Neurodegenerative diseases (NDDs) affect millions of people worldwide. They develop due to the pathological accumulation and aggregation of various misfolded proteins, axonal and synaptic loss and dysfunction, inflammation, cytoskeletal abnormalities, defects in DNA and RNA, and neuronal death. This leads to the activation of immune responses and the release of the antibodies against them. Recently, it has become clear that autoantibodies (Aabs) can contribute to demyelination, axonal loss, and brain and cognitive dysfunction. This has significantly changed the understanding of the participation of humoral autoimmunity in neurodegenerative disorders. It is crucial to understand how neuroinflammation is involved in neurodegeneration, to aid in improving the diagnostic and therapeutic value of Aabs in the future.
- neurodegenerative disorders
- autoantibodies
- biomarkers
- pathogenesis
- amyloid-β antibodies
1. Introduction: Neurodegenerative Diseases and the Role of the Immune System
2. Autoantibodies—Biology and Pathogenic Role in Neurodegenerative Diseases
3. Autoantibodies against Pathology-Related Molecules of the Most Common Neurodegenerative Diseases
3.1. Amyloid-β Autoantibodies and Microtubule Protein Tau Autoantibodies in Alzheimer’s Disease
3.2. α-Synuclein Autoantibodies in Parkinson’s Disease
3.3. Anti-Myelin Basic Protein (-MBP) and Anti-Myelin Oligodendrocyte Glycoprotein (MOG) Autoantibodies in Multiple Sclerosis
3.4. Neurofilament Autoantibodies in AD, PD, MS, and ALS
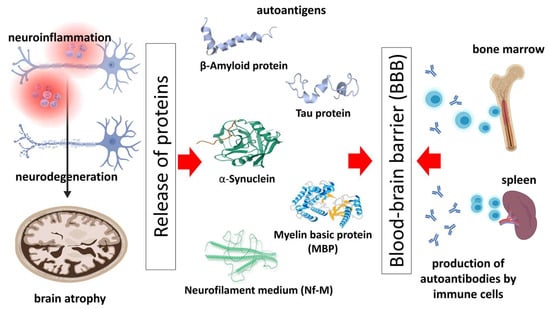
4. Autoantibodies as Biomarkers for Neurodegenerative Disease Diagnosis
This entry is adapted from the peer-reviewed paper 10.3390/antib12040081
References
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502.
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035.
- Dugger, B.N.; Hentz, J.G.; Adler, C.H.; Sabbagh, M.N.; Shill, H.A.; Jacobson, S.; Caviness, J.N.; Belden, C.; Driver-Dunckley, E.; Davis, K.J.; et al. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J. Neuropathol. Exp. Neurol. 2014, 73, 244–252, Erratum in J. Neuropathol. Exp. Neurol. 2014, 73, 732..
- Doty, K.R.; Guillot-Sestier, M.V.; Town, T. The role of the immune system in neurodegenerative disorders: Adaptive or maladaptive? Brain Res. 2015, 1617, 155–173.
- Long-Smith, C.M.; Sullivan, A.M.; Nolan, Y.M. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog. Neurobiol. 2009, 89, 277–287.
- Prokop, S.; Miller, K.R.; Heppner, F.L. Microglia actions in Alzheimer’s disease. Acta Neuropathol. 2013, 126, 461–477.
- Colangelo, A.M.; Alberghina, L.; Papa, M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci. Lett. 2014, 565, 59–64.
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934.
- Wang, W.; Li, Y.; Meng, X. Vitamin D and neurodegenerative diseases. Heliyon 2023, 9, e12877.
- Giannoccaro, M.P.; Verde, F.; Morelli, L.; Rizzo, G.; Ricciardiello, F.; Liguori, R. Neural Surface Antibodies and Neurodegeneration: Clinical Commonalities and Pathophysiological Relationships. Biomedicines 2023, 11, 666.
- Kocurova, G.; Ricny, J.; Ovsepian, S.V. Autoantibodies targeting neuronal proteins as biomarkers for neurodegenerative diseases. Theranostics 2022, 12, 3045–3056.
- Prüss, H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021, 21, 798–813.
- Marquetand, J.; van Lessen, M.; Bender, B.; Reimold, M.; Elsen, G.; Stoecker, W.; Synofzik, M. Slowly progressive LGI1 encephalitis with isolated late-onset cognitive dysfunction: A treatable mimic of Alzheimer’s disease. Eur. J. Neurol. 2016, 23, e28–e29.
