Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agronomy
|
Microbiology
As the global human population continues to increase, the use of saline–alkali land for food production is an important consideration for food security. In addition to breeding or cultivating salt-tolerant crop varieties, microorganisms are increasingly being evaluated for their ability to improve plant salt tolerance. Barley is one of the most important and salt-tolerant cereal crops and is a model system for investigating the roles of microorganisms in improving plant salt tolerance.
- Hordeum vulgare L.
- microbiome
- salt tolerance
- omics
1. Introduction
The effects of global climate change and anthropogenic activities have led to increasingly serious soil salinization issues that affect agricultural production and environments. A total of approximately 1 billion hectares of saline–alkali land has been estimated globally, with land in China accounting for approximately 10% of the global total [1]. As population growth and pressure on food security continue to increase, there is an increasing emphasis on the use of saline–alkali lands, especially for food production. However, in using saline–alkali land for crop production, the mechanism of salt tolerance must be understood so that effective measures can be taken to overcome the adverse effects of salt stress.
Studies of the molecular mechanisms underlying plant salt tolerances and the breeding/cultivation of salt-resistant (SR) crop varieties have been the primary means of mitigating salinization problems. However, increasing numbers of studies have shown that microorganisms play important roles in improving the salt tolerances of plants. These benefits arise from several plant processes, including nutrient acquisition promotion, ion homeostasis maintenance, increasing osmotic substance concentrations, scavenging excess reactive oxygen species (ROS), and regulating phytohormones (Figure 1), and the mechanisms of these microorganisms in improving plant salt tolerance have also been reviewed from different perspectives [2,3,4,5]. Therefore, the use of microorganisms to improve salt tolerance in plants has been gaining traction recently, as it is considered more environmentally friendly and sustainable.
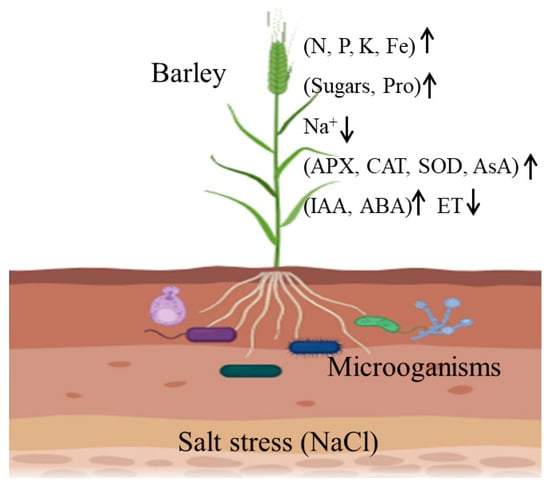
Figure 1. Mechanisms of microorganisms in the alleviation of salt stress in barley (created using biorender.com) (accessed on 14 November 2023).
2. Nutrient Acquisition Promotion
Salinized soils contain abundant Na+ and Cl+ concentrations that interfere with the absorption of other nutrients by plants, resulting in nutrient deficiencies and affecting normal plant growth and development [5]. In addition, changes in pH can contribute to the loss of certain nutrients or changes in their bioavailability, further affecting their absorption by plants. Microorganisms that live on or around the surfaces of plant roots or within plant roots can enhance nutrient absorption in plants. Under salt stress, the nutrients that microorganisms help plants absorb primarily include N, P, Fe, and K. In addition, the “Ion homeostasis maintenance” section explains the competition that occurs between K+ and Na+ absorption and K+ absorption.
Microorganisms can help plants absorb P primarily through three pathways. First, microorganisms convert insoluble inorganic phosphates into soluble forms via acidification, chelation, ion exchange, and reduction, thereby increasing available phosphates in soils [22,23,24]. Second, microorganisms decrease the threshold of phosphorus absorption in plants by expressing the high-affinity Pi transporter, PiPT [25]. Third, microorganisms improve phosphorus uptake by plants via interactions among root endophytic fungi and phosphorus-solubilizing bacteria [26]. In support of the above assertions, inoculation of barley with the novel rhizobacterium Siccibacter sp. strain C2 that was isolated from the rhizosphere of other plants significantly improved barley salt tolerance, along with demonstrating exceptional phosphorus-solubilizing capacity [20].
