Sulforaphane (SFN) is a naturally occurring compound found in cruciferous vegetables such as broccoli and cauliflower. It has been widely studied for its potential as a neuroprotective and anticancer agent. SFN has been shown to exert neuroprotective effects through the activation of the Nrf2 pathway, the modulation of neuroinflammation, and epigenetic mechanisms. In cancer treatment, SFN has demonstrated the ability to selectively induce cell death in cancer cells, inhibit histone deacetylase, and sensitize cancer cells to chemotherapy. SFN has also shown chemoprotective properties through inhibiting phase I metabolizing enzymes, modulating phase II xenobiotic-metabolizing enzymes, and targeting cancer stem cells.
1. Origin and Discovery
In the middle of the last century, sulforaphane (SFN; sulphoraphane in British English) was described as an antibiotic and was isolated from red cabbage and from hoary cress, a weed in rangelands of the western US [
1]. It was first synthesized by Talalay and Zhang, who were the first to isolate it from broccoli [
2]. SFN is a compound within the isothiocyanate (ITC) group of organosulfur compounds. ITCs are hydrolysis products of glucosinolates, secondary plant metabolites that are found in high concentrations in
Brassica vegetables [
3]. ITCs are known to be synthesized and stored as glucosinolates in plants and are released when damage to plant tissues occurs [
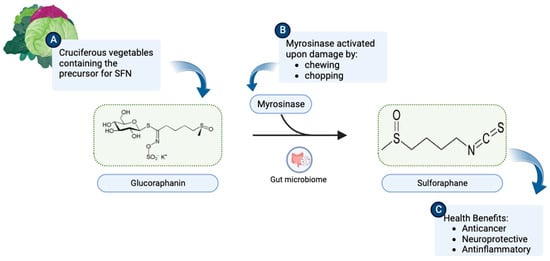
4]. The most characterized ITC compound is SFN, the hydrolysis product of glucoraphanin, and it generally is found in high concentrations in broccoli (
Figure 1) [
5]. SFN occurs in broccoli sprouts, which have been shown to be 20–50 times more effective in chemoprevention than mature heads [
6]. Among cruciferous vegetables, broccoli sprouts have the highest concentration the SFN precursor [
7], hence broccoli sprouts are preferred over other crucifers as a chemoprotective agent.
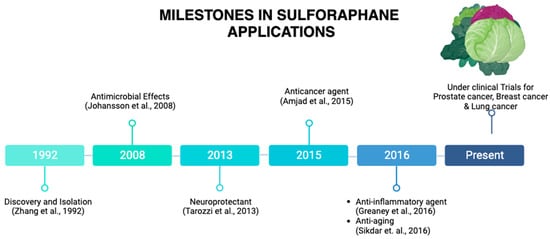
Figure 2 illustrates the journey of SFN from a promising naturally occurring compound to its status as a subject of ongoing research.
Figure 1. (
A) Cruciferous vegetables are a rich source of glucoraphanin. (
B) Upon chewing or chopping, the myrosinase enzyme present in plant tissues or intestinal flora catalyzes the breakdown of glucoraphanin to SFN (C
6H
11NOS
2). (
C) SFN consequently becomes available to exert health benefits. (Chemical structures of SFN and Glucoraphanin were sourced from their respective Wikipedia pages:
https://en.wikipedia.org/wiki/Sulforaphane and
https://en.wikipedia.org/wiki/Glucoraphanin.) This illustration was made with
Biorender.com (accessed on 8 August 2023).
Figure 2. Milestones in SFN applications. SFN was discovered in 1992. A remarkable milestone has been reached, from its applications as an antimicrobial agent, a neuroprotective agent, an anticancer agent, and anti-inflammatory agent to currently being under clinical trials for prostate cancer, breast cancer, and lung cancer, among others. This illustration was made with
Biorender.com (accessed on 8 August 2023) [
2,
8,
9,
10,
11,
12].
