Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Lymphedema is a progressive chronic condition affecting approximately 250 million people worldwide, a number that is currently underestimated. In Western countries, the most common form of lymphedema of the extremities is cancer-related and less radical surgical intervention is the main option to prevent it. Standardized protocols in the areas of diagnosis, staging and treatment are strongly required to address this issue.
- lymphedema
- imaging
- lymphoscintigraphy
1. Introduction
Lymphedema is characterized by the accumulation of protein-rich lymphatic fluid in the interstitium. Possible causes include congenital abnormalities, early and late primary conditions, and cancer-related or infective secondary lymphedema.
Cancer-related lymphedema ensues after the damage of lymphatic structures, as a consequence of lymph node surgical resection (axillary or inguinal) or direct tumor invasion.
The surgery for breast cancer treatment is the leading cause of cancer-associated lymphedema, because the estimated incidence after 3 years from sentinel lymph node dissection (SNLD) is 25%, rising to more than 30% after SNLD and radiotherapy [1].
Independent risk factors such as obesity, genetics and concomitant radiotherapy contribute to lymphedema development, along with the surgical technique and diagnostic methods used to plan it.
The coexistence of different underlying mechanisms may explain the delayed onset after surgery, irrespective of a clear clinical expression [2].
The clinical diagnosis is achieved by measuring differences between the limbs: in terms of volume, a difference higher than 100–200 cm3 is diagnostic; in terms of circumference, differences higher than 2 cm or an increase >10% are needed to recognize lymphedema [3].
These cut-offs are particularly effective in discriminating true lymphedema from simple differences in dominant and non-dominant arms, but potentially exclude mild forms of disease, even if symptomatic [3].
Many classifications are used to assess the severity of lymphedema; among them, the International Society of Lymphology (ISL) staging system is widely accepted [4].
Lymphedema is classified into four categories corresponding to subclinical (stage 0), mild (stage 1), moderate (stage 2) and severe (stage 3) according to symptoms and clinical presentation. Stage 0 is the earliest form presenting subjective symptoms as heaviness, tightness, fatigue and firmness in the absence of measurable swelling.
Stages 1 and 2 present measurable edema with spontaneous reversibility that improves with elevation or compression in stage 1, whereas irreversible edema irrespective of elevation or compression is seen in stage 2.
Moreover, the edema in stage 2 appears pitted, with indentations in early disease, but it evolves over time into a non-pitting pattern with the persistence of fluid stasis and subsequent fibrous changes. In stage 3, researchers see lymphostatic elephantiasis with the irreversible enlargement of limbs with severe fibrosis and skin changes such as acanthosis, fissures, ulceration, and rarely, lymphoangiosarcoma (Stewart–Treves Syndrome). This severe form is often complicated by recurrent episodes of infections/cellulitis. The ISL also defines arm measurements to correspond with the stage of lymphedema by changes in circumference from baseline. Stage 0 corresponds to no change in arm measurement. A change of sizes >5–10%, >10–20% and >20–40% corresponds to minimal, mild and moderate lymphedema, respectively. An increase >40% indicates severe disease.
Recent gradings have focused on quality of life rather than clinical manifestations, as reported in the Lymphedema Quality of Life Questionnaire for upper limbs (LYMQOL-UL) validated by Monticone et Al [5].
In recent years, new techniques of the study of the lymphatic system have been emerging with particular interest in sentinel lymph node mapping [6] and lymphedema diagnosis. The main features of the development process are the increase in spatial resolution, dose reduction and cost-efficacy.
The usage of imaging is becoming progressively wider these days to diagnose early forms of lymphedema and also to assess therapeutic intervention in advanced forms [7]. In recent years, two new techniques have shown promising results compared with the lymphoscintigraphic method, still the gold standard today, which are indocyanine green (ICG) fluorescence lymphangiography and magnetic resonance imaging (MRI) lymphangiography.
2. Lymphoscintigraphy
Scintigraphic studies of the lymphatic system began in the 1950s, and this continues to be the most widely used method in the world. The peculiar characteristics of radiocolloids make them ideal for visualizing a small vessel system without an autonomous pump system. The only forces that ensure lymphatic flow are hydrostatic and colloidal pressure [8].
There is a variety of radiopharmaceuticals used for studying the lymphatic system, not all of which are approved on different continents; for example, in Europe, the most widely used is 99mTc albumin nanocolloid [9].
Aggregates of human albumin and other formulations differ in size, and thus have to be filtered to obtain dimensions suitable for specific purpose. The smaller aggregates (less than 100 nm in diameter) show higher uptake in the lymphatic district, resulting in better image quality, while larger ones (200–1000 nm) are progressively trapped in lymph nodes, allowing sentinel node mapping [10].
The surface charge of the particles as well as the binding to specific receptors exposed in the lymphatic system are other relevant aspects that affect radiopharmaceutical bio-distribution [11].
The subcutaneous injection of radioactive molecules results in optimal lymphatic representation as regards transport kinetics and time of persistence in the site [12]. In contrast, intradermal injections exhibit greater uptake into the blood vessels, decreasing the residence time of the radiocolloid by increasing lymphatic drainage flow. This kinetic aspect allows for better quantitative analysis than qualitative [13].
Lymphoscintigraphy, always performed on both limbs, can define the severity of lymphedema based on imaging findings, such as asymmetry in the lymphatic vessels, the presence of lymphatic collaterals, delayed lymph flow, the absence of uptake in regional lymph nodes and dermal backflow [14].
For an accurate description of the technical characteristics and interpretations of lymphoscintigraphic examinations, please refer to the Genoa protocol set out by the authors Villa G. and Campisi C. [15].
Moreover, subfascial injections may be used to study deep lymphatic drainage.
Therefore, the site of injection depends on the type of radiopharma and scope of imaging (e.g., sentinel lymph node mapping vs. lymphedema). Thus, all previously reported techniques are feasible and indicated for specific conditions.
To date, lymphoscintigraphy remains an extremely accurate test with high sensitivity (up to 96%) and very high specificity (up to 100%) in the diagnosis of lymphedema, as assessed by Hassanein H. et al. [16] in one of the largest case studies of 227 patients enrolled between 2009 and 2016, with similar results to other studies based on large case series, such as the one conducted by Gloviczki P. et al. [17].
Imaging-based and clinical classifications are difficult to compare, even if they are based on similar lymphoscintigraphic findings. Clear correlations between stages in lymphoscintigraphic classifications and patient-reported symptoms have never been demonstrated; moreover, the ISL staging system is suboptimal for patients referred to surgery lacking anatomical knowledge.
Among the various classifications, the Taiwan Lymphoscintigraphy Staging (TLS) is one of the clearest, and was recently proposed by Pappalardo M. and Cheng M. [18]. This is based on three scintigraphic features: visualization of proximal/intermediate lymph nodes, linear lymphatic ducts, and dermal backflow. According to these, the classification recognizes three patterns: normal drainage, partial obstruction, and total obstruction. The last two patterns are further divided into three stages.
Over the years, thanks to microsurgical techniques applied to the lymphatic system such as lympho-venous anastomosis (LVA) and vascularized lymph nodes (VLN) for the treatment of lymphedematous pathology [19], lymphoscintigraphy has assumed a key role in planning surgical treatment.
Although there are no universally accepted classifications for this goal, Cheng M. et Al. proposed a comprehensive clinical imaging grading system called Cheng’s Lymphedema Grading System [20] that relates four parameters (circumferential difference (%), episodes of cellulitis (times/year), Taiwan Lymphoscintigraphy Staging and ICG lymphography) to the best possible treatment.
In relation to Cheng’s Lymphedema Grading System, for less severe cases, the most indicated treatment is complete decongestive therapy (CDT), which includes manual lymphatic drainage, band compression, exercise and skin care. Lympho-venous anastomosis (LVA) is performed in patients who do not want to wear elastic compression bands. For moderate–severe cases, a finer evaluation of the presence of functioning lymphatic ducts is mandatory, and only patients that demonstrate patent superficial lymphatic ducts are candidates for LVA. Vascularized lymph node transfer (VLN) is performed only if dermal backflow is present and the patients demonstrate nonfunctioning lymphatic ducts. In severe cases, the indication of VLN is always present, and association with additional surgical procedures such as liposuction or debulking surgery is often performed.
In severe cases, it is important to differentiate between obstructions in deep and superficial lymph vessels to establish multilevel surgical treatment [21]. However, it is not easy to assess differences with a planar method such as lymphoscintigraphy. For this purpose, methods such as SPECT, possibly associated with CT, have been used to improve spatial resolution, but without obtaining significant results [22].
Until now, however, there has been no close correlation between the results of pre- and post-operative lymphatic imaging methods and clinical objective findings, but in relation to the latter objective, based on preliminary studies on a small cohort of patients [23], it would appear that the lymphoscintigraphic method correlates the best with clinical and therapeutic outcomes.
The Quantitative Method: Alternative or Complement to Gold Standard?
Lymphoscintigraphy may be quantitative or qualitative. The differences in terms of uptake intensity as well as the transit time in proximal nodes, as well as the time for clearance and the time for appearance in blood, are some of the quantitative parameters that are measured (Figure 1 and Figure 2).
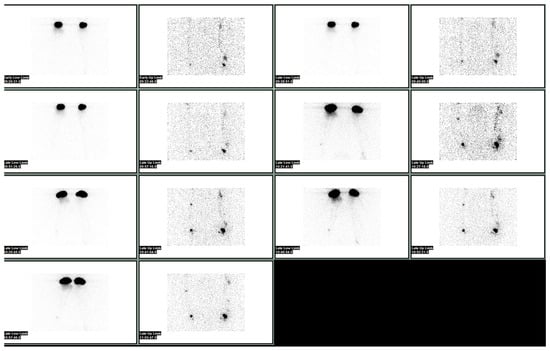
Figure 1. Normal lymphoscintigraphy of upper limbs performed after injection under the deep aponeurosis of the first interdigital spaces of hands bilaterally, and subsequently after a subdermal injection of the second, third and fourth interdigital spaces with 99mTc albumin nanocolloid.

Figure 2. Normal lymphoscintigraphy of the lower limbs performed after the injection under the deep aponeurosis of the third finger of feet bilaterally, and subsequently after a subdermal injection of the second, third and fourth interdigital spaces with 99mTc albumin nanocolloid.