Plants primarily absorb nitrogen (N) in the forms of nitrate (NO3−) and ammonium (NH4+), in addition to through organic compounds such as amino acids or peptides. Microorganisms primarily improve plant N uptake by (1) symbiosis via nitrogen-fixing rhizobia; (2) N-fixation by autogenous N-fixing bacteria (or non-symbiotic N-fixing bacteria); (3) by increasing the surface area of plant roots; or (4) by stimulating the expression of plant N metabolism-related enzymes that can improve N uptake and use efficiency by plants [27,28,29,30]. Non-symbiotic N-fixing bacteria are widely used to improve N uptake by plants under salt stress due to the limitations of symbiotic nodulation crops and the sensitivity of fungi to salt stress. For example, inoculation of the N-fixing bacterium Azospirillum brasilense into soils can significantly ameliorate the adverse effects of salt stress on barley growth and yield [9].
Iron (Fe) is also abundant in soils but not generally available to plants, especially under salt-stress conditions. Microorganisms can increase Fe availability by producing organic acids or secreting siderophores [31]. Concomitantly, siderophores can also control pathogenic bacterial growth by depriving them of Fe [32]. These processes benefit the growth and development of plants under salt stress. For example, the combined application of Azospirillum, mycorrhiza, and 0.9 g/L nano Fe oxide increased barley yield by approximately 15.45% under salt-stress conditions [33]. Further, Siccibacter sp. strain C2 can secrete siderophores, and inoculation with this rhizobacterium has been shown to significantly improve barley salt tolerance [20].
3. Ion Homeostasis Maintenance
Na+ accumulation increases its competition with K+ while also altering the cellular Na/K ratio and, in turn, disrupting enzyme activity, protein synthesis, turgor maintenance, photosynthesis, and stomatal motility [34]. A high Na/K ratio in plants indicates high salt stress, such that plants must maintain low levels of Na+ to resist their harmful effects on plants. Microorganisms can improve plant salt tolerance by limiting the uptake of Na+ in roots while increasing K+ uptake.
During salt stress, inoculation with arbuscular mycorrhizal fungi (AMF) significantly increases K+ uptake while decreasing Na+ uptake, thereby decreasing the Na/K ratio and improving the salt tolerance of plants [35,36]. Further, K+ uptake increased with increasing salinity due to the mycorrhiza [35]. The high-affinity K+ transporter HKT1 controls Na+ input in plant roots, while HKT1 overexpression in plants does not improve its salt tolerance, and inoculation with Bacillus subtilis GB03 can downregulate and upregulate HKT1 expression in roots and shoots, respectively, thereby reducing Na+ concentrations in the whole plant and improving plant salt tolerance [37]. These results highlight the complex roles of microorganisms in regulating salt tolerance in plants.
Colonization of Piriformospora indica in plant roots under salt stress resulted in a lower Na/K ratio, which might be due to the upregulation of HKT1 and the genes KAT1 and KAT2 that encode potassium channel proteins [38]. Inoculation of an endophytic fungus (Neotyphodium spp.) reduced Na+ concentrations in plant roots while increasing K+ concentrations in shoots, thereby increasing the K/Na ratio of plants and alleviating salt-stress damage to plants [39].
Several studies have evaluated these effects in barley. Sepehri et al. [19] observed similar results after inoculating the root endophytic fungus Serendipita indica into barley. However, no effect on the concentrations of Na+ and K+ was observed in leaves or leaf sheaths after inoculation with plant rhizosphere bacteria, although root Na+ uptake was reduced [13]. An additional study indicated that inoculation of PGPB reduced Na+ concentrations in both the roots and shoots of barley during salt stress while increasing the water potential of leaves, thereby alleviating salt-stress damage to plants [16]. Moreover, inoculation of microorganisms with the ability to produce biofilms also improved barley salt tolerance, although the precise effects of biofilms on Na+ content were not measured [14,15].
4. Increasing Osmotic Substance Concentrations
Under salt stress, plants typically synthesize many soluble small organic molecule compounds as osmolytes, like proline, glycine betaine, sugars, organic acids, polyamines, and amino acids. These osmolytes are also involved in scavenging ROS, the maintenance of membrane integrity, and the stabilization of enzymes, thereby also acting as osmoprotective compounds.