2. SFN as a Neuroprotective Agent
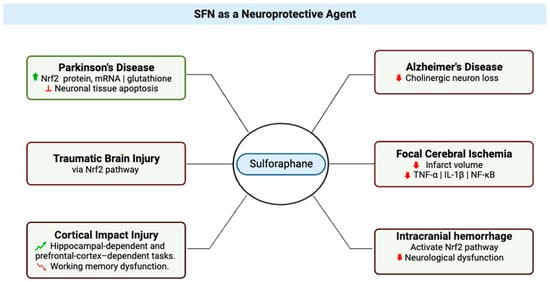
Neuroprotection refers to the mechanisms and strategies used to defend the central nervous system (CNS) (
Figure 3) against injury due to both acute (e.g., trauma or stroke) and chronic neurodegenerative disorders (e.g., dementia, Parkinson’s, Alzheimer’s, epilepsy) [
13]; by extension, neuroprotective agents comprise a category of agents that generally are used to protect neuronal structure and/or function. Research on the neuroprotective effects of SFN began in 2004 with studies that showed its effects protecting neurons [
14] and microglia [
15] against oxidative stress via activation of nuclear factor erythroid 2-related factor 2 (Nrf2). The research literature is replete with studies that support the vital role played by the Nrf2 pathway in the neuroprotective effects of SFN [
14,
16,
17,
18,
19,
20,
21], evidenced by lack of neuroprotection from toxins in Nrf2-knockout mice treated with SFN [
22,
23]. In a study of Parkinson’s disease that used a 6-hydroxydopamine-Parkinson’s disease mouse model, treatment of SH-SY5Y cells with SFN was found to have a protective effect on the neurons, which was attributed to the observed increases in active nuclear Nrf2 protein, Nrf2 mRNA, and total glutathione levels and inhibition of neuronal tissue apoptosis [
24]. A group studying SFN effects in traumatic brain injury confirmed that SFN showed neuroprotection in spinal cord injury, and it may be an emerging therapeutic agent in this setting [
25].
Figure 3. Multifaceted Neuroprotective Effects of Sulforaphane (SFN) in Diverse Neurological Conditions. The central node represents sulforaphane, while six distinct branches emanate from it, each depicting a specific condition where SFN exerts its therapeutic impact. Texts attached to each branch elaborate on the molecular mechanisms and outcomes associated with SFN’s effects in these conditions. Red signifies reduction or decrease in effects. Green indicates an increase or improvement in effects. This illustration was made with
Biorender.com (accessed on 8 August 2023) [
24,
25,
26,
27,
28,
29,
30,
31].
SFN also has been shown to exert neuroprotective effects in Alzheimer’s disease (AD) (
Figure 3); in brains of mice with Alzheimer’s disease-like lesions, SFN ameliorated neurobehavioral deficits by reducing cholinergic neuron loss. SFN is a potent inducer of the Nrf2 antioxidant response element (ARE) pathway, which plays a major role in upregulating cellular defenses to oxidative stress [
27].
Epigenetics emerges as a pivotal mechanism underlying the neuroprotective potential of SFN. In a study involving mouse neuroblastoma N2a cells expressing human Swedish mutant Aβ precursor protein (N2a/APPswe cells), SFN was observed to induce Nrf2 expression by reducing DNA methylation levels at the Nrf2 promoter [
20,
36]. The activation of the Nrf2 ARE pathway, subsequently leading to the upregulation of key downstream elements such as NAD(P)H quinone oxidoreductase 1, heme oxygenase 1, and glutathione peroxidase 1, plays a pivotal role in countering oxidative stress [
37].
Neuroinflammation, a pivotal factor in several neurological disorders, has also been a target of SFN’s neuroprotective effects [
35,
52,
53,
54,
55]. Microglia, the resident immune cells of the brain [
56], play a key role in neuroinflammation by secreting pro-inflammatory and anti-inflammatory mediators [
57]. Studies in hyperammonemic rat models revealed that SFN promotes microglia differentiation from pro-inflammatory M1 to anti-inflammatory M2 phenotypes, mitigating neuroinflammation [
35].