- McKeon, A.; Marnane, M.; O’connell, M.; Stack, J.P.; Kelly, P.J.; Lynch, T. Potassium channel antibody associated encephalopathy presenting with a frontotemporal dementia like syndrome. Arch. Neurol. 2007, 64, 1528–1530.
- Schumacher, H.; Meyer, T.; Prüss, H. GABAB receptor encephalitis in a patient diagnosed with amyotrophic lateral sclerosis. BMC Neurol. 2019, 19, 41.
- May, C.; Nordhoff, E.; Casjens, S.; Turewicz, M.; Eisenacher, M.; Gold, R.; Brüning, T.; Pesch, B.; Stephan, C.; Woitalla, D.; et al. Highly immunoreactive IgG antibodies directed against a set of twenty human proteins in the sera of patients with amyotrophic lateral sclerosis identified by protein array. PLoS ONE 2014, 9, e89596.
- Tüzün, E.; Gezen-Ak, D.; Tzartos, J.; Dursun, E.; Giriş, M.; Zisimopoulou, P.; Karagiorgou, K.; Yetimler, B.; Küçükali, C.I.; Idrisoğlu, H.A. LRP4 antibody positive amyotrophic lateral sclerosis patients display neuropil-reactive IgG and enhanced serum complement levels. Immunol. Lett. 2018, 203, 54–56.
- Landa, J.; Gaig, C.; Planagumà, J.; Saiz, A.; Antonell, A.; Sanchez-Valle, R.; Dalmau, J.; Graus, F.; Sabater, L. Effects of IgLON5 Antibodies on Neuronal Cytoskeleton: A Link between Autoimmunity and Neurodegeneration. Ann. Neurol. 2020, 88, 1023–1027.
- Rocchi, A.; Sacchetti, S.; De Fusco, A.; Giovedi, S.; Parisi, B.; Cesca, F.; Höltje, M.; Ruprecht, K.; Ahnert-Hilger, G.; Benfenati, F. Autoantibodies to synapsin I sequestrate synapsin I and alter synaptic function. Cell Death Dis. 2019, 10, 864.
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.; Merlini, G.; Saraiva, M.J.; Westermark, P.; Nomenclature Committee of the International Society of Amyloidosis. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid 2012, 19, 167–170.
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507–511.
- Wells, C.; Brennan, S.; Keon, M.; Ooi, L. The role of amyloid oligomers in neurodegenerative pathologies. Int. J. Biol. Macromol. 2021, 181, 582–604.
- Katsinelos, T.; Tuck, B.J.; Mukadam, A.S.; McEwan, W.A. The Role of Antibodies and Their Receptors in Protection Against Ordered Protein Assembly in Neurodegeneration. Front. Immunol. 2019, 10, 1139.
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851.
- Sun, B.L.; Wang, L.H.; Yang, T.; Sun, J.Y.; Mao, L.L.; Yang, M.F.; Yuan, H.; Colvin, R.A.; Yang, X.Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018, 163–164, 118–143.
- Ahn, J.H.; Cho, H.; Kim, J.-H.; Kim, S.H.; Ham, J.-S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66.
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78.
- Kheirkhah, R.; DeMarshall, C.; Sieber, F.; Oh, E.; Nagele, R.G. The origin and nature of the complex autoantibody profile in cerebrospinal fluid. Brain Behav. Immun. Health. 2020, 2, 100032.
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503.
- Gaskin, F.; Finley, J.; Fang, Q.; Xu, S.; Fu, S.M. Human antibodies reactive with beta-amyloid protein in Alzheimer’s disease. J. Exp. Med. 1993, 177, 1181–1186.
- Du, Y.; Dodel, R.; Hampel, H.; Buerger, K.; Lin, S.; Eastwood, B.; Bales, K.; Gao, F.; Moeller, H.J.; Oertel, W.; et al. Reduced levels of amyloid beta-peptide antibody in Alzheimer disease. Neurology 2001, 57, 801–805.
- Weksler, M.E.; Relkin, N.; Turkenich, R.; LaRusse, S.; Zhou, L.; Szabo, P. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp. Gerontol. 2002, 37, 943–948.
- Baril, L.; Nicolas, L.; Croisile, B.; Crozier, P.; Hessler, C.; Sassolas, A.; McCormick, J.B.; Trannoy, E. Immune response to Abeta-peptides in peripheral blood from patients with Alzheimer’s disease and control subjects. Neurosci. Lett. 2004, 355, 226–230.
- Qu, B.X.; Gong, Y.; Moore, C.; Fu, M.; German, D.C.; Chang, L.Y.; Rosenberg, R.; Diaz-Arrastia, R. Beta-amyloid auto-antibodies are reduced in Alzheimer’s disease. J. Neuroimmunol. 2014, 274, 168–173.
