Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Plants are increasingly used for the production of high-quality biological molecules for use as pharmaceuticals and biomaterials in industry. Plants have proved that they can produce life-saving therapeutic proteins (Elelyso™—Gaucher’s disease treatment, ZMapp™—anti-Ebola monoclonal antibodies, seasonal flu vaccine, Covifenz™—SARS-CoV-2 virus-like particle vaccine); however, some of these therapeutic proteins are difficult to bring to market, which leads to serious difficulties for the manufacturing companies.
- plant-derived biologics
- plant-based expression systems
- plant molecular farming
1. Introduction
Protein-based biologics are the fastest-growing class of pharmaceutical products, manufactured from engineered biological sources. Plants can be engineered to produce various types of biologics (antibodies, vaccines, enzymes, therapeutic proteins, hormones, and cytokines), as well as recombinant proteins for cosmetics, food, and the chemical industry, in addition to research and diagnostic purposes. The process is known as plant molecular farming (PMF) [1,2,3,4]. PMF offers a safe, cost-effective, and scalable production of unique multimeric proteins, ensuring fast and global-scale deployment of biologics and other valuable recombinant proteins [5]. These advantages make plants an efficient alternative to the traditional expression systems such as bacterial, yeast, insect, and mammalian cells, which cannot fully satisfy global needs for biologics and industrial proteins [6,7,8]. The successful implementation of well-established practices, such as plant cell engineering and optimization of biosynthetic pathways through cell medium improvement or genome editing, increased yields by using different methods of expression, or improving downstream processing (DSP), has led to optimization and maturation of PMF processes [9,10,11]. Companies producing plant-derived recombinant proteins for industrial purposes have seen sustainable success overall. In the pharmaceutical sector, however, this success has been limited to just a few notable examples (Elelyso™, ELFABRIOTM, and growth factors). This is most likely due to a lack of regulatory frameworks, an inability of plant-based expression systems to compete with established industrial platforms, and the reluctance of large pharmaceutical companies to reorganize their manufacturing processes [12,13].
2. Types of Plant-Derived Biologics
Plant-derived protein-based biologics represent a diverse array of molecules that can be produced using plants as an expression system [14,15,16]. They often have highly complex structures containing additional moieties (glycocarbohydrates and fatty acids) and can be divided into several major categories: monoclonal antibodies (mAbs), vaccines, enzymes for replacement therapies, receptor modulators, and bioactive small molecules [17].
2.1. Antibodies and Antibody Fragments
Plant-based expression systems have been leveraged for producing monoclonal antibodies and antibody fragments, known as “plantibodies.” Their applications include therapeutics, diagnostics, and research. Plantibodies have already successfully targeted infectious agents, cancer biomarkers, and therapeutic entities [33]. During the 2014 Ebola outbreak, for instance, the plant-based production of ZMapp (a cocktail of monoclonal antibodies) significantly increased the survival rate of infected patients [34], though the small number of patients treated makes a statistical evaluation of efficacy difficult. Plantibodies offer potential advantages in terms of reduced production costs, scalability, and customization.
2.2. Vaccines and VLPs
Plants present an appealing platform for vaccine production due to their ability to express immunogenic viral and bacterial antigens, as well as highly organized virus-like particles (VLPs) [35,36,37,38,39]. VLPs are self-assembling virus coat proteins resembling viruses but devoid of genetic material, which makes them safe for use in vaccines and nanoparticle production. Plant-derived VLPs and chimeric VLPs (composed of structural proteins or immunogenic epitopes from different viruses) are used as immune modulators and self-adjuvants in order to provoke strong immune responses against different viral diseases, as well as others such as cancer, allergies, and autoimmune diseases [5,40,41,42,43]. Plant-derived vaccines offer a multitude of benefits, including reduced production costs, improved stability, minimal cold chain requirements, and the possibility of oral delivery. Notably, edible vaccines have emerged, utilizing genetically engineered plants to express vaccine antigens. This innovative approach holds great promise for transforming vaccine delivery, particularly in developing countries where oral administration can eliminate the need for injections and cold chain storage. However, a major disadvantage is controlling the vaccination dose [8,36,44].