The quantitative method is more sensitive when used in the diagnosis of lymphedema, especially in the earliest stages, where slight differences are difficult to assess. The lack of standardized protocol and inconclusive results have negatively affected the use of quantitate parameters in clinical practice.
A recent study by Kwon et al. [24] has opened up the possibility of using quantitative parameters to predict surgical procedure outcomes. The aim of the study was to investigate factors predicting early and late treatment outcomes using lymphoscintigraphic factors before LVA.
The authors suggest that dermal backflow is a significant positive-predictive qualitative factor; moreover, the surgical effect is higher in patients with both proximal and distal dermal backflow. These finding may be related to the regurgitation of lymphatic fluids into subcutaneous tissues at high pressures due to the occlusion of lymphatic vessels. LVA restores lymphatic flow by reducing the pressures in the lymphatic district and producing significant clinical effects.
The authors have analyzed other quantitative parameters over different times (1 h and 2 h) such as lymph node uptake ratio, extremity uptake ratio and injection site clearance ratio. All of these have been correlated to the treatment response and to the volume difference ratio at 3 months and 1 year after surgery
The results between quantitative parameters and volume difference ratio, evaluated with Sperman’s rank correlation coefficient, were statistically significant only for extremity uptake ratio at 2 h (2 h EUR) in relation to volume difference ratio after 3 months (p = 0.016), and even more so at 1 year (p = 0.001). Regarding the relationship between quantitative parameters and therapeutic response, assessed by Mann–Whitney test, the results show statistical significance only for 2 h EUR in relation to therapeutic response at 1 year. In addition, patients with a high 2 h EUR showed greater volume reduction than patients with a low 2 h EUR (p = 0.027). All other quantitative parameters assessed at 1 and 2 h did not show statistical significance.
The study presents some limitations, such as the small group of patients (17) and non-homogeneity regarding the etiology of lymphedema (primary or secondary); however, it shows an advantage in terms of using both the dermal backflow pattern and extremity uptake ratio (EUR) at preoperative lymphoscintigraphy to predict therapy response in patients who will undergo to LVA. These results are in line with those of other similar studies.
At first, Yoo JN. et al. correlated quantitative and qualitative lymphoscintigraphic results to the arm circumferences in breast cancer patients with lymphedema secondary to ALD [25]. Here, 72 patients with cancer-related lymphedema were divided into three qualitative groups, defined as follows: normal pattern with normal lymphatic system, with the visualization of superficial lymphatic system and normal axillary lymph nodes; a decreased function group that showed decreased visualization of lymphatic channels or delayed lymphatic flow, and an obstruction group that showed abnormal dermal backflow or few or no axillary lymph nodes. The authors also measured arm circumference at five standardized levels, and calculated the maximal circumference differences (MCDs) in the most symptomatic area. After this, they went on to correlate these results with the results of quantitative analyses calculated by quantitative asymmetry index (QAI) in both arms in three ROIs per arm, respectively—one circular ROI for axillary lymph nodes and two rectangular ROIs for the upper arm and the forearm region, excluding the elbow and hand. The results show a direct correlation between QAI and qualitative patterns. In fact, patients with obstructive patterns showed lower QAI in the axillary lymph node ROI and higher QAI in the upper limb ROI. MCD also appears to be inversely related to the QAI of the axillary lymph node ROI. Regarding the decreased function pattern, axillary QAI is found to be reduced, and is the lowest amongst all measures, even compared to the normal pattern in the QAI of upper limb ROI. All this explains the reduced function of the lymphatic system with reduced axillary flow through the remaining lymph nodes and increased flow due to new lymphatic collaterals in the upper arm [26].
Kim P. et al. evaluated the use of the quantitative method in 201 patients without lymphedema after unilateral breast cancer surgery. They observed a higher probability of developing lymphedema (OR = 0.14, CI = 0.04–0.46) in patients presenting abnormal ratios of radioactivity between affected arms and normal axilla (RRA) [27].
Szuba A. and Strauss W. [28] enrolled 90 patients with lymphedema after breast cancer therapy. The severity of lymphedema was evaluated by lymphoscintigraphy with the quantification of RRA. Patients were re-evaluated after the completion of therapy for lymphedema. The authors concluded that there is a correlation between the ARR and the percentage reduction in edema volume after therapy.
Newly available software permits the extraction of new quantitative parameters. A recent example has been proposed by Keramida G. et al. The lymphatic drainage efficiency (LDE) measures the percentage of injected activity accumulating in ilio-inguinal nodes, and has shown potential value in clinical research [29].
All these studies show that the quantitative approach should be systematically placed side by side with the classical qualitative protocol, because it effectively illustrates lymphedema severity, and it may be of use in negative cases determined using qualitative methods.
At last, lymphoscintigraphy appears to be the best method for the evaluation of primary and secondary lymphedema with low radiation doses (about 1 mSv), high sensitivity, and even higher specificity.
Lymphoscintigraphy is not only useful for the diagnosis and staging of lymphedema severity, but also for the prediction of worsening or improvements after therapy. These abilities are displayed whether using the qualitative or the quantitative approach, although the latter is less diffuse, especially in the case of bilateral lymphedema. Quantitative lymphoscintigraphy should be seriously considered for implementation, but the lack of widely accepted protocol makes it difficult to introduce this method into clinical practice.
