Prostate cancer (PC) is the second most common type of cancer and the leading cause of death among men worldwide. Preventing the progression of cancer after treatments such as radical prostatectomy, radiation therapy, and hormone therapy is a major concern faced by prostate cancer patients. Inflammation, which can be caused by various factors such as infections, the microbiome, obesity and a high-fat diet, is considered to be the main cause of PC. Inflammatory cells are believed to play a crucial role in tumor progression. Therefore, nonsteroidal anti-inflammatory drugs (NSAIDs) along with their effects on the treatment of inflammation-related diseases, can prevent cancer and its progression by suppressing various inflammatory pathways. Evidence shows that nonsteroidal anti-inflammatory drugs are effective in the prevention and treatment of prostate cancer.
- prostate cancer
- nonsteroidal anti-inflammatory drugs
- NSAID
- inflammation
1. Introduction
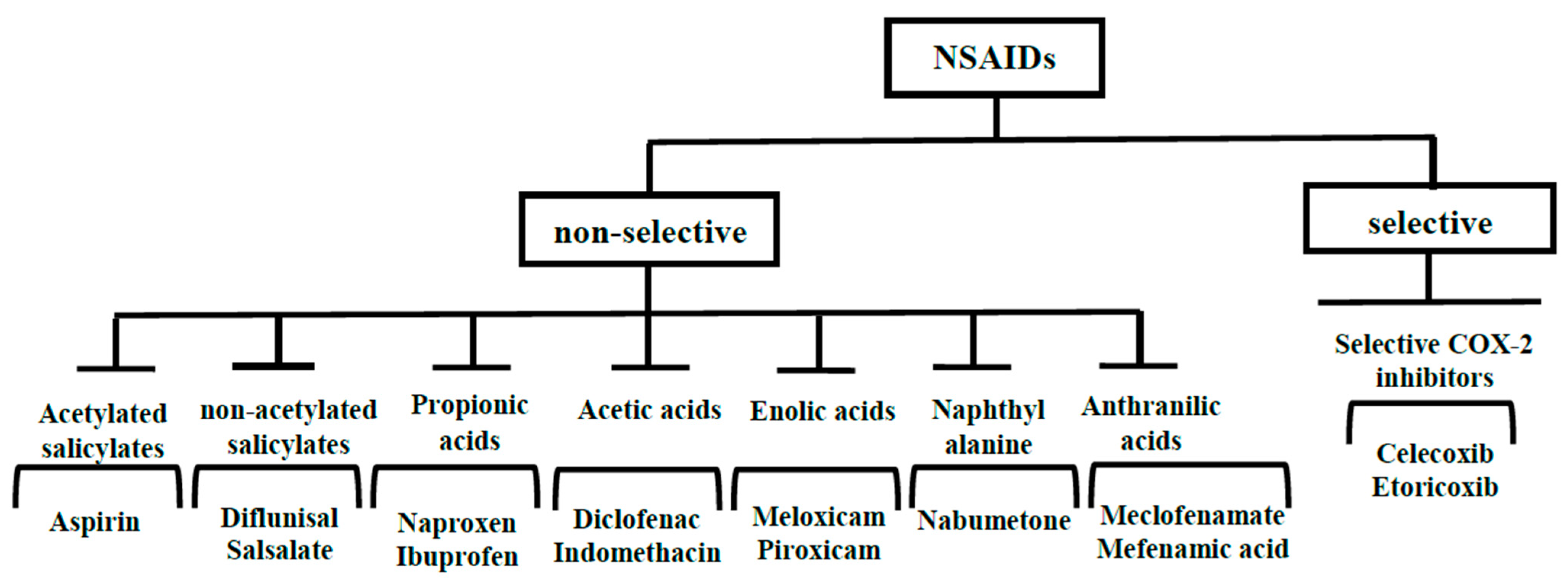
2. NSAIDs
2.1. Aspirin
2.2. Ibuprofen
2.3. Naproxen
2.4. Diclofenac
2.5. Indomethacin
2.6. Mefenamic Acid
2.7. Celecoxib
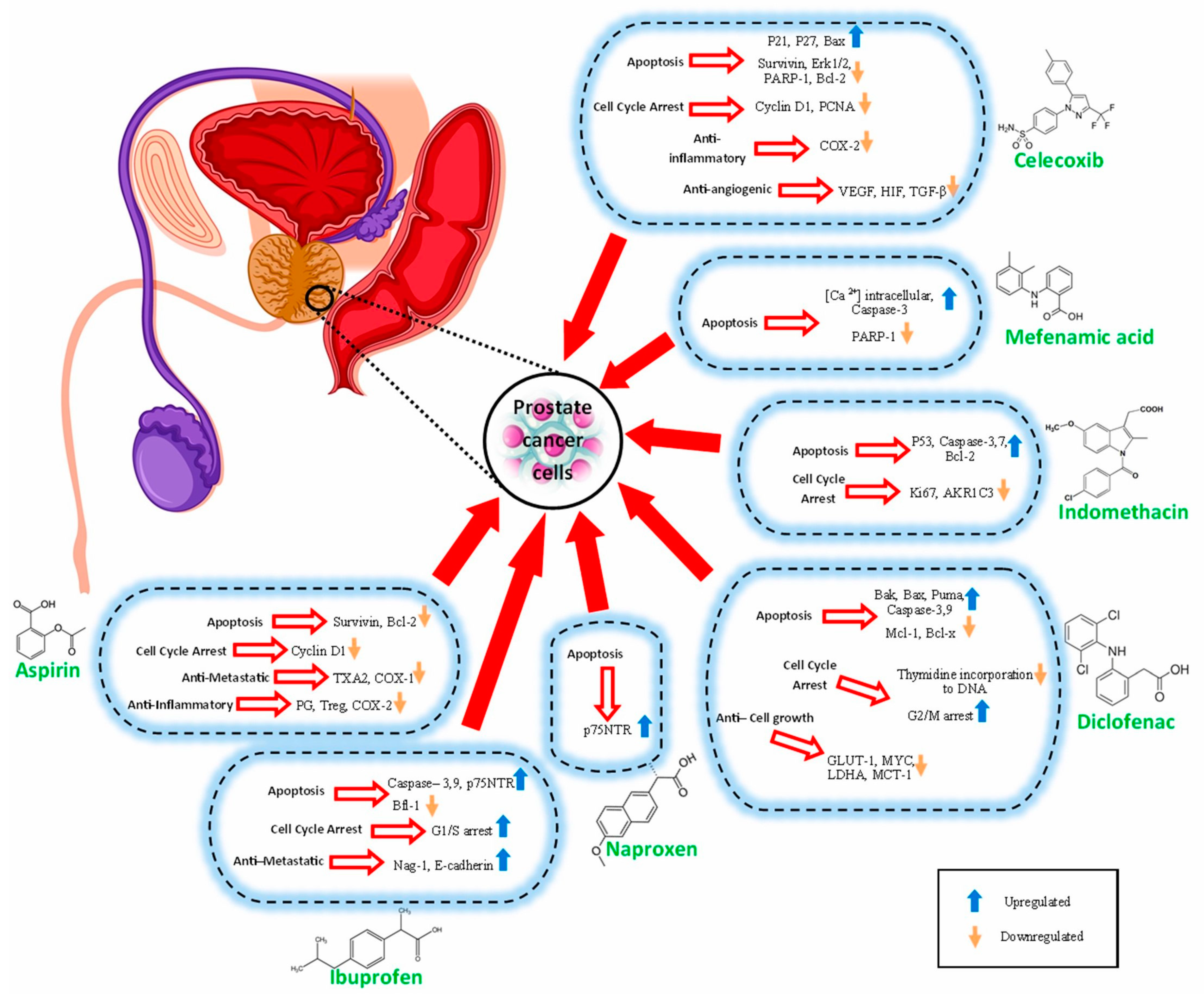
The possible anti-prostate cancer mechanisms of NSAIDs that have been discussed in various investigations are summarized in Table 1 and Figure 2.
|
Effects NSAIDs |
Apoptosis |
Cell Cycle Arrest |
Anti-Metastatic |
Anti-Cell Growth |
Anti-Inflammatory |
Anti-Angiogenic |
|---|---|---|---|---|---|---|
|
ASP |
Survivin, Bcl-2 |
Cyclin D1 |
TXA2, COX-1 |
PG, Treg, COX-2 |
||
|
IBN |
Caspase-3,9, p75NTR Bfl-1 |
G1/S arrest |
Nag-1, E-cadherin |
|||
|
NAP |
p75NTR |
|||||
|
DCF |
Bak, