Increasing attention has been paid to understanding the causes of infertility, which is being recognized as a growing health problem affecting large numbers of couples worldwide. Male infertility is a contributing factor in approximately 30–40% of cases, and one of its etiological causes is sexually transmitted infections (STIs). Among sexually transmitted pathogens, human papillomavirus (HPV) can contribute in various ways to the failure of spontaneous and assisted reproduction, acting in the different phases of conception, especially in the early ones. In particular, HPV infection can affect sperm DNA integrity, sperm motility, count, viability, and morphology and can induce the production of anti-sperm antibodies (ASAs).
- infertility
- HPV infection
- sperm parameters
- HPV
- DNA Fragmentation
1. Introduction
2. HPV Impact on Sperm Parameters
| Type of Infection | Effect | Reference |
|---|---|---|
| HPV positivity | Increased risk of DFI > 30%, asthenozoospermia, ASAs production, and negative ART outcome (alteration in fertilization, implantation, and development of the embryo). | [27][28][29] |
| hrHPV genotype | Reduced sperm count and motility alterations. | [26][30] |
| Multiple HPV infections |
Hypospermia, abnormal viscosity, and increased seminal pH. | [23] |
| HPV16 or HPV31 | Sperm genomic DNA breaks and increased apoptotic events. | [31] |
| HPV16 and HPV18 |
Exonic modification of p53 gene. | [32] |
3. Correlation between HPV and Sperm DNA Fragmentation
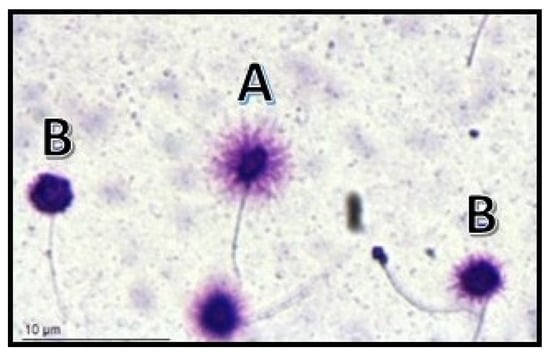
This entry is adapted from the peer-reviewed paper 10.3390/ijms242417562
References
- Tahmasbpour, E.; Balasubramanian, D.; Agarwal, A. A multi-faceted approach to understanding male infertility: Gene mutations, molecular defects and assisted reproductive techniques (ART). J. Assist. Reprod. Genet. 2014, 31, 1115–1137.
- Practice Committee of the American Society for Reproductive Medicine Diagnostic evaluation of the infertile male: A committee opinion. Fertil. Steril. 2015, 103, e18–e25.
- Lyu, Z.; Feng, X.; Li, N.; Zhao, W.; Wei, L.; Chen, Y.; Yang, W.; Ma, H.; Yao, B.; Zhang, K.; et al. Human papillomavirus in semen and the risk for male infertility: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 714.
- Caliskan, Z.; Kucukgergin, C.; Aktan, G.; Kadioglu, A.; Ozdemirler, G. Evaluation of sperm DNA fragmentation in male infertility. Andrologia 2022, 54, e14587.
- de Kretser, D.M. Male infertility. Lancet Infect. Dis. 1997, 349, 787–790.
- Goulart, A.C.X.; Farnezi, H.C.M.; França, J.P.B.M.; Santos, A.D.; Ramos, M.G.; Penna, M.L.F. HIV, HPV and Chlamydia trachomatis: Impacts on male fertility. J. Bras. Reprod. Assist. 2020, 24, 492–497.
- Gizzo, S.; Ferrari, B.; Noventa, M.; Ferrari, E.; Patrelli, T.S.; Gangemi, M.; Nardelli, G.B. Male and couple fertility impairment due to HPV-DNA sperm infection: Update on molecular mechanism and clinical impact—Systematic review. BioMed Res. Int. 2014, 2014, 20–22.
- Capra, G.; Notari, T.; Buttà, M.; Serra, N.; Rizzo, G.; Bosco, L. Human Papillomavirus (HPV) Infection and Its Impact on Male Infertility. Life 2022, 12, 1919.
- Dunne, E.F.; Park, I.U. HPV and HPV-associated diseases. Infect. Dis. Clin. N. Am. 2013, 27, 765–778.
- Das, S.; Doss, C.G.P.; Fletcher, J.; Kannangai, R.; Abraham, P.; Ramanathan, G. The impact of human papilloma virus on human reproductive health and the effect on male infertility: An updated review. J. Med. Virol. 2023, 95, e28697.
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221.
- Gheit, T. Mucosal and cutaneous human papillomavirus infections and cancer biology. Front. Oncol. 2019, 9, 355.
- Giuliano, A.R.; Nielson, C.M.; Flores, R.; Dunne, E.F.; Abrahamsen, M.; Papenfuss, M.R.; Markowitz, L.E.; Smith, D.; Harris, R.B. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: The HPV detection in men study. J. Infect. Dis. 2007, 196, 1146–1152.