3. New Emerging Techniques for Lymphedema Assessment in Real Clinical Practice
Over the past two decades, new techniques have emerged for the study of the lymphatic system, and particularly lymphedematous pathology. One of these is indocyanine green fluorescence lymphangiography, which has better spatial resolution than classical lymphoscintigraphy but a limitation in terms of anatomic coverage, including limited depth in skin studies.
More recently, magnetic resonance lymphangiography (MRL) has been developed for lymphedema screening and follow-up. This has been made possible by new MRI protocols both with and without contrast, achieving incredible anatomical detail thanks to high-field machines. Noninvasive MRL (NIMRL), using heavily T2-weighted sequences with very long TR/TE, has the advantages of reducing the method time, eliminating the radioactive dose given to the patient and clinicians, and not using contrast agents, avoiding adverse reactions [30].
Also, in contrast-enhanced MRI, new contrast agents are being investigated that have been specifically created for MRL, such as Ultrasmall Superparamagnetic Iron Oxide Nanoparticles (USPIO) that are selectively captured in the lymphatic system and lymph nodes [30]; however, today, MR lymphoangiography is most frequently performed via the subcutaneous injection of gadolinium-based contrast agent. This provides high spatial resolution 3D imaging of the lymphatic vessels, thus selecting patients who can benefit from surgical treatments such as LVA and helping surgeons to study lymphatic structures in the anatomic region of interest [31]. A detailed description of the imaging protocols of the various MRL techniques is given in the article by Guerrini S. et al. [32].
MRL can also assess alterations in flow dynamics, as proposed by Borri et al. [33] in a study that describes a five-parameter model that can predict flow velocity, and outlines the difference between healthy and affected arms (9.7 cm/min in the unaffected arm vs. 2.1 cm/min in the affected arm) as an additional quantitative parameter to stratify patients who are candidates for different treatment approaches.
Recently, Kim G. [34] proposed a new MRI-based staging system. Although validated on a small number of patients (45) with secondary lymphedema in the upper limb, this staging method is proposed as an accurate non-invasive marker for therapeutic planning. The grading system is based on an evaluation of STIR-weighted images on axial sections at three levels: elbow, 5–8 cm proximal to the radius head, and 5–8 cm distal from the olecranon. Here, on the three sections, the percentage of circumferential subcutaneous fluid infiltration was assessed and graduated. Stage 0 was assigned when no subcutaneous tissue infiltration was present at any level, stage 1 was given when circumferential infiltration did not exceed 50% in any section, stage 2 was when the circumferential fluid infiltration was greater than 50% in any of the sections, and finally stage 3 was given when all three sections demonstrated circumferential fluid infiltration greater than 75%. All patients were evaluated with ICG lymphography, lymphoscintigraphy, the Lymphedema Quality of Life Questionnaire (LYMQOL), International Society of Lymphology (ISL) staging and quantitative measurements (limb volume and L-Dex®). The study showed a strong correlation between advanced stages under MRI and those under ISL, as well as with dermal backflow shown by lymphoscintigraphy. A correlation was also shown between advanced stages under MRI and abnormal ICG lymphography patterns, larger percentage differences in limb volume and higher L-Dex® ratios. Therefore, this staging method showed encouraging results when used in MRI-based evaluations, with excellent interpretive reproducibility and correlation with other methods, in addition to the use of no contrast agent, but with all the advantages that it provides. Certainly, validation on a larger number of patients is needed. Other sequences (also with contrast agents) could be evaluated to show greater correlation, even in early stages of lymphedema.
In recent years, the MRI method used for the diagnosis and stratification of lymphedema is taking on an increasing role, and at the same time, efforts have been made to increase its sensitivity by combining it with a complementary method, such as indocyanine green (ICG) fluorescent lymphography.
ICG fluorescence lymphography is a relatively recent and very promising method, especially when used in the early stages of lymphedema or when combined with other methods. This method, which was initially develop to identify the sentinel lymph node, has not only been evaluated as a staging modality, but also been used to assess candidacy for surgical intervention in lymphedema. The technique is based on the use of contrast agents such as indocynine green, exploiting their ability to absorb light at a certain wavelength in the near-infrared spectrum and simultaneously (in real-time) visualize its uptake and transit due to induced fluorescence through a dedicated camera. This method demonstrates a high spatial resolution that can allow for the precise localization of functional superficial lymphatic vessels, their transport capacity, any collateral lymphatic vessels, and the presence of dermal backflow that represents the pathological conditions of the underlying lymphatic vessels. Although this is a very sensitive method capable of demonstrating alterations in the lymphatic pathway even before volumetric changes, it has many disadvantages, the most important of which is the loss of visualization of vessels located more than 2 cm under the skin; in fact, the method is only able to study the most superficial lymphatic structures.
