1. Structure of Connexin
Connexins are transmembrane proteins. Approximately 20 types of connexin genes have been reported in humans and mice [
1,
2]. The corresponding genes are abbreviated as “GJ” (for gap junction), while the most commonly used protein nomenclature employs “Cx” (for connexin). Connexin proteins are often identified by the molecular mass of the predicted polypeptide in kilodaltons (e.g., connexin43, Cx43) [
3]. Different connexin sequences have been used to recognize connexin subfamilies, with five connexin subfamilies defined (α, β, γ, δ, and ε in mice or GJA, GJB, GJC, GJD, and GJE in humans) [
1], each of them containing distinct biophysical properties and expression patterns with various biological functions. All connexins have similar topological structural characteristics, consisting of four transmembrane domains: the amino (NT) and carboxyl termini (CTs) in the cytoplasm, a cytoplasmic loop (CL), and two extracellular loops (EL1 and EL2) [
4,
5]. The CTs are considered regulatory regions for multiple post-translational modifications and protein binding sites, exhibiting the greatest heterogeneity in amino acid sequences [
6]. Every six connexin monomers form a hexagonal lattice of protein subunits, as seen under electron crystallography, called connexons or hemichannels (HCs) [
7]. After trafficking to the membrane docks, two HCs from adjacent cells with a “head-to-head” pattern form an intercellular channel called a GJ [
8]. These GJs and HCs allow the passage of small molecules (Mw < 1 kDa), including ions, essential metabolites, and second messengers such as Ca
2+, IP3, nicotinamide adenine dinucleotide (NAD), prostaglandin E
2 (PGE
2), ATP, ADP, cAMP, and cGMP [
9]. This type of intercellular communication is known to be essential for various physiological activities, including cell growth, proliferation, and differentiation. It also plays crucial roles in tissue homeostasis, tumorigenicity, fibrosis, and wound healing.
2. Channel-Dependent Functions of Cx in Fibrosis, EMTs, and Wound Healing
2.1. Connexin GJs and HCs in Eye Diseases
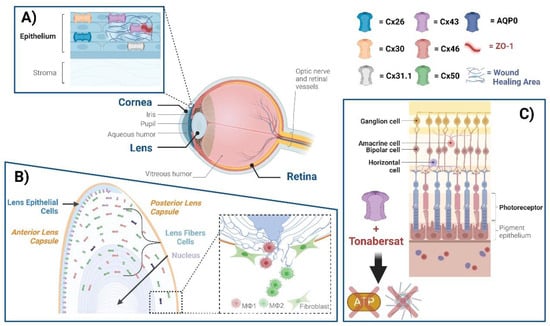
2.1.1. Cornea
The eye comprises the cornea, lens, uvea, retina, and several other subcomponents, within which various connexins exist. GJ communication in the human corneal epithelium is mediated by Cx26, Cx30, Cx31.1, and Cx43 [
21,
22]. Specifically, Cx43 has been associated with inflammatory responses and wound healing in injured corneas [
23,
24]. Cx43 knockdown can accelerate wound healing in the corneal endothelium and promote re-epithelialization by suppressing stromal oedema and inflammatory responses [
25,
26]. The interaction between Cx43 and zonula occludens-1 (ZO-1), a scaffolding protein that plays a role in regulating GJ assembly, has been extensively studied [
27,
28,
29,
30]. The alpha-carboxy terminus 1 (αCT1) peptide, derived from the carboxyl terminus of Cx43, disrupts Cx43 interaction with ZO-1, leading to an enhancement of GJs [
31]. Treatment with αCT1 may play a role in the early stages of migration during corneal healing, as well as in affecting EMT pathway genes [
32,
33]. Cx43 antisense oligodeoxynucleotides (AsODNs), which knockdown Cx43 expression, have been shown to have a definite therapeutic effect in a reproducible model of corneal wound healing [
26]. Cx43 AsODN-treated corneas show improved corneal clarity, faster re-epithelialization, and reduced inflammation. Additionally, the reduction in Cx43 GJ function with AsODNs in human nonhealing ocular burn wounds could enhance the recovery of limbal reperfusion and reduce inflammation, holding therapeutic potential for the treatment of severe, often unresponsive corneal injuries. This is achieved by targeting the downregulation of Cx43 to enable vascular recovery, making Cx43 a possible key factor in the treatment of wounds [
34]. Moore et al. also found a downregulation of Cx43 in corneal wound healing. Between the 24- and 72-h time points, as the wound is healing, Cx43 is upregulated as cell–cell adhesion and GJ formation recur. By day 21, there was a downregulation in Cx43 [
32]. The critical point is the time for treatment. Early use increases GJs, which are closely related to inhibiting inflammation and reducing edema. But in later stages, reducing over-expressed Cx43 can promote repair, speed healing, and prevent excessive scarring. Gap27 is a mimicking sequence on the second external loop of the Cx43 HC [
9,
35]. Its use in the early stages of wound healing for superficial corneal epithelial wounds has been proposed for promoting corneal epithelial healing, including persistent corneal ulcers, limbal stem cell deficiency, and dry eye syndrome. However, prolonged and frequent treatment in stromal wounds could elicit an undesirable inflammatory response [
36].