- Laprise, C.; Trottier, H.; Monnier, P.; Coutlée, F.; Mayrand, M.H. Prevalence of human papillomaviruses in semen: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 640–651.
- Schillaci, R.; Capra, G.; Bellavia, C.; Ruvolo, G.; Scazzone, C.; Venezia, R.; Perino, A. Detection of oncogenic human papillomavirus genotypes on spermatozoa from male partners of infertile couples. Fertil. Steril. 2013, 100, 1236–1240.
- Pérez-Andino, K.R.J.; Buck, C.B. Adsorption of Human Papillomavirus 16 to Live Human Sperm. PLoS ONE 2009, 4, e5847.
- Kaspersen, M.D.; Larsen, P.B.; Ingerslev, H.J.; Fedder, J.; Petersen, G.B.; Bonde, J.; Höllsberg, P. Identification of multiple HPV types on Spermatozoa from human sperm donors. PLoS ONE 2011, 6, e18095.
- Foresta, C.; Patassini, C.; Bertoldo, A.; Menegazzo, M.; Francavilla, F.; Barzon, L.; Ferlin, A. Mechanism of human papillomavirus binding to human spermatozoa and fertilizing ability of infected spermatozoa. PLoS ONE 2011, 6, e15036.
- Gimenes, F.; Souza, R.P.; Bento, J.C.; Teixeira, J.J.; Maria-Engler, S.S.; Bonini, M.G.; Consolaro, M.E. Male infertility: A public health issue caused by sexually transmitted pathogens. Nat. Rev. Urol. 2014, 11, 672–687.
- Gomez, L.M.; Ma, Y.; Ho, C.; McGrath, C.M.; Nelson, D.B.; Parry, S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum. Reprod. 2008, 23, 709–715.
- Garolla, A.; Pizzol, D.; Bertoldo, A.; De Toni, L.; Barzon, L.; Foresta, C. Association, prevalence, and clearance of human papillomavirus and antisperm antibodies in infected semen samples from infertile patients. Fertil. Steril. 2013, 99, 125–131.e2.
- WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021.
- Damke, E.; Kurscheidt, F.A.; Balani, V.A.; Takeda, K.I.; Irie, M.M.; Gimenes, F.; Consolaro, M.E. Male partners of infertile couples with seminal infections of human papillomavirus have impaired fertility parameters. BioMed Res. Int. 2017, 2017, 11–17.
- Weinberg, M.; Nahshon, C.S.-S.; Feferkorn, I.; Bornstein, J. Evaluation of human papilloma virus in semen as a risk factor for low sperm quality and poor in vitro fertilization outcomes: A systematic review and meta-analysis. Fertil. Steril. 2020, 113, 955–969.e4.
- Moghimi, M.; Zabihi-Mahmoodabadi, S.; Kheirkhah-Vakilabad, A.; Kargar, Z. Significant correlation between high-risk hpv dna in semen and impairment of sperm quality in infertile men. Int. J. Fertil. Steril. 2019, 12, 306–309.
- Lai, Y.M.; Soong, Y.K.; Lee, J.F.; Yang, F.P.; Huang, H.Y.; Pao, C.C. The effect of human papillomavirus infection on sperm cell motility. Fertil. Steril. 1997, 67, 1152–1155.
- Moreno-Sepulveda, J.; Rajmil, O. Seminal human papillomavirus infection and reproduction: A systematic review and meta-analysis. Andrology 2021, 9, 478–502.
- Foresta, C.; Noventa, M.; De Toni, L.; Gizzo, S.; Garolla, A. HPV-DNA sperm infection and infertility: From a systematic literature review to a possible clinical management proposal. Andrology 2015, 3, 163–173.
- Mastora, E.; Kitsou, C.; Evangelou, T.; Zikopoulos, A.; Zagorianakou, N.; Georgiou, I. Presence of HPV 16 and HPV 18 in spermatozoa and embryos of mice. In Vivo 2021, 35, 3203–3209.
- Piroozmand, A.; Nasab, S.D.M.; Erami, M.; Hashemi, S.M.A.; Khodabakhsh, E.; Ahmadi, N.; Vahedpoor, Z. Distribution of human papillomavirus and antisperm antibody in semen and its association with semen parameters among infertile men. J. Reprod. Infertil. 2020, 21, 183–188.
- Connelly, D.A.; Chan, P.J.; Patton, W.C.; King, A. Human sperm deoxyribonucleic acid fragmentation by specific types of papillomavirus. Am. J. Obstet. Gynecol. 2001, 184, 1068–1070.
- Lee, C.A.; Huang, C.T.F.; King, A.; Chan, P.J. Differential effects of human papillomavirus DNA types on p53 tumor-suppressor gene apoptosis in sperm. Gynecol. Oncol. 2002, 85, 511–516.