Furthermore, VLPs can be used as nanoparticles for drug delivery systems [45].
2.3. Therapeutic Enzymes
Plants efficiently produce various recombinant enzymes with clinically improved profiles. For example, ElelysoTM (β-glucocerebrosidase) has an improved profile compared to its CHO-made counterpart because the final plant-derived β-glucocerebrosidase contains terminal mannose residues, which are a key factor in the success of enzyme replacement therapy for Gaucher’s disease treatment [46,47]. Further, plant-made enzymes can be delivered orally, due to the cellulose wall of plant cells that makes them resistant to degradation [48]. Recombinant protein medications are shielded from stomach acids and digestive enzymes by the plant cell wall polymers, which have β 1,4 and β 1,6-glycosidic linkages that are resistant to hydrolysis [49]. Commensal microorganisms in the intestinal epithelium break down the plant cell wall, releasing the bioencapsulated recombinant proteins, which are recognized by the gut-associated lymphoid tissues (GALT) and induce an adaptive immune response [50,51]. Utilization of plant expression systems offers numerous advantages and has demonstrated promising outcomes in both preclinical and clinical studies, presenting a potential alternative to traditional biologic production methods [52,53,54]. Notably, plant-based expression systems have facilitated the cost-effective production of complex recombinant proteins, revolutionizing human and animal health management [5,55,56].
2.4. Receptor Modulators
Plant-based expression systems have been successfully used for the production of various small polypeptides and glycoproteins involved in the regulation processes in mammalian cells, such as cytokines and hormones [57,58]. Human growth hormone (hGH) produced in N. benthamiana plants demonstrated its biological activity in a hypophysectomized rat [58]. Cytokines are signaling proteins that help control inflammation in the body and can be used in the treatment of cancer, immune disorders, and various other related diseases. Erythropoietin [59], IL-2 [60], IL-4 [61], IL-12 [62], IL-13 [63], IL-18 [64], cardiotrophin 1 [65], human granulocyte-macrophage colony-stimulating factor (GM-CSF) [66,67,68,69], tumor necrosis factor-alpha (TNF) [70], interferon-alpha [71], human fibroblast growth factor 8b [72], and insulin-like growth factor 1 [73] have been expressed in different plant species using various methods for increasing recombinant protein yield and stability.
2.5. Small Molecules
Plants are a natural source of many medicinal compounds based on secondary metabolites, such as triterpenoids, alkaloids, and phenolics. These are synthesized through multi-step biosynthetic pathways involving a variety of different enzyme activities, and the active products have a highly specific stereochemistry. Such medicinal compounds can be extracted from the native species; however, in many cases, propagation of the plants and the low yields of extractable compounds and their subsequent purification make this an expensive procedure. The alternative of complete chemical synthesis is generally impractical due to the complex structures of the compounds. For these reasons, attention has turned to the detailed characterization of the biosynthetic pathways for plant-derived metabolites with the aim of reconstructing them in more tractable organisms through the co-expression of the relevant biosynthetic enzymes. Such an approach also allows variants of naturally produced molecules to be produced through the combinatorial expression of different enzymes.
A dramatic early example of this approach was the reconstruction of the biosynthetic pathway for the anti-malarial compound artemisinin in yeast [74]. Subsequently, the relevant enzymes have been transferred to other organisms, including plants more tractable than Artemisia annua, especially N. benthamiana (reviewed by Zhao et al., 2022) [75]. Though stable transformation has been used in plants, the “go-to” method is transient expression of the relevant enzymes. Not only is this much quicker than stable transformation, but it also allows a combinatorial approach for making a wide variety of related compounds that have differing bioactivities [76,77]. This ability has been exploited to great effect in the case of triterpenes [78,79], culminating in the transient combinatorial expression of 16 enzymes in N. benthamiana to produce a saponin molecule suitable for further bioengineering to produce adjuvants for use with vaccines [80]. This approach has also been used in studies to elucidate the biosynthetic pathway of the anticancer drugs vinblastine [81,82] and paclitaxel [83]. Though, at the time of writing, none of the molecules produced in this way have made it to deployment in medicine, the technology has great promise.
