes require DDS because they are too large to enter the animal membrane. Moreover, gene therapy for cancer treatment is challenging due to the unstable and flexible nature of genes [179]. Dendronized chitosan-based poly amidoamine deoxycholic acid (PAMAM-CS-DCA) NPs were synthesized [189,190,191]. These chitosan-based NPs exhibited low toxicity in healthy cells and a good gene transfection efficiency. Low doses of DOX increased the gene expression and synergetic ability, demonstrating great potential for the codelivery of genes and drugs. The overall results exhibited higher efficiency against tumor cells. Similarly, a double-walled microsphere was synthesized, and chitosan-based DNA nanocomplexes, including the p53 gene, were loaded for combined chemo- and gene therapy [192,193]. Another study described the chitosan-based NPs for the codelivery of DOX and siRNA. In this approach, carboxymethyl dextran (CMD) chitosan-based NPs were prepared to load the DOX and siRNA. The effect of these NPs on the epithelial–mesenchymal transition (EMT) gene expression and cell growth in HCT-116 cell lines was also explained [194]. In addition, another study elaborated the lung cancer therapy with HMGA2 (high-mobility group A2) suppressing small interfering RNA (siRNA) along with DOX by using chitosan-based NPs [195,196]. The findings showed that the codelivery of HMGA2, siRNA, and DOX through innovative CMDTMChiNPs was a novel therapeutic method with prodigious potential effectiveness for lung cancer treatment. The increased activity level and expression of P-glycoprotein were responsible for increasing the drug resistance [197,198]. To solve this, chitosan–g-D-α-tocopheryl polyethylene glycol (TPGS) NPs were produced by using evaporation techniques. TPGS NPs have the ability to control the P-glycoprotein activity and significantly decrease the ATP level, which is beneficial in preventing DOX efflux [199]. Chitosan–dextran–sulfate-coated PLGA–PVA (poly lactic-co-glycolic acid–polyvinyl alcohol) NPs for DOX distribution were used to test the effectiveness of the chitosan NPs by preventing DOX progression [200]. All the above-mentioned examples prove the effectiveness of codelivering DOX and genes using chitosan NPs as a carrier agent.
2.7. Immunotherapeutic-Modified Chitosan NPs
Cancer leads to a significant proliferation of abnormal cells and immunosuppressive cells that inhibit the immune response [202,203,204]. Reforming the immune environment of tumors can enhance the efficiency of the immune response against tumors. [205,206,207]. Recently, tumor immunotherapy has gained a lot of attention for cancer treatment [208,209]. However, the efficiency and toxicity effects of simple immunotherapy are minimal [210,211]. Cancerous cells are present at the core tumor mass, and the treatment efficiency is restricted [212]. Due to this condition, combination therapy is the most effective method of cancer treatment [213,214]. A facile approach was developed using CMC derivatives (M2pep-CMCS) targeting tumor-linked macrophages 2 (TAM2) and cyclodextrin derivative (R6RGD-CM βCD) with the tumor target [215]. DOX was loaded onto cyclodextrin-derivative NPs, and R848 was loaded onto CMC-derivative NPs, demonstrating good absorption. These NPs enormously increased the expression levels of cleaved Caspase 3, indicating enhanced cell apoptosis. Similarly, these NPs also altered the shape of tumor-linked macrophages. Overall, these materials are considered an effective delivery carrier in cancer treatment. Another study has investigated enhancing the immune response against cancer treatment, in which TMC-based NPs were synthesized and fabricated with DOX and interleukin-2 (rhIL-2) [216]. These NPs proficiently delivered the hydrophobic DOX and hydrophilic interleukin-2 to attain the combination therapy against the tumor microenvironment. DOX was attached to the TMC NPs with covalent bonds that led to the pH-sensitive release, while interleukin-2 bonded through electrostatic forces. The diameter of these NPs was about 200 nm, and a zeta potential > 20 mV was recorded. For the targeted delivery, folate modification was used to accumulate the drugs at the target site. The final results showed that the combinational therapy of interleukin-2 and DOX-based TMC NPs have the potential to kill the tumor cells and enhanced the immune response against cancer.
3. Combined Delivery with Other Anticancer Drugs
A large number of studies have focused on combining chemotherapeutic drugs and chemosensitizers due to the resistance of tumor cells to DOX alone. The combined effect of anticancer drugs may improve the tumor suppression efficiency and minimize side effects [217,218,219,220]. Overall, the codelivery of drugs with chitosan NPs has proven the efficiency of this method against various cancerous treatments by reducing the drug resistance without harming the noncancerous cells [221]. In this aspect, research was conducted on breast cancer treatment. The codelivery of DOX and Tanshinone IIA (TSIIA) (a bioactive compound isolated from Chinese herbs known as “Danshen”) was achieved using CMC chitosan-based hypoxia NPs for breast cancer treatment [222]. Hypoxia-responsive NPs are designed to respond to low-oxygen levels, a condition known as hypoxia, typically found in tumor microenvironments. These NPs showed an improvement in release efficiency and an increase in the cytotoxicity of DOX against the tumor microenvironment. The immunofluorescence staining of the tumor section confirmed that the combined nanoparticles exerted a synergistic antitumor effect by inhibiting tumor. Consequently, CMC chitosan-based NPs exhibited promising properties for drug delivery against breast cancer. Degradation of the delivery agent is crucial for DDS at specific target sites and to prevent the presence of the delivery agent’s byproduct in the body [223]. Cancerous cells produce glutathione (GSH) 7 to 10 times more than healthy cells, which is highly beneficial for disulfide bond degradation [224,225]. Thus, a disulfide-bond-based DDS proves to be very helpful for enhancing the stability of delivery agents and facilitating degradation at the targeted tumor site in cancer therapy [226,227,228]. To achieve this purpose, another approach was established, wherein redox-sensitive chitosan/stearic acid NPs (CSSA NPs) were synthesized for DOX and curcumin delivery in cancer treatment [229]. The degradable CSSA NPs had a size of about 200 nm and were synthesized based on disulfide cross-linking. The hydrophilic DOX and hydrophobic curcumin drugs were encapsulated onto the CSSA NPs. Codelivery of therapeutic drugs through this approach increased the efficiency of the cancer treatment. These NPs exhibited a low drug release in the normal cells, while approximately 98% of DOX and 96% of curcumin were released in the tumor cells under a GSH reducing environment. Consequently, this method has demonstrated enhanced encapsulation, release of dual drugs, and cytotoxicity for cancer treatment. Another method was established for CD44 receptor targeting, reducing multidrug resistance (MDR) and enhancing the drug release and cytotoxicity in tumor cells for breast cancer treatment [230]. The NPs consisted of three layers: a poly core, liposome, and chitosan, respectively. These NPs (Ch-MLNPs) were loaded with DOX, silybin, and paclitaxel. These three drugs were released at target-specific sites exploiting CD44 receptors in breast cancer cells. In vivo, studies showed the good efficiency of these three-layered NPs against breast cancer and lowered the MDR.
This entry is adapted from the peer-reviewed paper 10.3390/molecules29010031
This entry is offline, you can click here to edit this entry!