Various classifications have been proposed for the assessment of lymphedema severity, making it difficult to compare the ICG fluorescence method with others. One of the most widely used, and internationally accepted, is that of the group from the Department of Plastic Surgery of University of Texas M. D. Anderson Cancer Center, set out by authors Chang D., Suami H. and Skoracki R. [35]. The classification proposed by the authors came from a prospective study of 100 patients undergoing LVA. The aim was to evaluate not only the efficacy of surgical treatment at the various stages of lymphedema, but also to establish the role of ICG lymphography in the assessment and selection of patients. The MD Anderson lymphedema classification (MDAC) is divided into five stages based on the descriptive features of ICG lymphography. Stage 0: Many patent lymphatic vessels, no dermal backflow, normal contractility. Stage 1: Many patent lymphatic vessels, minimal, patchy dermal backflow, slightly delayed contractility. Stage 2: Moderate patent lymphatic vessels, segmental dermal backflow, moderately delayed contractility. Stage 3: Few patent lymphatic vessels, extensive dermal backflow involving the entire arm, minimal contractility. Stage 4: No patent lymphatic vessels, severe dermal backflow in the entire extremity and dorsum extending to the digits (finger/toe sign) and volar (palm/sole sign), no contractility. Stage 5: No patent lymphatic vessels, no dye movement, no contractility.
There is great concordance between the ICG fluorescence and lymphoscintigraphic methods, as shown in a recent study by Akita S. et al. [36]. The authors compared ICG to lymphoscintigraphy when used in 169 extremities with lymphedema after lymph node dissection, demonstrating sensitivity, specificity and accuracy of 97%, 54% and 81%, respectively. The authors reported a sensitivity of ICG of 97%, a specificity of 92% and an accuracy of 95% in patients in the early stage, and discordant findings were revealed between the two techniques. According to these findings, they suggest the use of ICG as the first procedure.
The knowledge of the lymphatic system’s anatomy and its change after surgery is essential, and it is crucial to recognize regenerative lymphatic collaterals in order to explain lymphedema severity. ICG lymphography appears to be effective in this context.
Suami H. et al. proposed that the mechanism of lymphatic drainage is an additional factor in determining the degree of severity of lymphedema. These authors, in a recent study [37], reviewed a series of images obtained by lymphoscintigraphy and ICG lymphography in patients undergoing lymph node dissection. They proposed three types of possible mechanisms of afferent lymphatic vessel regrowth: new lymphatic vessels, dermal backflow, or a combination of these. They suppose that the mechanism of regeneration proceeding through dermal backflow is associated with more severe lymphedema because of the smaller size of lymphatic capillaries.
The combined usage of ICG and MRL represents an opportunity to achieve maximum benefits in accurate preoperative studies on patients who are candidates for LVA.
MRI is, in fact, able to depict deep structures not visible to ICG lymphography, and is used for initial evaluations of the patient. This approach was proposed by Pons G. et al. [38] in a prospective study on 82 patients. They obtained a high rate of success in performing LVA thanks to the precise spatial localization achieved with MRL.
In recent years, contrast enhancement ultra sonography (CEUS), which has already shown potential use in sentinel lymph node mapping [39], has been considered for the preoperative evaluation of lymphatic vessels that are not detected by other techniques, such as ICG lymphography. CEUS uses lipid or protein microbubbles containing inert gases as contrast agents, but few studies have proven its efficacy in lymphedema.
A recent study by Jang S.et al. [40] evaluated, in 11 women with breast cancer treatment-related lymphedema (BCRL), whether CEUS can be used to identify target lymphatic vessels before LVA surgery. ICG lymphography was performed in 10 women and failed to identify any targets in 5 of them, but CEUS was able to identify all lymphatic channels. This shows how the use of CEUS may help surgeons with preoperative planning when ICG lymphography is inadequate. A single study is not enough to support the inclusion of CEUS in clinical practice, as it lacks standardized protocols. More prospective studies on a larger and more varied case series are therefore needed to evaluate the efficacy of CEUS, which, due to its low cost and availability, could bring numerous advantages.
AI and Machine Learning: The Distant Future of Diagnostic Imaging
Medicine, particularly diagnostic imaging, as in many other fields, has been affected by the influence of artificial intelligence (AI), which finds applications from radiomics to machine learning based on modern neural networks. Radiomics is a quantitative approach to medical imaging, which aims at enhancing the existing data available to clinicians by means of advanced mathematical analyses [41].
There are few studies demonstrating the real potential of applying AI in the context of lymphedema. An early example is a recent study by Son H. et al. [42], which showed the application potential of deep-learning (DL)-based algorithms for the early identification of lymphedema-induced fibrosis by computer tomography (CT). The study evaluated 27 patients with lymphedema by analyzing a total of 2138 CT cross-sectional images. Then, the results of the algorithms were compared, based on four indices, with those obtained from the two gold-standard methods for fibrosis identification, which are standardized circumference difference ratio (SCDR) and bioelectrical impedance (BEI). The results obtained showed good correlation with traditional methods, although the study shows many limitations.
In another study by Nowak S. et al. [43], the authors evaluated the effectiveness of a DL pipeline that can assess shape, volume, and asymmetry based on an MRI of the lower extremities of patients with lymphedema. The authors retrospectively evaluated 45 patients, obtaining results that will facilitate a standardized analysis of volume and tissue distribution, which could help in the diagnosis of lymphedema or its monitoring.
Deep learning algorithms can also help achieve reproducibility in operator-dependent methods, such as echography. In fact, the goal of a study by Goudarzi S. et al. was precisely to establish automatic segmentation within a dataset of 39 patients. This may, in the future, make the staging of BCRL more convenient and accessible [44].