2.6. Bioactive Proteins from Plants
Plant-derived biologics also encompass bioactive molecules, such as lectins. Lectins are natural proteins that can bind carbohydrates that are highly specific for the sugar groups of other molecules. Some of the lectins have potent antimicrobial activity through binding to carbohydrates on microbial surfaces and inducing changes in cell permeability and pore formation [84]. Their biological activities make them useful as microbicides, antitumor agents, and vaccine adjuvants [85,86,87]. Mistletoe lectins (ML-I, ML-II, and ML-III) were transiently expressed in N. benthamiana and demonstrated anticancer activity [88].
3. Strengths, Weaknesses, Opportunities, and Threats (SWOT) Analysis of Biologics
One of the primary advantages of harnessing plant-derived biologics lies in their inherent safety profile and reduced unwanted immunogenicity, which paves the way for enhanced patient tolerance and minimized adverse effects [89]. The plant glycan moieties’ immunogenicity has been the subject of extensive studies, and various animal models have been investigated to elucidate the immunogenicity of plant-derived glycoproteins [90,91]. The (1,2) xylose and (1,3) fucose structures have been recognized as cross-reactive carbohydrate determinants as a result of the discovery of IgE antibodies in allergy patients that cross-react with these structures on glycoproteins from a variety of species [92]. Plant-derived taliglucerase alfa (TGA), Protalix, contains tri-mannose glycoform with the addition of β(1,2) xylose and α(1,3) fucose, which are present at above 90% of the total glycan pool. No overtly adverse effects that could be related to these N-glycan residues have been observed in a clinical trial with TG involving healthy human volunteers, and no anti-drug antibodies have been found [93].
Additionally, whole plants and plant cell suspension cultures, which are free from animal pathogens and toxins, are suitable for oral delivery of biologics without purification or with minimal purification [48,94]. Production of oral biopharmaceuticals in edible plant tissues has proven to be efficacious in several clinical vaccinations for disease prevention [95,96,97,98]. The ability of plant cell walls to protect the plant-made biologic from enzymatic degradation in the gastrointestinal tract and the ability of these biologics to reach the lymphoid tissue in the gut in their active form can lead to the induction of oral tolerance, the prevention of unwanted immune responses, and the prevention of allergic responses [99,100,101,102]. Furthermore, the cost-effective production and scalability of these biopharmaceuticals present an opportunity to address the escalating healthcare costs and global disparities in access to essential medications [103,104]. By capitalizing on the inherent adaptability of plants, scientists can usher in a new era of personalized medicine, where targeted therapies are tailored to individual patients, boosting treatment outcomes and improving overall patient well-being [105]. In light of these remarkable advancements, it becomes increasingly evident that plant-derived biologics represent a transformative force in the field of biopharmaceuticals, offering a multifaceted solution to the pressing challenges faced by modern medicine [106]. Noteworthy stories, such as the development of plant-based antibodies or VLPs for treating cancer and the utilization of plant-produced vaccines for infectious diseases, underscore the efficacy and potential of these biologics [107,108,109,110,111,112,113,114]. Moreover, the environmental sustainability of plant-derived biologics deserves recognition. Their production systems boast lower carbon footprints, reduced energy requirements, and decreased reliance on non-renewable resources compared to other biological production methods. Addressing regulatory and safety considerations is paramount. Adherence to guidelines established by regulatory agencies ensures the approval and quality control of these biologics; however, unwavering vigilance is still necessary to guarantee safety and efficacy throughout the production process. Further, they also have weaknesses in terms of time consumption, variable yields, regulatory considerations, and protein degradation. Threats include intellectual property barriers, competition, and public perception. Continued research, development, and regulatory efforts are crucial to overcome these challenges and fully realize the potential of plant-derived biologics [15,115].