- Brettschneider, S.; Morgenthaler, N.G.; Teipel, S.J.; Fischer-Schulz, C.; Bürger, K.; Dodel, R.; Du, Y.; Möller, H.J.; Bergmann, A.; Hampel, H. Decreased serum amyloid β1-42 autoantibody levels in Alzheimer’s disease, determined by a newly developed immuno-precipitation assay with radiolabeled amyloid β1-42 peptide. Biol. Psychiatry 2005, 57, 813–816.
- Lv, J.; Yao, Z.; Quan, W.; Cao, Z.; Xu, J.; Luo, J. Low avidity and level of serum anti-Abeta antibodies in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 127–132.
- Sohn, J.H.; So, J.O.; Hong, H.J.; Kim, J.W.; Na, D.R.; Kim, M.; Kim, H.; Nam, E.; Ha, H.J.; Kim, Y.H.; et al. Identification of autoantibody against beta-amyloid peptide in the serum of elderly. Front. Biosci. (Landmark Ed.) 2009, 14, 3879–3883.
- Nath, A.; Hall, E.; Tuzova, M.; Dobbs, M.; Jones, M.; Anderson, C.; Woodward, J.; Guo, Z.; Fu, W.; Kryscio, R.; et al. Autoantibodies to amyloid beta-peptide (Abeta) are increased in Alzheimer’s disease patients and Abeta antibodies can enhance Abeta neurotoxicity: Implications for disease pathogenesis and vaccine development. Neuromolecular Med. 2003, 3, 29–40.
- McMahon, M.J.; O’kennedy, R. Polyreactivity as an acquired artefact, rather than a physiologic property, of antibodies: Evidence that monoreactive antibodies may gain the ability to bind to multiple antigens after exposure to low pH. J. Immunol. Methods 2000, 241, 1–10.
- Li, X.-W.; Li, X.-X.; Liu, Q.-S.; Cheng, Y. Blood and Cerebrospinal Fluid Autoantibody to Aβ Levels in Patients with Alzheimer’s Disease: A Meta-Analysis Study. J. Mol. Neurosci. 2020, 70, 1208–1215.
- Bartos, A.; Fialová, L.; Švarcová, J. Lower Serum Antibodies Against Tau Protein and Heavy Neurofilament in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 751–760.
- Bartos, A.; Fialová, L.; Švarcová, J.; Ripova, D. Patients with Alzheimer disease have elevated intrathecal synthesis of antibodies against tau protein and heavy neurofilament. J. Neuroimmunol. 2012, 252, 100–105.
- Klaver, A.C.; Coffey, M.P.; Bennett, D.A.; Loeffler, D.A. Specific serum antibody binding to phosphorylated and non-phosphorylated tau in non-cognitively impaired, mildly cognitively impaired, and Alzheimer’s disease subjects: An exploratory study. Transl. Neurodegener. 2017, 6, 32.
- Krestova, M.; Hromadkova, L.; Bilkova, Z.; Bartos, A.; Ricny, J. Characterization of Isolated Tau-Reactive Antibodies From the Ivig Product, Plasma of Patients with Alzheimer’s Disease and Cognitively Normal Individuals. J. Neuroimmunol. 2017, 313, 16–24.
- Kuhn, I.; Rogosch, T.; Schindler, T.I.; Tackenberg, B.; Zemlin, M.; Maier, R.F.; Dodel, R.; Kronimus, Y. Serum titers of autoantibodies against α-synuclein and tau in child- and adulthood. J. Neuroimmunol. 2018, 315, 33–39.
- Skillbäck, T.; Farahmand, B.Y.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; Schott, J.M.; et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 2015, 138, 2716–2731.
- Kolarova, M.; García-Sierra, F.; Bartos, A.; Ricny, J.; Ripova, D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimer’s Dis. 2012, 2012, 731526.
- Hromadkova, L.; Kolarova, M.; Jankovicova, B.; Bartos, A.; Ricny, J.; Bilkova, Z.; Ripova, D. Identification and characterization of natural antibodies against tau protein in an intravenous immunoglobulin product. J. Neuroimmunol. 2015, 289, 121–129.
- Rosenmann, H.; Meiner, Z.; Geylis, V.; Abramsky, O.; Steinitz, M. Detection of circulating antibodies against tau protein in its unphosphorylated and in its neurofibrillary tangles-related phosphorylated state in Alzheimer’s disease and healthy subjects. Neurosci. Lett. 2006, 410, 90–93.