The application of AI is not only limited to the field of diagnostic imaging, but, as far as lymphedema is concerned, it can be applied to geographic prevalence studies for forms of primary lymphedema secondary to filariasis; it here affects the field of rehabilitation by evaluating in real time the patient’s movements and guiding them better in their daily exercises, and is also relevant to the field of robotic surgery, and finally early detection through predictive patterns in image recognition. All these fields of application have been covered in a recent review by Elday A.S. et al. [45].
4. The Role of Imaging in the Prevention and Treatment of Lymphedema
Imaging is taking on an increasingly central role in patient assessments—not only for diagnostic purposes, as it was until a few decades ago, but also now in prevention and, when this is no longer feasible, in therapy. While the number of staging classifications of lymphedema has increased with the assessment of patients over time, the same has not happened for the imaging-related risk group classifications. This lack plays a key role in hindering the development of cancer-related lymphedema, because it makes it impossible to define an individual diagnostic–therapeutic protocol based on personal risk.
Of the predictive factors associated with an increased risk of developing lymphedema, most are related either to individual factors, such as weight and age, or to cancer-related factors such as stage, lymph node dissection, and radiation therapy. Thus, predictive factors related to imaging parameters are lacking, although some authors report an association with the dermal backflow sign. However, many consider dermal backflow to be a true early sign of lymphedema, even if it is clinically silent, rather than a predictive parameter.
The role of imaging in treating lymphedema has benefited from new techniques in the area of lymphatic vessel anatomization, as demonstrated by the use of MRL in the pre-operative setting; similarly, ICG lymphography has demonstrated its ability to precisely identify lymphatic vessels to be subjected to reconstructive surgery in real time. Nuclear medicine has shown great potential in adding pathophysiological data to anatomical imaging [46][47][48][49][50]. These advancements are of value in many clinical scenarios where scintigraphy guides the treatment, as demonstrated by radioguided surgery (RGS) [51][52].
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10121407
References
- McLaughlin, S.A.; Wright, M.J.; Morris, K.T.; Giron, G.L.; Sampson, M.R.; Brockway, J.P.; Hurley, K.E.; Riedel, E.R.; Van Zee, K.J. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J. Clin. Oncol. 2008, 26, 5213.
- Petrek, J.A.; Senie, R.T.; Peters, M.; Rosen, P.P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001, 92, 1368–1377.
- Segerstrom, K.; Bjerle, P.; Graffman, S.; Nystrom, A. Factors that influ- ence the incidence of brachial oedema after treatment of breast cancer. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1992, 26, 223–227.
- Committee, E. The diagnosis and treatment of peripheral lymph- edema: 2016 consensus document of the International Society of Lymphology. Lymphology 2016, 49, 170–184.
- Monticone, M.; Ferriero, G.; Keeley, V.; Brunati, R.; Liquori, V.; Maggioni, S.; Restelli, M.; Giordano, A.; Franchignoni, F. Lymphedema quality of life questionnaire (LYMQOL): Cross-cultural adaptation and validation in Italian women with upper limb lymphedema after breast cancer. Disabil. Rehabil. 2022, 44, 4075–4080.
- Cuccurullo, V.; Rapa, M.; Catalfamo, B.; Cascini, G.L. Role of Nuclear Sentinel Lymph Node Mapping Compared to New Alternative Imaging Methods. J. Pers. Med. 2023, 13, 1219.
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819.
- Spiegel, M.; Vesti, B.; Shore, A.; Franzeck, U.K.; Becker, F.; Bollinger, A. Pressure of lymphatic capillaries in human skin. Am. J. Physiol. Circ. Physiol. 1992, 262, H1208–H1210.
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356.
- De Cicco, C.; Cremonesi, M.; Luini, A.; Bartolomei, M.; Grana, C.; Prisco, G.; Galimberti, V.; Calza, P.; Viale, G.; Veronesi, U.; et al. Lymphoscintigraphy and Radioguided Biopsy of the Sentinel Axillary Node in Breast Cancer. J. Nucl. Med. 1998, 39, 2080–2084.
- Ballinger, J.R. Challenges in Preparation of Albumin Nanoparticle-Based Radiopharmaceuticals. Molecules 2022, 27, 8596.
- Partsch, H. Assessment of abnormal lymph drainage for the diagnosis of lymphedema by isotopic lymphangiography and by indirect lymphography. Clin. Dermatol. 1995, 13, 445–450.
- McNeill, G.C.; Witte, M.H.; Witte, C.L.; Williams, W.H.; Hall, J.N.; Patton, D.D.; Pond, G.D.; Woolfenden, J.M. Whole-body lymphangioscintigraphy: Preferred method for initial assessment of the peripheral lymphatic system. Radiology 1989, 172, 495–502.
- Szuba, A.; Shin, W.S.; Strauss, H.W.; Rockson, S. The third circulation: Radionuclide lymphoscintigraphy in the evaluation of lymphedema. J. Nucl. Med. 2003, 44, 43–57.
- Villa, G.; Campisi, C.C.; Ryan, M.; Boccardo, F.; Di Summa, P.; Frascio, M.; Sambuceti, G. Procedural Recommendations for Lymphoscintigraphy in the Diagnosis of Peripheral Lymphedema: The Genoa Protocol. Nucl. Med. Mol. Imaging 2019, 53, 47–56.