2.1.2. Lens
The lens epithelial cells express Cx43 and Cx50, while fiber cells express Cx46 and Cx50 [
37]. Past research primarily focuses on the function and regulation of GJs because these channels play essential roles in maintaining lens cell homeostasis, metabolic coupling, and preventing the accumulation of reactive oxidants. Connexin mutations are being identified and linked to numerous types of human congenital cataracts [
38,
39]. Abnormal activities of GJs and/or HCs would greatly compromise these crucial functions. Our group has previously reported that the G143R mutation increases the interaction between the intracellular loop domain and Cx46, which is associated with the reduction in GJs but an increase in HC function [
40]. Additionally, Cx50 HCs activated by H
2O
2 mediate glutathione (GSH) transport and protect lens fiber cells from oxidative stress [
41]. Mechanically activated Cx HCs and GSH transport can reduce H
2O
2- and UVB-induced intracellular reactive oxygen species (ROS), further mitigating cellular apoptosis and death [
42]. Our recent study confirmed that protein kinase A (PKA) activation reduces cataracts induced by oxidative stress, increases GJs/HCs in Cx50, Cx46, or Cx50 and Cx46 co-expressing cells, and decreases ROS levels and cell death [
43].
The researchers proposed a new molecular mechanism of HCs in lens epithelial cells that protects the lens against oxidative stress. The researchers found that Cx43 HCs mediate the exchange of oxidants and antioxidants in lens epithelial cells undergoing oxidative stress. These transporting activities facilitate a reduction in intracellular ROS accumulation and maintain intracellular glutathione levels through the exchange of redox metabolites and changes in anti-oxidative gene expression. Additionally, Cx43 HCs can be regulated by the intracellular redox state, and this regulation is mediated by residue Cys260 located at the Cx43 C-terminus [
19]. All these findings demonstrate that GJs between lens fiber cells are an important mechanism for maintaining lens transparency by transmitting redox metabolites and that HCs assist in delivering nutrients and anti-oxidants into lens epithelial and fiber cells.
Posterior capsular opacification (PCO), a common complication following cataract surgery, results from the proliferation and degenerative fibrosis of residual lens epithelial cells [
44]. The type-2 EMT and fibrosis are closely related to lens epithelial cells. Numerous studies have implicated transforming growth factor-β (TGF-β) in the fibrotic formation of PCO, including isoforms of TGFβ1 and TGFβ2 [
45,
46]. Additionally, there is a link between the inflammatory response to a lens injury and the mechanisms responsible for TGFβ1/β2 activation and the induction of fibrosis [
47,
48]. Moreover, cell adhesion signaling has also been implicated in the regulation of the EMT and fibrosis in the lens [
49]. However, there is limited knowledge regarding the role of connexin channels in fibrosis. It is not clear whether GJs/HCs participate in the TGF-β-induced EMT of lens epithelial cells in response to an injury, including capsular scarring after cataract lens removal and intraocular lens implantation. Although TGF-β1 at a low concentration of 0.4 ng/mL markedly increases the expression of EMT markers, this amount is not sufficient to enhance GJ intercellular communication (GJIC) in chick embryonic lens epithelial cells. This finding has led to the deduction that the upregulation of GJIC by TGF-β is not an obvious downstream consequence of the TGF-β-induced EMT [
50].