- Pascual, G.; Wadia, J.S.; Zhu, X.; Keogh, E.; Kükrer, B.; van Ameijde, J.; Inganäs, H.; Siregar, B.; Perdok, G.; Diefenbach, O.; et al. Immunological memory to hyperphosphorylated tau in asymptomatic individuals. Acta Neuropathol. 2017, 133, 767–783.
- Fein, J.A.; Sokolow, S.; Miller, C.A.; Vinters, H.V.; Yang, F.; Cole, G.M.; Gylys, K.H. Co-localization of amyloid beta and tau pathology in Alzheimer’s disease synaptosomes. Am. J. Pathol. 2008, 172, 1683–1692.
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508.
- Selkoe, D.J. Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004, 6, 1054–1061.
- Marsh, S.E.; Blurton-Jones, M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimer’s Res. Ther. 2012, 4, 11.
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872, Erratum in Neurology 2005, 65, 1992.
- Meneses, A.; Koga, S.; O’leary, J.; Dickson, D.W.; Bu, G.; Zhao, N. TDP-43 Pathology in Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 84.
- Borghi, R.; Marchese, R.; Negro, A.; Marinelli, L.; Forloni, G.; Zaccheo, D.; Abbruzzese, G.; Tabaton, M. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson’s disease and normal subjects. Neurosci. Lett. 2000, 287, 65–67.
- Mori, F.; Tanji, K.; Yoshimoto, M.; Takahashi, H.; Wakabayashi, K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp. Neurol. 2002, 176, 98–104.
- El-Agnaf, O.M.A.; Salem, S.A.; Paleologou, K.E.; Cooper, L.J.; Fullwood, N.J.; Gibson, M.J.; Curran, M.D.; Court, J.A.; Mann, D.M.A.; Ikeda, S.-I.; et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003, 17, 1945–1947.
- Miller, D.W.; Hague, S.M.; Clarimon, J.; Baptista, M.; Gwinn-Hardy, K.; Cookson, M.R.; Singleton, A.B. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology 2004, 62, 1835–1838.
- Barbour, R.; Kling, K.; Anderson, J.P.; Banducci, K.; Cole, T.; Diep, L.; Fox, M.; Goldstein, J.M.; Soriano, F.; Seubert, P.; et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 2008, 5, 55–59.
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A role for α-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002, 22, 3090–3099.
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Park. Dis. 2017, 7, 433–450.
- Scott, D.; Roy, S. A-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 2012, 32, 10129–10135.
- Cheng, F.; Vivacqua, G.; Yu, S. The role of α-synuclein in neurotransmission and synaptic plasticity. J. Chem. Neuroanat. 2011, 42, 242–248.
- Allen Reish, H.E.; Standaert, D.G. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Park. Dis. 2015, 5, 1–19.
- Pandey, M.K. The Role of Alpha-Synuclein Autoantibodies in the Induction of Brain Inflammation and Neurodegeneration in Aged Humans. Front. Aging Neurosci. 2022, 14, 902191.
- Stephenson, J.; Nutma, E.; Van Der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219.
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2019, 36, 1–12.
- Chahine, L.M.; Beach, T.G.; Adler, C.H.; Hepker, M.; Kanthasamy, A.; Appel, S.; Pritzkow, S.; Pinho, M.; Mosovsky, S.; Serrano, G.E.; et al. Central and peripheral α-synuclein in Parkinson disease detected by seed amplification assay. Ann. Clin. Transl. Neurol. 2023, 10, 696–705.
- Schulz-Schaeffer, W.J. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010, 120, 131–143.
- Irizarry, M.C.; Growdon, W.; Gomez-Isla, T.; Newell, K.; George, J.M.; Clayton, D.F.; Hyman, B.T. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J. Neuropathol. Exp. Neurol. 1998, 57, 334–337.
- Orr, C.F.; Rowe, D.B.; Mizuno, Y.; Mori, H.; Halliday, G.M. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain 2005, 128, 2665–2674.
- Heinzel, S.; Gold, M.; Deuschle, C.; Bernhard, F.; Maetzler, W.; Berg, D.; Dodel, R. Naturally occurring alpha-synuclein autoantibodies in Parkinson’s disease: Sources of (error) variance in biomarker assays. PLoS ONE 2014, 9, e114566.