- Hassanein, A.H.; Maclellan, R.A.; Grant, F.D.; Greene, A.K. Diagnostic accuracy of lymphoscintigraphy for lymphedema and analysis of false negative tests. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1396.
- Gloviczki, P.; Calcagno, D.; Schirger, A.; Pairolero, P.C.; Cherry, K.J.; Hallett, J.W.; Wahner, H.W. Noninvasive evaluation of the swollen extremity: Experiences with 190 lymphoscintigraphic examinations. J. Vasc. Surg. 1989, 9, 683–690.
- Pappalardo, M.; Cheng, M.H. Lymphoscintigraphy for the diagnosis of extremity lymphedema: Current controversies regarding protocol, interpretation, and clinical application. J. Surg. Oncol. 2020, 121, 37–47.
- Cheng, M.H.; Chang, D.W.; Patel, K.M. Principles and Practice of Lymphedema Surgery; Elsevier: Oxford, UK, 2016.
- Cheng, M.H.; Pappalardo, M.; Lin, C.; Kuo, C.F.; Lin, C.Y.; Chung, K.C. Validity of the novel Taiwan lymphoscintigraphy staging and correlation of cheng lymphedema grading for unilateral extremity lymphedema. Ann. Surg. 2018, 268, 513–525.
- Campisi, C.C.; Ryan, M.; Villa, G.; Di Summa, P.; Cherubino, M.; Boccardo, F.; Campisi, C. Rationale for study of the deep subfascial lymphatic vessels during lymphoscintigraphy for the diagnosis of peripheral lymphedema. Clin. Nucl. Med. 2019, 44, 91–98.
- Fujiyoshi, T.; Mikami, T.; Hashimoto, K.; Asano, S.; Adachi, E.; Kagimoto, S.; Yabuki, Y.; Kitayama, S.; Matsubara, S.; Maegawa, J.; et al. Pathological changes in the lymphatic system of patients with secondary lower limb lymphedema based on single photon-emission computed tomography/computed tomography/lymphoscintigraphy images. Lymphat. Res. Biol. 2021, 20, 144–152.
- Sacks, G.A.; Sandler, M.P.; Born, M.L.; Claton, J.A.; Franklin, J.D.; Partain, C.L. Lymphoscintigraphy as an adjunctive procedure in the perioperative assessment of patients undergoing microlymphaticovenous anastomoses. Clin. Nucl. Med. 1983, 8, 309–311.
- Kwon, H.R.; Hwang, J.H.; Mun, G.-H.; Hyun, S.H.; Moon, S.H.; Lee, K.-H.; Choi, J.Y. Predictive role of lymphoscintigraphy undergoing lymphovenous anastomosis in patients with lower extremity lymphedema: A preliminary study. BMC Med. Imaging 2021, 21, 188.
- Yoo, J.N.; Cheong, Y.S.; Min, Y.S.; Lee, S.W.; Park, H.Y.; Jung, T.D. Validity of quantitative lymphoscintigraphy as a lymphedema assessment tool for patients with breast cancer. Ann. Rehabil. Med. 2015, 39, 931–940.
- Chiewvit, S.; Kumnerdnakta, S. Lymphoscintigraphic Findings That Predict Favorable Outcome after Lymphaticovenous Anastomosis. Lymphology 2017, 50, 1–8.
- Kim, P.; Lee, J.K.; Lim, O.K.; Park, H.K.; Park, K.D. Quantitative Lymphoscintigraphy to Predict the Possibility of Lymphedema Development After Breast Cancer Surgery: Retrospective Clinical Study. Ann. Rehabil. Med. 2017, 41, 1065–1075.
- Szuba, A.; Strauss, W.; Sirsikar, S.P.; Rockson, S.G. Quantitative radionuclide lymphoscintigraphy predicts outcome of manual lymphatic therapy in breast cancer-related lymphedema of the upper extremity. Nucl. Med. Commun. 2002, 23, 1171–1175.
- Keramida, G.; Wroe, E.; Winterman, N.; Aplin, M.; Peters, A.M. Lymphatic drainage efficiency: A new parameter of lymphatic function. Acta Radiol. 2018, 59, 1097–1101.
- Bellin, M.F.; Beigelman, C.; Precetti-Morel, S. Iron oxide-enhanced MR lymphography: Initial experience. Eur. J. Radiol. 2000, 34, 257–264.
- Neligan, P.C.; Kung, T.A.; Maki, J.H. MR lymphangiography in the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 18–22.
- Guerrini, S.; Gentili, F.; Mazzei, F.G.; Gennaro, P.; Volterrani, L.; Mazzei, M.A. Magnetic resonance lymphangiography: With or without contrast? Diagn. Interv. Radiol. 2020, 26, 587–595.
- Borri, M.; Schmidt, M.A.; Gordon, K.D.; Wallace, T.A.; Hughes, J.C.; Scurr, E.D.; Koh, D.-M.; Leach, M.O.; Mortimer, P.S. Quantitative Contrast-Enhanced Magnetic Resonance Lymphangiography of the Upper Limbs in Breast Cancer Related Lymphedema: An Exploratory Study. Lymphat. Res. Biol. 2015, 13, 100–106.