- Rintala, M.A.M.; Grénman, S.E.; Pöllänen, P.P.; Suominen, J.J.O.; Syrjänen, S.M. Detection of high-risk HPV DNA in semen and its association with the quality of semen. Int. J. STD AIDS 2004, 15, 740–743.
- Luttmer, R.; Dijkstra, M.G.; Snijders, P.J.; Hompes, P.G.; Pronk, D.T.; Hubeek, I.; Berkhof, J.; Heideman, D.A.; Meijer, C.J. Presence of human papillomavirus in semen in relation to semen quality. Hum. Reprod. 2016, 31, 280–286.
- Luttmer, R.; Dijkstra, M.G.; Snijders, P.J.; Jordanova, E.S.; King, A.J.; Pronk, D.T.; Foresta, C.; Garolla, A.; Hompes, P.G.; Berkhof, J.; et al. Presence of human papillomavirus in semen of healthy men is firmly associated with HPV infections of the penile epithelium. Fertil. Steril. 2015, 104, 838–844.e8.
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469.
- Ricardo, L.H.J. Male Accessory Glands and Sperm Function. In Spermatozoa-Facts and Perspectives; IntechOpen: London, UK, 2018; pp. 101–116.
- Foresta, C.; Bertoldo, A.; Garolla, A.; Pizzol, D.; Mason, S.; Lenzi, A.; De Toni, L. Human papillomavirus proteins are found in peripheral blood and semen Cd20 + and Cd56 + cells during Hpv-16 semen infection. BMC Infect. Dis. 2013, 13, 593.
- Wang, S.; Liu, L.; Zhang, A.; Song, Y.; Kang, J.; Liu, X. Association between human papillomavirus infection and sperm quality: A systematic review and a meta-analysis. Andrologia 2021, 53, e14034.
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917.
- Yang, H.; Li, G.; Jin, H.; Guo, Y.; Sun, Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl. Androl. Urol. 2019, 8, 356–365.
- Baldi, E.; Muratori, M. Genetic Damage in Human Spermatozoa; Springer International: Cham, Switzerland, 2019.
- Ferrigno, A.; Ruvolo, G.; Capra, G.; Serra, N.; Bosco, L. Correlation between the DNA fragmentation index (DFI) and sperm morphology of infertile patients. J. Assist. Reprod. Genet. 2021, 38, 979–986.
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127.
- Benchaib, M.; Lornage, J.; Mazoyer, C.; Lejeune, H.; Salle, B.; Guerin, J.F. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil. Steril. 2007, 87, 93–100.
- Henkel, R.; Kierspel, E.; Hajimohammad, M.; Stalf, T.; Hoogendijk, C.; Mehnert, C.; Menkveld, R.; Schill, W.B.; Kruger, T.F. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod. Biomed. Online 2003, 7, 477–484.
- Barratt, C.L.; Aitken, R.J.; Björndahl, L.; Carrell, D.T.; de Boer, P.; Kvist, U.; Lewis, S.E.; Perreault, S.D.; Perry, M.J.; Ramos, L.; et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications-a position report. Hum. Reprod. 2010, 25, 824–838.
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management—Meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326.
- Rathke, C.; Baarends, W.M.; Awe, S.; Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta—Gene Regul. Mech. 2014, 1839, 155–168.
- Gallegos, G.; Ramos, B.; Santiso, R.; Ph, D.; Fern, L. Sperm DNA fragmentation in infertile men with genitourinary infection by Chlamydia trachomatis and Mycoplasma. Fertil. Steril. 2008, 90, 328–334.
- Kang, X.; Xie, Q.; Zhou, X.; Li, F.; Huang, J.; Liu, D.; Huang, T. Effects of hepatitis B virus S protein exposure on sperm membrane integrity and functions. PLoS ONE 2012, 7, e33471.
- Cortés-Gutiérrez, E.I.; Dávila-Rodríguez, M.I.; Fernández, J.L.; de la O-Pérez, L.O.; Garza-Flores, M.E.; Eguren–Garza, R.; Gosálvez, J. The presence of human papillomavirus in semen does not affect the integrity of sperm DNA. Andrologia 2017, 49, e12774.
- Williams, V.M.; Filippova, M.; Filippov, V.; Payne, K.J.; Duerksen-Hughes, P. Human Papillomavirus Type 16 E6* Induces Oxidative Stress and DNA Damage. J. Virol. 2014, 88, 6751–6761.
- Kato, Y.; Shigehara, K.; Nakagawa, T.; Nakata, H.; Iijima, M.; Nakashima, K.; Kawaguchi, S.; Izumi, K.; Kadono, Y.; Mizokami, A. Human papillomavirus detected in sperm of Japanese infertile males affects reproductive parameters. Int. J. Infect. Dis. 2021, 112, 294–299.
- Szabó, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle-, environmental-, and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 5.
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod Fertil. 2023, 4, e230024.