- Woulfe, J.M.; Duke, R.; Middeldorp, J.M.; Stevens, S.; Vervoort, M.; Hashimoto, M.; Masliah, E.; Chan, P.; Di Monte, D.A.; Langston, J.W.; et al. Absence of elevated anti-alpha-synuclein and anti-EBV latent membrane protein antibodies in PD. Neurology 2002, 58, 1435.
- Smith, L.M.; Schiess, M.C.; Coffey, M.P.; Klaver, A.C.; Loeffler, D.A. A-Synuclein and anti-α-synuclein antibodies in Parkinson’s disease, atypical Parkinson syndromes, REM sleep behavior disorder, and healthy controls. PLoS ONE 2012, 7, e52285.
- Yanamandra, K.; Gruden, M.A.; Casaite, V.; Meskys, R.; Forsgren, L.; Morozova-Roche, L.A. A-Synuclein Reactive Antibodies as Diagnostic Biomarkers in Blood Sera of Parkinson’s Disease Patients. PLoS ONE 2011, 6, e18513.
- Horvath, I.; Iashchishyn, I.A.; Forsgren, L.; Morozova-Roche, L.A. Immunochemical Detection of α-Synuclein Autoantibodies in Parkinson’s Disease: Correlation between Plasma and Cerebrospinal Fluid Levels. ACS Chem. Neurosci. 2017, 8, 1170–1176.
- Bryan, T.; Luo, X.; Forsgren, L.; Morozova-Roche, L.A.; Davis, J.J. The robust electrochemical detection of a Parkinson’s disease marker in whole blood sera. Chem. Sci. 2012, 3, 3468–3473.
- Besong-Agbo, D.; Wolf, E.; Jessen, F.; Oechsner, M.; Hametner, E.; Poewe, W.; Reindl, M.; Oertel, W.H.; Noelker, C.; Bacher, M.; et al. Naturally occurring α-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology 2013, 80, 169–175.
- Maetzler, W.; Berg, D.; Synofzik, M.; Brockmann, K.; Godau, J.; Melms, A.; Gasser, T.; Hörnig, S.; Langkamp, M. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J. Alzheimer’s Dis. 2011, 26, 171–179.
- Gruden, M.A.; Sewell, R.D.; Yanamandra, K.; Davidova, T.V.; Kucheryanu, V.G.; Bocharov, E.V.; Bocharova, O.A.; Polyschuk, V.V.; Sherstnev, V.V.; Morozova-Roche, L.A. Immunoprotection against toxic biomarkers is retained during Parkinson’s disease progression. J. Neuroimmunol. 2011, 233, 221–227.
- Akhtar, R.S.; Licata, J.P.; Luk, K.C.; Shaw, L.M.; Trojanowski, J.Q.; Lee, V.M. Measurements of auto-antibodies to α-synuclein in the serum and cerebral spinal fluids of patients with Parkinson’s disease. J. Neurochem. 2018, 145, 489–503.
- Koehler, N.K.; Stransky, E.; Shing, M.; Gaertner, S.; Meyer, M.; Schreitmüller, B.; Leyhe, T.; Laske, C.; Maetzler, W.; Kahle, P.; et al. Altered serum IgG levels to α-synuclein in dementia with Lewy bodies and Alzheimer’s disease. PLoS ONE 2013, 8, e64649.
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in multiple sclerosis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2023, 15, e1583.
- Sandi, D.; Fricska-Nagy, Z.; Bencsik, K.; Vécsei, L. Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy-Kynurenines Are Important Players. Molecules 2021, 26, 3423.
- Pette, M.; Fujita, K.; Kitze, B.; Whitaker, J.N.; Albert, E.; Kappos, L.; Wekerle, H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology 1990, 40, 1770.
- O’Connor, K.C.; Chitnis, T.; E Griffin, D.; Piyasirisilp, S.; Bar-Or, A.; Khoury, S.; Wucherpfennig, K.W.; A Hafler, D. Myelin basic protein-reactive autoantibodies in the serum and cerebrospinal fluid of multiple sclerosis patients are characterized by low-affinity interactions. J. Neuroimmunol. 2003, 136, 140–148.
- Berger, T.; Rubner, P.; Schautzer, F.; Egg, R.; Ulmer, H.; Mayringer, I.; Dilitz, E.; Deisenhammer, F.; Reindl, M. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 2003, 349, 139–145.
- Angelucci, F.; Mirabella, M.; Frisullo, G.; Caggiula, M.; Tonali, P.A.; Batocchi, A.P. Serum levels of anti-myelin antibodies in relapsing-remitting multiple sclerosis patients during different phases of disease activity and immunomodulatory therapy. Dis. Markers 2005, 21, 49–55.