- Kim, G.; Smith, M.P.; Donohoe, K.J.; Johnson, A.R.; Singhal, D.; Tsai, L.L. MRI staging of upper extremity secondary lymphedema: Correlation with clinical measurements. Eur. Radiol. 2020, 30, 4686–4694.
- Chang, D.W.; Suami, H.; Skoracki, R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast. Reconstr. Surg. 2013, 132, 1305–1314.
- Akita, S.; Mitsukawa, N.; Kazama, T.; Kuriyama, M.; Kubota, Y.; Omori, N.; Koizumi, T.; Kosaka, K.; Uno, T.; Satoh, K. Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 792–798.
- Suami, H.; Koelmeyer, L.; Mackie, H.; Boyages, J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surg. Oncol. 2018, 27, 743–750.
- Pons, G.; Clavero, J.A.; Alomar, X.; Rodríguez-Bauza, E.; Tom, L.K.; Masia, J. Preoperative planning of lymphaticovenous anastomosis: The use of magnetic resonance lymphangiography as a complement to indocyanine green lymphography. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 884–891.
- Huang, Y.; Zheng, S.; Lin, Y. Accuracy and Utility of Preoperative Ultrasound-Guided Axillary Lymph Node Biopsy for Invasive Breast Cancer: A Systematic Review and Meta-Analysis. Comput. Intell. Neurosci. 2022, 2022, 3307627.
- Jang, S.; Lee, C.U.; Hesley, G.K.; Knudsen, J.M.; Brinkman, N.J.; Tran, N.V. Lymphatic Mapping Using US Microbubbles before Lymphaticovenous Anastomosis Surgery for Lymphedema. Radiology 2022, 304, 218–224.
- Panico, A.; Gatta, G.; Salvia, A.; Grezia, G.D.; Fico, N.; Cuccurullo, V. Radiomics in Breast Imaging: Future Development. J. Pers. Med. 2023, 13, 862.
- Son, H.; Lee, S.; Kim, K.; Koo, K.I.; Hwang, C.H. Deep learning-based quantitative estimation of lymphedema-induced fibrosis using three-dimensional computed tomography images. Sci. Rep. 2022, 12, 15371.
- Nowak, S.; Henkel, A.; Theis, M.; Luetkens, J.; Geiger, S.; Sprinkart, A.M.; Pieper, C.C.; Attenberger, U.I. Deep learning for standardized, MRI-based quantification of subcutaneous and subfascial tissue volume for patients with lipedema and lymphedema. Eur. Radiol. 2023, 33, 884–892.
- Goudarzi, S.; Whyte, J.; Boily, M.; Towers, A.; Kilgour, R.D.; Rivaz, H. Segmentation of Arm Ultrasound Images in Breast Cancer-Related Lymphedema: A Database and Deep Learning Algorithm. IEEE Trans. Biomed. Eng. 2023, 70, 2552–2563.
- Eldaly, A.S.; Avila, F.R.; A Torres-Guzman, R.; Maita, K.; Garcia, J.P.; Serrano, L.P.; Forte, A.J. Artificial intelligence and lymphedema: State of the art. J. Clin. Transl. Res. 2022, 8, 234–242.
- Briganti, V.; Cuccurullo, V.; Di Stasio, G.D.; Mansi, L. Gamma emitters in pancreatic endocrine tumors imaging in the pet era: Is there a clinical space for 99mTc-peptides? Curr. Radiopharm. 2019, 12, 156–170.
- Cuccurullo, V.; Manti, F.; De Risi, M.; Cascini, G.L. DG-CT/PET false positive case in hip prosthesis: A clue to avoid error. Radiol. Case Rep. 2021, 16, 2601–2604.
- Cuccurullo, V.; Di Stasio, G.D.; Manti, F.; Arcuri, P.; Damiano, R.; Cascini, G.L. The Role of Molecular Imaging in a Muscle-Invasive Bladder Cancer Patient: A Narrative Review in the Era of Multimodality Treatment. Diagnostics 2021, 11, 863.
- Parmeggiani, D.; Gambardella, C.; Patrone, R.; Polistena, A.; De Falco, M.; Ruggiero, R.; Cirocchi, R.; Sanguinetti, A.; Cuccurullo, V.; Accardo, M.; et al. Radioguided thyroidectomy for follicular tumors: Multicentric experience. Int. J. Surg. 2017, 41 (Suppl. S1), S75–S81.
- Cuccurullo, V.; Di Stasio, G.D.; Mansi, L. Radioguided surgery with radiolabeled somatostatin analogs: Not only in GEP-NETs. Nucl. Med. Rev. 2017, 20, 49–56.
- Cuccurullo, V.; Cioce, F.; Sica, A.; Gatta, G.; Rubini, G. Gastroenteric diseases in the third millennium: A rational approach to optimal imaging technique and patient selection. Recent. Progress. Med. 2012, 103, 426–430.
- Cuccurullo, V.; Di Stasio, G.; Prisco, M.; Mansi, L. Is there a clinical usefulness for radiolabeled somatostatin analogues beyond the consolidated role in NETs? Indian J. Radiol. Imaging 2017, 27, 509–516.
This entry is offline, you can click here to edit this entry!