3. SFN as an Anticancer Agent
In recent years, SFN has gained attention for its potential use as an anticancer agent. In a study in 2015, Ullah proposed three major factors that enhance the plausibility of clinical applications and the translational value. First, normal cells are relatively resistant to SFN-induced cell death [
60], an important feature for potential anticancer agents, and recent in vitro work demonstrated that SFN suppressed metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway [
61]. Second, SFN has good bioavailability; it can reach high intracellular and plasma concentrations, and it has been detected in breast tissues after a single oral administration [
62,
63]. Finally, a study by Myzak et al. in 2007 provided evidence that histone deacetylase was inhibited after human subjects ingested 68 g of broccoli sprouts, indicating that SFN provides anticancer pharmacological effects at levels that humans can readily ingest.
Ullah proposed a mechanism by which SFN exerts its chemopreventive effects. In this model, low to moderate levels of reactive oxygen species (ROS) are active participants in cellular functions and act as signaling molecules that sustain cellular proliferation and differentiation and that activate responses to oxidative stress [
64]. Under normal, unstressed conditions, the cellular NRF2 level is very low [
65], but it significantly increases upon exposure to electrophilic chemicals or ROS [
66]. In response to oxidative or electrophilic stress, Nrf2 stimulates antistress signaling and consequently inhibits carcinogenesis [
67]. Under conditions of stress, Kelch-like ECH-Associated Protein 1 (KEAP1), which is a cytosolic inhibitor of Nrf2, is oxidized, leading to stabilization and translocation of Nrf2 into the nucleus, where the transcription factor activates expression of genes crucial to antioxidant defense [
68].
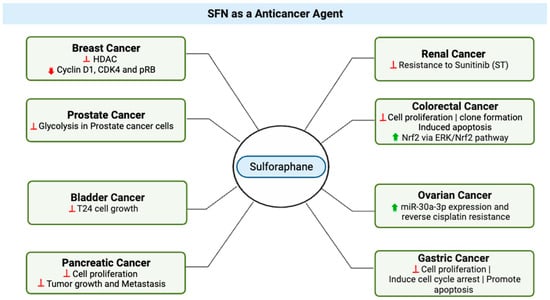
In bladder cancer (
Figure 4), an in vitro study highlighted SFN’s dose-dependent effects on cell growth, presenting opportunities for targeted therapeutic strategies [
71]. In this study, at higher concentrations, ranging from 10–160 μM and after 24 to 48 h of treatment, SFN demonstrated a significant inhibitory effect on T24 cell growth. However, it is important to consider that at lower doses, specifically 2.5 μM, SFN resulted in a slight increase in cell proliferation by 5.18–11.84% within a 6 to 48 h treatment window [
71]. These results suggest that SFN’s effects on cell growth are dose-dependent, with potential implications for further research and development of targeted therapeutic strategies in the context of bladder cancer.
Figure 4. SFN’s Anticancer Effects Across Diverse Cancer Types. The various cancer types for which sulforaphane (SFN) exhibits potent anticancer properties. Red signifies reduction or decrease in effects. Green indicates an increase or improvement in effects. This illustration was made with
Biorender.com (accessed on 8 August 2023) [
44,
70,
71,
72,
73,
74,
75,
76,
77].
Interesting, insights from breast cancer cell research revealed that SFN orchestrates DNA methylation through the modulation of DNA methyltransferase and histone deacetylase levels, coupled with the downregulation of cyclin D1, CDK4, and pRB, thereby promoting breast cancer cell apoptosis [
72].
Sunitinib (ST), an established therapy for renal cell carcinoma (RCC), faces limitations as a standalone treatment due to tumor reactivation and resistance [
78]. To address this, an in vitro study investigated the combination of ST with sulforaphane (SFN). SFN emerged as a critical enhancer of ST’s efficacy by suppressing resistance in RCC cells, offering a potent approach to overcome ST monotherapy limitations. Short-term SFN application reduced cell numbers across diverse lines, sensitizing RCC cells to ST. Long-term SFN use exhibited greater effectiveness, particularly in 786O cells, where the ST-SFN combination outperformed SFN alone [
74].