- Olsson, T.; Baig, S.; Hojeberg, B.; Link, H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann. Neurol. 1990, 27, 132–136.
- Reindl, M.; Linington, C.; Brehm, U.; Egg, R.; Dilitz, E.; Deisenhammer, F.; Poewe, W.; Berger, T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: A comparative study. Brain 1999, 122, 2047–2056.
- Warren, K.G.; Catz, I.; Johnson, E.; Mielke, B. Anti-myelin basic protein and anti-proteolipid protein specific forms of multiple sclerosis. Ann. Neurol. 1994, 35, 280–289.
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747.
- Tran, G.T.; Hodgkinson, S.J.; Carter, N.; Killingsworth, M.; Spicer, S.T.; Hall, B.M. Attenuation of experimental allergic encephalomyelitis in complement component 6-deficient rats is associated with reduced complement C9 deposition, P-selectin expression, and cellular infiltrate in spinal cords. J. Immunol. 2002, 168, 4293–4300.
- Rus, H.; Cudrici, C.; Niculescu, F. C5b-9 complement complex in autoimmune demyelination and multiple sclerosis: Dual role in neuroinflammation and neuroprotection. Ann. Med. 2005, 37, 97–104.
- Gay, F.W. Early cellular events in multiple sclerosis. Intimations of an extrinsic myelinolytic antigen. Clin. Neurol. Neurosurg. 2006, 108, 234–240.
- Coyle, P.K.; Procyk-Dougherty, Z. Multiple sclerosis immune complexes: An analysis of component antigens and antibodies. Ann. Neurol. 1984, 16, 660–667.
- Geffard, M.; Boullerne, A.; Brochet, B. Seric immune complexes in multiple sclerosis do not contain MBP epitopes. Brain Res. Bull. 1993, 30, 365–368.
- Hedegaard, C.J.; Chen, N.; Sellebjerg, F.; Sørensen, P.S.; Leslie, R.G.Q.; Bendtzen, K.; Nielsen, C.H. Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: A role in regulating cytokine responses to MBP. Immunology 2009, 128 (Suppl. S1), e451–e461.
- Villar, L.; Garcia-Barragan, N.; Espiño, M.; Roldán, E.; Sadaba, M.; Gómez-Rial, J.; Gonzalez-Porque, P.; Alvarez-Cermeno, J. Influence of oligoclonal IgM specificity in multiple sclerosis disease course. Mult. Scler. J. 2008, 14, 183–187.
- Ambrosius, W.; Michalak, S.; Kozubski, W.; Kalinowska, A. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease: Current Insights into the Disease Pathophysiology, Diagnosis and Management. Int. J. Mol. Sci. 2020, 22, 100.
- Jarius, S.; Paul, F.; Aktas, O.; Asgari, N.; Dale, R.C.; de Seze, J.; Franciotta, D.; Fujihara, K.; Jacob, A.; Kim, H.J.; et al. MOG encephalomyelitis: International recommendations on diagnosis and antibody testing. J. Neuroinflammation 2018, 15, 134.
- Mak, G.; Menon, S.; Lu, J.Q. Neurofilaments in neurologic disorders and beyond. J. Neurol. Sci. 2022, 441, 120380.
- Perrot, R.; Berges, R.; Bocquet, A.; Eyer, J. Review of the multiple aspects of neurofilament functions and their possible contribution to neurodegeneration. Mol. Neurobiol. 2008, 38, 27–65.
- Gafson, A.R.; Barthélemy, N.R.; Bomont, P.; Carare, R.O.; Durham, H.D.; Julien, J.P.; Kuhle, J.; Leppert, D.; Nixon, R.A.; Weller, R.O.; et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020, 143, 1975–1998.
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589.
- Gentil, B.J.; Tibshirani, M.; Durham, H.D. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015, 360, 609–620.
- Bocquet, A.; Berges, R.; Frank, R.; Robert, P.; Peterson, A.C.; Eyer, J. Neurofilaments bind tubulin and modulate its polymerization. J. Neurosci. 2009, 29, 11043–11054.
- Schwartz, M.L.; Shneidman, P.S.; Bruce, J.; Schlaepfer, W.W. Stabilization of neurofilament transcripts during postnatal development. Brain Res. Mol. Brain Res. 1994, 27, 215–220.
- Yuan, A.; Nixon, R.A. Specialized roles of neurofilament proteins in synapses: Relevance to neuropsychiatric disorders. Brain Res. Bull. 2016, 126, 334–346.
- Zetterberg, H.; Burnham, S.C. Blood-based molecular biomarkers for Alzheimer’s disease. Mol. Brain 2019, 12, 26.