2.1.3. Retina
Connexins are present in the five neuronal types of the retina, vascular endothelial cells, pericytes, glial cells, and the retinal pigment epithelium (RPE) [
51,
52]. The neuronal types of the retina are composed of photoreceptors and horizontal, bipolar, amacrine, and ganglion cells. There is a large array of connexins on these cells in mice and humans, with previous research demonstrating the presence of several Cx subtypes, including Cx35, Cx36, Cx43, Cx45, Cx57, Cx59, among others [
52,
53,
54,
55]. Furthermore, studies have largely focused on the role connexins play in material transport and signaling transmission. Cxs are reported to play crucial roles in the fibrotic development of several chronic retinal diseases, such as diabetic retinopathy (DR), retinal ischemia, age-related macular degeneration (AMD), and RPE-related diseases [
51,
56]. It is also worth noting that all these diseases are associated with astrocytes, Müller cells, microglia, RPE, and vessel endothelial cells in conjunction with inflammatory processes and vascular responses [
51]. However, the participation of neuronal retinal Cxs in wound healing and fibrosis has not been directly reported.
Cx43, the most ubiquitously expressed isoform in these three types of glial cells, RPE, and endothelial cells, plays an essential role in multiple cellular processes [
53,
57,
58]. As an example, Tien et al. previously reported findings indicating that the high glucose-induced downregulation of Cx43 expression and GJIC may contribute to the breakdown of endothelial barrier tight junctions associated with DR [
59]. They then showed that Cx43 protein expression and GJIC were significantly reduced in diabetic retinas compared to non-diabetic retinas. These results signify that the reduction in Cx43 is associated with increased vascular cell death in human diabetic retinas [
60]. In other studies, Toychiev et al. investigated the outcome of an increase in Cx43 expression and GJ coupling during an acute ischemia/reperfusion injury, which exacerbated ganglion cell loss. The inhibition of astrocytic Cx43 channels may represent a useful strategy for promoting RGC survival in pathologic conditions [
61].
GJs and functional HCs are major focus areas of prior research. Cx HCs have a low probability of being open under normal physiological conditions. However, when open in an undocked form, these HCs allow for the ubiquitous release of paracrine and autocrine signals [
62,
63]. Overactivated HCs have been associated with secondary tissue injuries, including edema, compromised vascular integrity, and retinal damage, by allowing the passage of molecules and ions between the cell cytoplasm and the extracellular milieu. They can also affect downstream GJ communication [
51,
56,
64]. During inflammation, HC-mediated ATP release is highly prominent [
65]. Certain peptides, such as low concentrations of Peptide5 and Gap19, have been used to block Cx43 HCs specifically without affecting GJ coupling. These tools appear to provide viable therapeutic options for treating retinal ischemia and retinal inflammatory diseases associated with Cx43 HC opening [
62,
66,
67]. Meanwhile, the novel benzopyran derivative Tonabersat, a sodium channel blocker that also inhibits Cx43 HCs, is used in some inflammatory-related retinal diseases, including AMD. Previous work has shown that orally administered Tonabersat improved clinical signs in animal models of both DR and dry AMD and was associated with reduced inflammation. It was discovered that Tonabersat significantly preserved the function of the retina, particularly the function of photoreceptors and bipolar cells in the inner retina [
68]. Tonabersat has also been shown to prevent the formation of NOD-like receptor protein 3 (NLRP3), an ATP-mediated inflammasome, and the cleaved caspase-1 complex assembly, as well as the release of the proinflammatory cytokines IL-1β, VEGF, and IL-6 [
69]. Non-obese diabetic mice have been used to evaluate the ocular safety and efficacy of Tonabersat [
70]. Overall, drug treatment significantly reduces macrovascular abnormalities, hyperreflective foci, sub-retinal fluid accumulation, vascular leakage, inflammation, and inflammasome activation. Lyon H et al. have found that Tonabersat also attenuates both TGF-β2 release and RPE EMTs under disease-mimicking conditions, providing a therapeutic target for diseases, such as DR, underlined by the EMT [
71]. The demonstrated efficacy of Tonabersat could be attributed in part to the inhibition of Cx43 HCs. All these findings suggest that Cx HCs inhibitors may be effective tools and hold potential as therapeutics for multiple eye diseases related to inflammation or fibrosis.
2.2. Connexin GJ/HCs in the Central Nervous System
A disruption of the balance of GJs, HCs, and Cxs affects tissue homeostasis and allows cells to progress toward pathological conditions with varying degrees of severity in the central nervous system (CNS) [
72]. The most abundant Cx in the brain is Cx43. Cx43 is prominently expressed in astrocytes but is also present in microglial cells [
73,
74]. Neuronal survival and physiological functions strictly depend on the maintenance of the blood–brain barrier (BBB). Astrocytes, which establish an elaborate network at the BBB, play critical roles in neuroprotection [
75,
76].