Many microorganisms can produce proline. The proBA genes of Bacillus subtilis strain 93151 have been overexpressed in Arabidopsis thaliana by using the 35S promoter, leading to significantly higher proline content in transgenic plants than in control plants, with salt and drought stress tolerance both being enhanced in the former [42]. Moreover, inoculation of the rhizosphere bacteria Staphylococcus haemolyticus strain ST-9 and Bacillus subtilis strain RH-4 isolated from the rhizosphere of Heleochloa schocnoides significantly improved the proline content and salt tolerance of chickpea plants [43]. However, some studies have suggested that proline content in rice increases under salt stress, while inoculation with plant growth-promoting bacteria (PGPB) or their mixtures with endophytic bacteria (e.g., Pseudomonas pseudoalcaligenes and Bacillus pumilus) reduces proline content in rice but enhances rice salt tolerance [44]. Consequently, the role of microorganisms in proline accumulation within salt-stressed plants remains controversial, including for barley plants. Inoculation of Azospirillum brasilense into barley improved its salt tolerance, with decreased proline accumulation implicated as one of the underlying causes [9], consistent with the results of Badawy et al. [18]. Nevertheless, proline production was also significantly higher in plants during salt-stress alleviation and after inoculation of the bacterial strains Bacillus mojavensis S1, B. pumilus S2, and Pseudomonas fluorescens S3 into soils [16].
Soluble sugars (e.g., glucose, sucrose, dextrin, and maltose) also act as osmoprotective compounds by stabilizing cell membranes and protoplasts while also protecting enzymes from high intracellular concentrations of inorganic ions [45]. Under high salinity conditions, plant sucrose is broken down to meet glucose requirements [46]. Inoculation of cucumber seedling leaves with the siderophore-producing bacterial strain Trichoderma asperellum Q1 significantly increased the soluble sugar concentrations in both control and salt-stress conditions [47]. Likewise, barley inoculated with Piriformospora indica under variable salt stresses exhibited enhanced salt tolerance and increased soluble sugar concentrations [12]. Further, seawater stress led to decreased soluble sugar concentrations in barley, while inoculation with Aspergillus ochraceus effectively alleviated the adverse effects of salt stress on barley and also increased soluble sugar concentrations in barley leaves under different treatments, thereby suggesting a role of soluble sugars in barley salt tolerance [18].
5. Scavenging Excess ROS
Salt stress induces ROS production, including production of superoxide radicals (O2−), hydroxyl radicals (OH−), and hydrogen peroxide (H2O2), resulting in oxidative damage to lipids, proteins, and DNA [48]. Two approaches are primarily used by plants to cope with the adverse effects of excessive ROS, namely enzymatic and non-enzymatic antioxidant systems [2]. Enzymatic systems primarily include ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), dehydroascorbate reductase (DHAR), and monodehydroascorbate reductase (MDAR). In addition, non-enzymatic antioxidant systems primarily include ascorbic acid (AsA), glutathione (GSH), carotenoids, and several types of osmolytes. Microorganisms can activate antioxidant defense mechanisms by upregulating the activities of antioxidant enzymes or antioxidant concentrations in plants to resist damage caused by excessive ROS [5].
Inoculation with Trichoderma asperelloides T203 affects the expression of genes associated with osmotic protection and oxidative stress in the roots of two kinds of plants, with genes encoding monodehydroascorbate reductase (MDAR) significantly upregulated in the plants and increased ascorbic acid concentrations also observed [49]. Further, inoculation with Piriformospora indica increased the concentration of non-enzymatic antioxidants like carotenoids and proline in rice enduring salt stress, thereby improving its salt tolerance [50]. Inoculation with Trichoderma longibrachiatum T6 also increased the expression of genes encoding the antioxidant enzymes superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) in wheat seedlings undergoing salt stress, thereby improving their salt tolerance [51]. Inoculation with the SR PGPB Dietzia natronolimnaea STR1 enhanced the gene expression of multiple antioxidant enzymes like APX, MnSOD, CAT, POD, GPX, and GR, thereby increasing proline content and protecting wheat from the adverse effects of salt stress [52]. Moreover, inoculation with Azospirillum lipoferum FK1 increased the levels of enzymatic and non-enzymatic antioxidants in chickpea seedling leaves, thereby improving their salt tolerance [53]. In addition, the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) increased in the roots of wheat during salt stress after inoculation with the PGPB Nesterenkonia rhizosphaerae wp-8, while the proline contents of leaves also significantly increased and malondialdehyde concentrations significantly decreased.