For colorectal cancer, Hao et al.’s study (2020) demonstrated SFN’s potential as a chemopreventive agent (
Figure 4), acting through the modulation of the ERK/Nrf2 pathway and impacting cell proliferation, apoptosis, and migration [
76]. Additionally, SFN hindered the motility and migration of colorectal cancer cells. Mechanistically, SFN led to dose-dependent upregulation of nuclear factor, erythroid 2 like 2 (Nrf2) and UDP glucuronosyltransferase 1A (UGT1A) expression. This effect was mediated through the ERK/Nrf2 signaling pathway, as ERK inhibition attenuated SFN-induced upregulation of Nrf2 and UGT1A, along with mitigating increased intracellular ROS levels [
13]. In sum, SFN emerges as a promising agent for colorectal cancer chemoprevention, acting through modulation of Nrf2-mediated detoxification and anti-proliferative pathways.
4. Chemoprotectant Properties of SFN
Cancer chemoprevention is defined as the use of dietary or pharmacological agents to prevent, block, or reverse the process of tumor development before clinical manifestation of the disease [
79]. Chemoprotectants are natural or synthetic chemical compounds that can ameliorate, mimic, or inhibit the toxic or adverse effects of structurally different chemotherapeutic agents, radiation therapy, cytotoxic drugs, or naturally occurring toxins, without compromising the anticancer or antitumor potential of the chemotherapeutic drugs [
80]. In vitro, SFN has been shown to be a potent chemopreventive agent and has been demonstrated to target multiple cellular mechanisms [
81].
Inhibition of phase I metabolizing enzymes was reported by Langouet et al. as the primary mechanism of chemoprotection by SFN. A secondary mechanism has been proposed, in which phase II xenobiotic-metabolizing enzymes are modulated, and binding of carcinogens to DNA is directly inhibited [
84]. As a result, cellular pro-inflammatory responses are suppressed, which inhibits formation of DNA adducts and reduces the mutation rate [
84]. A tertiary chemoprevention mechanism also has been proposed, in which SFN abrogates tumorigenesis and progression of metastasis by targeting cancer stem cells in pancreatic and prostate cancer [
83].
5. Effects of SFN on Tumors, Chemotherapy, Radiation Therapy, and Cardiotoxicity
Laboratory and animal studies have provided evidence that SFN has anticancer properties, but more research is needed to understand its effects on humans. Studies have proposed that SFN may prevent the growth of certain types of tumors, such as breast and prostate tumors, by causing cell death and stopping the formation of new blood vessels, which are required for tumor growth.
Research supports the proposition that SFN may improve the effectiveness of chemotherapy by increasing cancer cell sensitivity to the drugs used to treat them [
87], which is known as “chemo-sensitization”. One way in which SFN may sensitize cancer cells to chemotherapy is by inhibiting the Nrf2 pathway [
87].
Some studies have suggested that SFN may have beneficial effects on the body after radiation therapy, although more research is needed to confirm these findings. Radiation therapy is a common treatment for cancer that uses high-energy radiation to kill cancer cells, but it can also damage healthy cells and tissues in the area being treated, leading to side effects such as skin irritation, fatigue, and a risk of developing secondary tumors. Preliminary studies have suggested that SFN may help protect healthy cells and tissues from the harmful effects of radiation [
97].