4. Manufacturing of Plant-Based Production Systems
Many of the original technical weaknesses of PMF, such as low yields, low recovery of recombinant proteins after downstream processing, and the impact of specific glycosylation in plants, have been overcome in some cases. Introducing technologies, such as stable plastid transformation, plant cell cultures, transient expression, agrobacterium infiltration, modeling of the plant glycosylation pathway, plant genome editing for improving the biosynthetic pathways in plants, increasing protein stability, and the production in current Good Manufacturing Practice (cGMP), will lead to scaling up the manufacturing of functional, unique recombinant proteins in plants [7,116,117,118,119,120,121]. The main steps in the development of PDB are presented in Figure 1.
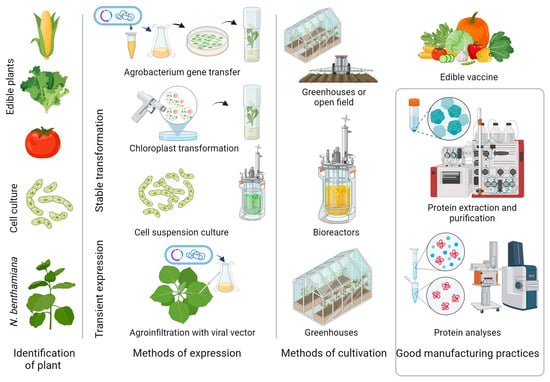
Figure 1. Main steps in the production of biologics in plants. Valuable recombinant proteins are produced in stable transgenic whole plants (nuclear or chloroplast) using stable transgenic plant cell cultures and transient expression via agroinfiltration or modified plant viruses.
The extraordinary variety of plant species with specific useful characteristics enables the development of a wide variety of plant production systems [12]. Tobacco, maize, rice, potato, tomato, carrot, and lettuce are commonly used plant species for stable (nucleus or chloroplast) transformation [120,122,123,124,125,126]. Plant cell culture systems such as ProCellEx® and tobacco cell cultures (BY-2 and NT-1) covered the criteria for a high yield, precise control of environments and cell growth conditions, production according to good manufacturing practices, and regulatory compliance [127]. These advantages of plant cell systems have brought them to market with two products (β-glucocerebrosidase and a vaccine against Newcastle disease virus) approved, respectively, by the US Food and Drug Administration (FDA) and the US Department of Agriculture (USDA) [27,128]. Plant cell suspension cultures are a connecting link between plants and current commercial cell-based production platforms. Furthermore, higher plants are not the only ones used for the production of therapeutic protein cell suspension systems: Chlamydomonas reinhardtii (alga), Lemna minor (duckweed), and Physcomitrella patens (moss) [129]. In addition to expression within living cells, a cell-free system based on BY-2 cells harvested in the exponential growth phase has been investigated as a means of synthesizing proteins. Yields of up to 270 μg/mL have been obtained when producing the yellow fluorescent protein (eYFP) using this approach [130].
The tobacco relative, Nicotiana benthamiana, is the most frequently used plant in molecular farming to produce recombinant proteins using transient expression [131,132,133]. N. benthamiana is characterized by the accumulation of a large biomass in a short period, reduced gene silencing, and being the most suitable plant species for transient expression using plant virus-based vectors and agrobacterium infiltration [35,134,135,136,137]. Utilization of plant viruses, such as tobacco mosaic virus (TMV) or cowpea mosaic virus (CPMV), as full virus vectors for delivering foreign genes into plants began in the 1980s [138,139,140,141]. Later on, these first-generation (gene substitution or insertion vectors) were replaced by deconstructed vector systems [142,143,144]. The deconstructed vectors contain only the viral genome components essential for effective protein expression, allowing the production of up to 80% of total soluble protein (magnICON) [145]. Furthermore, multiple gene expression leads to the accumulation of multi-subunit proteins such as VLPs, IgA, and IgM [146]. Plants with engineered post-translational modifications (glycosylation mutants and expression of recombinant glycosylation enzymes) have been developed for the proper production of recombinant glycoproteins [147,148,149]. New techniques such as CRISPR/Cas9 genome editing have been used to engineer the endogenous N-glycosylation machinery of the plants to generate N. benthamiana with deficient α-1,3-fucosyltransferase and β-1,2-xylosyltransferase activity [150].
This entry is adapted from the peer-reviewed paper 10.3390/ijms242417575
This entry is offline, you can click here to edit this entry!