Cx HCs form an integrated network of channels in neurons and astrocytes. Most cell types in the CNS exhibit HC activity, while most studies revolve around Cx43 HCs in astrocytes [
76]. The Cx43-HC-mediated release of gliotransmitters (ATP/glutamate) results in structural neuronal alterations and increased oxidative stress [
77]. While strategies that promote axonal growth are still limited, other treatment paradigms that limit secondary damage, especially maintaining a balance between pro- and anti-inflammatory factors in incomplete spinal cord injuries (SCIs), can potentially allow patients to retain greater function post-injury [
78,
79]. During inflammation, Ca
2+ signaling in the astrocyte network is elevated, resulting in increased ATP production and release through the opening of HCs. ATP stimulates purinoceptors, leading to increased Ca
2+ release from internal stores [
80]. This extracellular Ca
2+ signaling attenuates intercellular Ca
2+ signaling, causing reduced cell communication via GJs [
81,
82]. Similar to the treatment of retinal diseases mentioned before, GJ and HC inhibitors strongly stimulate research toward the development of new modulators to be used against CNS disorders [
83]. On one hand, Cx43 blockers can be used to deal with neuropathic pain after SCIs [
84,
85]. On the other hand, channel inhibitors can reduce the spread of damage and improve functional recovery after an injury [
86,
87]. A spinal injection of Gap26 and Gap27 effectively reduces neuropathic pain symptoms (mechanical allodynia) by decreasing the release of the chemokines CCL2 and CXCL1 in the late phase (21 days) [
85]. Peptide 5, by preventing HCs from opening, significantly reduces the degree of swelling and the loss of neurons in a concentration- and time-dependent manner [
86]. Cx43 AsODN treatment can reduce glial cell activation, neutrophil recruitment, and the functional consequences of a spinal cord injury. Within 24 h of a compression injury, rats treated with Cx43 AsODNs scored higher than sense- and vehicle-treated controls on behavioral tests of locomotion. In rats tested for locomotor ability over the next 28 days, the initial improvement in scores was sustained [
87]. Our group has developed a novel monoclonal antibody, MHC1, that targets Cx43 and specifically inhibits HC opening but has no effect on GJ coupling in two incomplete SCI mouse models. The researchers have shown that a single antibody administration within 30 min after an SCI significantly decreased secondary injury, improved locomotion function, attenuated gliosis, preserved white and gray matter, and protected neurons [
88] (
Figure 1).
Figure 1. Connexin GJs and HCs in eye diseases. The eye comprises the cornea, lens, retina, and several other subcomponents, within which various connexins exist. (A) Cx26, Cx30, Cx31.1, and Cx43 mediate GJ communication in the human corneal epithelium. ZO-1 is a scaffolding protein that plays a role in regulating GJ assembly. A disruption of Cx43 interaction with ZO-1 leads to the enhancement of GJs; targeting this disruption could be a means of enhancing wound healing. (B) Cx50 and AQP0, located at lens fibers, respectively, mediate cell–cell adhesion, maintaining lens fiber integrity. Deficiency of Cx50 and AQP0 leads to a loss in cell–cell adhesion, resulting in alterations of lens structures. At the posterior part of the lens, newly formed fibers cannot bundle properly, leading to lens posterior extrusion and capsule rupture. Macrophages are recruited to the vitreous cavity adjacent to the ruptured posterior capsule. M1 macrophages mainly mediate the removal of the tissue mass, while M2 macrophages play a crucial role in posterior capsule sealing and fibrosis. (C) The neuronal types of the retina are composed of photoreceptors, horizontal cells, bipolar, amacrine, and ganglion cells, all of which contain connexins. The participation of neuronal retinal Cxs in wound healing and fibrosis has not been directly reported. Tonabersat, a sodium channel blocker inhibiting Cx43 HCs, is used in some inflammatory-related retinal diseases. Tonabersat has also been shown to prevent the formation of NOD-like receptor protein 3 (NLRP3), an ATP-mediated inflammasome, as well as the release of other proinflammatory cytokines. Throughout the lens tissue, targeting Cxs for inflammation or fibrosis may hold therapeutic potential.
This entry is adapted from the peer-reviewed paper 10.3390/biom13121796