Cardiotoxicity is the occurrence of heart dysfunction as electric or muscle damage, resulting in heart toxicity [
92]. Several studies have demonstrated the protective role of SFN in cardiotoxicity. For example, in a study by Bose et al. [
99] with a breast cancer model in rats, they found that SFN reduced cardiac oxidative stress (a contributing factor to cardiotoxicity) induced by doxorubicin (DOX). When DOX was administered alone, only an 11% survival rate was observed as compared to a 62% survival rate observed when DOX was combined with SFN
6. SFN on Metastasis
SFN exhibits anticancer properties at various stages of carcinogenesis in prostate, lung, colon, and breast cancers [
104,
105,
106]. Early research has focused on the ability of SFN to activate nuclear factor (erythroid-derived 2)-like 2 (Nrf2). SFN was shown to be effective in preventing breast cancer at different stages of carcinogenesis by increasing the levels of antioxidants and phase II detoxifying enzymes via the activation of the nuclear factor erythroid 2-related factor 2 [
107]. SFN has been shown to alter key mechanisms in vivo and in vitro which impact induction of cell cycle arrest and apoptosis and inhibition of histone deacetylase. SFN inhibits transforming growth factor-β1 (TGF-β1)-induced migration and invasion in human triple negative breast cancer (TNBC) cells [
61]. Sulforaphane (SFE), an SFN derivative, has been shown to reduce TNBC proliferation by mediating ERG1/PTEN axis [
107]. Sulforaphane exerted its anti-metastatic effects on non-small cell lung cancer through down-regulation of miR-616-5p, which was identified as a marker associated with risk of relapse and metastasis in patients [
108]. SFN has been reported to inhibit histone deacetylase (HDAC) enzymes, alter histone acetylation, and affect gene regulation. Natural inhibitors of HDAC have received considerable interest as anticancer agents because of their ability to induce p21Cip1/Waf1, leading to cell cycle arrest and apoptosis [
62]. SFN inhibits HDAC activity in prostate cancer cells, in mouse xenografts, and in human peripheral blood mononuclear cells [
109]. In colon cancers, SFN blocks cells’ progression and angiogenesis by inhibiting HIF-1α and VEGF expression [
110].
7. SFN Bioavailability and Pharmacokinetics
In mammals, SFN is metabolized rapidly via a conjugation reaction with glutathione. SFN is metabolized through the mercapturic acid pathway, starting with GSH conjugation by glutathione S-transferase and subsequently generating SFN-cysteine followed by SFN-N-acetylcysteine [
111]. Pharmacokinetic studies in rodents have focused on either free SFN or its metabolite SFN-glutathione. Following consumption of broccoli, sulforaphane is excreted in urine predominantly as a conjugate with N-acetyl cysteine. In plasma, it has been found that approximately 50% of sulforaphane is found unconjugated with other thiols [
104]. In rats, after an oral dose of 50 μmol of SFN, the plasma concentration of SFN can peak at 20 μM at 4 h and decline with a half-life of about 2.2 h [
112]. SFN is well absorbed in the intestine, with an absolute bioavailability of approximately 82%. Elimination of was characterized by a long terminal phase; no major difference was evident in plasma concentrations between 6 and 24 h following intravenous administration or oral administration [
113].
8. Ongoing and Completed Clinical Trials on SFN
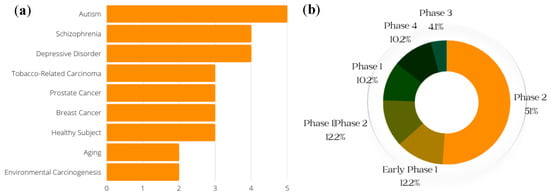
Clinical trials are an important part of the research process, and drug development benefits from both favorable and unfavorable results. Even when studies do not yield the predicted outcomes, trial results can help point scientists in the correct direction [
72]. (Clinical trials of SFN that were withdrawn are not included in the data presented here.) The conditions with the greatest number of ongoing or previous clinical trials on SFN, including breast cancer and prostate cancer, are shown in
Figure 5a; the general phases of clinical trials on SFN (both past and current) are presented in
Figure 5b.
Figure 5. Conditions for which clinical trials with SFN have been registered. (Generated with data from ClinicalTrials.gov) (a) Conditions and respective numbers of clinical trials. The x-axis represents the number of studies that have recorded clinical trials with SFN and the conditions on the y-axis. (b) Breakdown of the phases of SFN clinical trials. This gives a general breakdown of how far SFN clinical trials have gone, with a majority of studies at the Phase 2 level.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28196902