In barley, many studies have investigated the relationship between improving salt tolerance by inoculation of microorganisms and antioxidants. Barley salt tolerance was improved after inoculation with Piriformospora indica and might be related to increased antioxidant capacity due to activation of the glutathione–ascorbic acid cycle [7,8]. Inoculation with the PGPB Pseudomonas fluorescens SBW25 and Pseudomonas putida KT2440 to investigate improved salt tolerance in barley revealed that both led to the upregulation of genes encoding GR and that strain SBW25 could also enhance the expression of genes encoding CAT2. Consequently, these bacteria could enhance plant salt tolerance by regulating the expression of genes encoding antioxidant enzymes [17]. Further, inoculation with Siccibacter C2 might alleviate oxidative stress by reducing hydrogen peroxide and malondialdehyde concentrations resulting from salt stress, thereby improving barley salt tolerance [20]. Further, inoculation with P. macrospinosa, N. goegapense, and N. chichastianum all increased the activities of the antioxidant enzymes SOD, CAT, and POX in barley [21]. However, the improvement of barley salt tolerance after inoculation with Azospirillum brasilense could be related to decreased antioxidant enzyme activity that was more effective in the SS rice variety [9]. In addition to the above, inoculation with Aspergillus ochraceus mitigated the harmful effects of seawater on the growth and physiologic status of barley plants while also leading to increases in proline, malondialdehyde, and hydrogen peroxide concentrations, in addition to antioxidant enzyme activities [37]. The results above demonstrate that varying relationships exist between microbial-induced changes in antioxidants and barley salt tolerances.
6. Regulating Phytohormones
Phytohormones play important roles in regulating the growth and development of plants while also helping alleviate abiotic stresses. Phytohormones are commonly considered plant growth regulators that primarily include abscisic acid (ABA), gibberellin (GA), ethylene (ET), cytokinins, and auxin (especially indoleacetic acid or IAA). Some microorganisms can produce or regulate phytohormones that can help plants tolerate or avoid salt stress. For example, inoculation with Pseudomonas putida Rs-198 increased the germination rate and seedling growth of cotton during salt stress while concomitantly leading to increased IAA concentrations and decreased ABA concentrations in plants [56]. Inoculation of cucumbers with three plant growth-promoting bacteria, namely Burkholdera cepacia SE4, Promicromonospora sp. SE188, and Acinetobacter calcoaceticus SE370 strain, to evaluate their effects during salt stress suggested that they might be associated with the downregulation of ABA and the upregulation of SA and GA4 in plants [57]. Further, Trichoderma asperellum Q1 can produce IAA, GA, and ABA, and inoculation of plants with this strain increased the concentrations of these three phytohormones and alleviated salt damage to cucumber seedlings [58]. Transcriptome analysis also revealed that inoculation with Bacillus amyloliquefaciens FZB42 induced transcription of ET and jasmonic acid (JA)-related genes in Arabidopsis thaliana during salt stress, while the inoculation of Arabidopsis-related mutations further confirmed that strain FZB42 might induce salt tolerance in plants by activating plant ET and JA signaling rather than through an ABA-dependent pathway [59]. However, inoculation with the PGPB B. amyloliquefaciens RWL-1 strain that can produce ABA in rice plants experiencing increasing salt concentration stress led to decreased ability to produce ABA (and decreased concentrations in plants) but significantly improved salt tolerance, with SA concentrations also increasing [60]. Thus, plants often exhibit variable concentrations of phytohormones after inoculation with microorganisms, and inoculation with microorganisms that produce certain phytohormones does not necessarily lead to their increased concentrations in plants. Consequently, the specific relationships among microbial hormone production, their regulation of plant endogenous hormones, and plant salt tolerance require further study.
PGPB that produce 1-aminocyclopropane-1-carboxylate (ACC) deaminase are thought to reduce ethylene levels and improve plant growth under salt stress because the salt tolerance of barley was improved after inoculation with some ACC deaminase-producing microorganisms [11]. In one study, barley seedlings inoculated with Hartmannibacter diazotrophicus E19T were exposed to 200 mM and 400 mM NaCl for 2 h, revealing reduced ethylene release and improved salt tolerance, indicating that this might be due to ACC deaminases produced by microorganisms [45]. An investigation of the molecular mechanism of Pseudomonas fluorescens SBW25 and Pseudomonas putida T2440 in improving salt tolerance of barley indicated the presence of greater downregulated genes associated with ABA biosynthesis and regulation but more upregulated genes related to JA, ethylene, and SA biosynthesis [17]. Further, inoculation of the PGPB Bacillus mojavensis S1 and Pseudomonas fluorescens S3 alleviated damage from salt stress to barley and induced plants to produce large amounts of IAA [38].
This entry is adapted from the peer-reviewed paper 10.3390/life14010006
This entry is offline, you can click here to edit this entry!