- Novakova, L.; Axelsson, M.; Velayudhan, L.; Rabinovici, G.D.; Miller, B.; Pariante, C.; Nikkheslat, N.; Resnick, S.M.; Thambisetty, M.; Schöll, M.; et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 2021, 12, 3400.
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 2017, 88, 930–937.
- Ehling, R.; Lutterotti, A.; Wanschitz, J.; Khalil, M.; Gneiss, C.; Deisenhammer, F.; Reindl, M.; Berger, T. Increased frequencies of serum antibodies to neurofilament light in patients with primary chronic progressive multiple sclerosis. Mult. Scler. J. 2004, 10, 601–606.
- Fialová, L.; Bartos, A.; Švarcová, J.; Zimova, D.; Kotoucova, J.; Malbohan, I. Serum and cerebrospinal fluid light neurofilaments and antibodies against them in clinically isolated syndrome and multiple sclerosis. J. Neuroimmunol. 2013, 262, 113–120.
- Amor, S.; van der Star, B.J.; Bosca, I.; Raffel, J.; Gnanapavan, S.; Watchorn, J.; Kuhle, J.; Giovannoni, G.; Baker, D.; Malaspina, A.; et al. Neurofilament light antibodies in serum reflect response to natalizumab treatment in multiple sclerosis. Mult. Scler. J. 2014, 20, 1355–1362.
- Kuhle, J.; Barro, C.; Disanto, G.; Mathias, A.; Soneson, C.; Bonnier, G.; Yaldizli, Ö.; Regeniter, A.; Derfuss, T.; Canales, M.; et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult. Scler. J. 2016, 22, 1550–1559.
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017, 81, 857–870.
- Novakova, L.; Zetterberg, H.; Sundström, P.; Axelsson, M.; Khademi, M.; Gunnarsson, M.; Malmeström, C.; Svenningsson, A.; Olsson, T.; Piehl, F.; et al. Monitoring disease activity in multiple sclerosis. Using serum neurofilament light protein. Neurology 2017, 89, 2230–2237.
- Barro, C.; Benkert, P.; Disanto, G.; Tsagkas, C.; Amann, M.; Naegelin, Y.; Leppert, D.; Gobbi, C.; Granziera, C.; Yaldizli, Ö.; et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018, 141, 2382–2391.
- Piehl, F.; Kockum, I.; Khademi, M.; Blennow, K.; Lycke, J.; Zetterberg, H.; Olsson, T. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult. Scler. 2018, 24, 1046–1054.
- Lu, X.Y.; Chen, X.X.; Huang, L.D.; Zhu, C.Q.; Gu, Y.Y.; Ye, S. Anti-alpha-internexin autoantibody from neuropsychiatric lupus induce cognitive damage via inhibiting axonal elongation and promote neuron apoptosis. PLoS ONE 2010, 5, e11124.
- Oron, L.; Dubovik, V.; Novitsky, L.; Eilam, D.; Michaelson, D.M. Animal model and in vitro studies of anti neurofilament antibodies mediated neurodegeneration in Alzheimer’s disease. J. Neural. Transm. Suppl. 1997, 49, 77–84.
- Stubbs, E.B.; Lawlor, M.W.; Richards, M.P.; Siddiqui, K.; Fisher, M.A.; Bhoopalam, N.; Siegel, G.J. Anti-neurofilament antibodies in neuropathy with monoclonal gammopathy of undetermined significance produce experimental motor nerve conduction block. Acta Neuropathol. 2003, 105, 109–116.
- Soussan, L.; Tchernakov, K.; Bachar-Lavi, O.; Yuvan, T.; Wertman, E.; Michaelson, D.M. Antibodies to different isoforms of the heavy neurofilament protein (NF-H) in normal aging and Alzheimer’s disease. Mol. Neurobiol. 1994, 9, 83–91.
- Elizan, T.S.; Casals, J.; Yahr, M.D. Antineurofilament antibodies in postencephalitic and idiopathic Parkinson’s disease. J. Neurol. Sci. 1983, 59, 341–347.
- Karcher, D.; Federsppiel, B.S.S.; Lowenthal, F.D.; Frank, F.; Lowenthal, A. Anti-neurofilament antibodies in blood of patients with neurological diseases. Acta Neuropathol. 1986, 72, 82–85.
- Kronimus, Y.; Albus, A.; Balzer-Geldsetzer, M.; Straub, S.; Semler, E.; Otto, M.; Klotsche, J.; Dodel, R.; Landscape Consortium; Mengel, D. Naturally Occurring Autoantibodies against Tau Protein Are Reduced in Parkinson’s Disease Dementia. PLoS ONE 2016, 11, e0164953.
- Liu, T.W.; Chen, C.M.; Chang, K.H. Biomarker of Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4148.
- Couratier, P.; Yi, F.H.; Preud’homme, J.L.; Clavelou, P.; White, A.; Sindou, P.; Vallat, J.M.; Jauberteau, M.O. Serum autoantibodies to neurofilament proteins in sporadic amyotrophic lateral sclerosis. J. Neurol. Sci. 1998, 154, 137–145.
- Fialová, L.; Švarcová, J.; Bartos, A.; Ridzoň, P.; Malbohan, I.; Keller, O.; Rusina, R. Cerebrospinal fluid and serum antibodies against neurofilaments in patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2010, 17, 562–566.
- Puentes, F.; Topping, J.; Kuhle, J.; van der Star, B.J.; Douiri, A.; Giovannoni, G.; Baker, D.; Amor, S.; Malaspina, A. Immune reactivity to neurofilament proteins in the clinical staging of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2014, 85, 274–278.
- Niebroj-Dobosz, I.; Dziewulska, D.; Janik, P. Auto-antibodies against proteins of spinal cord cells in cerebrospinal fluid of patients with amyotrophic lateral sclerosis (ALS). Folia Neuropathol. 2006, 44, 191–196.
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Elastin-Derived Peptides in the Central Nervous System: Friend or Foe. Cell. Mol. Neurobiol. 2022, 42, 2473–2487.
- DeMarshall, C.; Sarkar, A.; Nagele, E.P.; Goldwaser, E.; Godsey, G.; Acharya, N.K.; Nagele, R.G. Utility of autoantibodies as biomarkers for diagnosis and staging of neurodegenerative diseases. Int. Rev. Neurobiol. 2015, 122, 1–51.
- Yin, W.; Stover, C.M. The potential of circulating autoantibodies in the early diagnosis of Alzheimer’s disease. AIMS Allergy Immunol. 2017, 1, 62–70.
- Gao, V.; Briano, J.A.; Komer, L.E.; Burré, J. Functional and Pathological Effects of α-Synuclein on Synaptic SNARE Complexes. J. Mol. Biol. 2023, 435, 167714.
- Hampel, H.; O’Bryant, S.E.; Molinuevo, J.L.; Zetterberg, H.; Masters, C.L.; Lista, S.; Kiddle, S.J.; Batrla, R.; Blennow, K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018, 14, 639–652.
- Obrocki, P.; Khatun, A.; Ness, D.; Senkevich, K.; Hanrieder, J.; Capraro, F.; Mattsson, N.; Andreasson, U.; Portelius, E.; Ashton, N.J.; et al. Perspectives in fluid biomarkers in neurodegeneration from the 2019 biomarkers in neurodegenerative diseases course-a joint PhD student course at University College London and University of Gothenburg. Alzheimer’s Res. Ther. 2020, 12, 20.
- Nagele, E.; Han, M.; DeMarshall, C.; Belinka, B.; Nagele, R. Diagnosis of Alzheimer’s Disease Based on Disease-Specific Autoantibody Profiles in Human Sera. PLoS ONE 2011, 6, e23112.
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS ONE 2013, 8, e60726.
- Reddy, M.M.; Wilson, R.; Wilson, J.; Connell, S.; Gocke, A.; Hynan, L.; German, D.; Kodadek, T. Identification of Candidate IgG Antibody Biomarkers for Alzheimer’s Disease Through Screening of Synthetic Combinatorial Libraries. Cell 2011, 144, 132–142.
- Leslie, D.; Lipsky, P.; Notkins, A.L. Autoantibodies as predictors of disease. J. Clin. Investig. 2001, 108, 1417–1422.
- Bingley, P.J.; Bonifacio, E.; Williams, A.J.K.; Genovese, S.; Bottazzo, G.F.; Gale, E.A.M. Prediction of IDDM in the general population: Strategies based on combinations of autoantibody markers. Diabetes 1997, 46, 1701–1710.
- Cummings, J. Anti-Amyloid Monoclonal Antibodies are Transformative Treatments that Redefine Alzheimer’s Disease Therapeutics. Drugs 2023, 83, 569–576.
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2022, 13, 80.
- Gklinos, P.; Papadopoulou, M.; Stanulovic, V.; Mitsikostas, D.D.; Papadopoulos, D. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals 2021, 14, 92.
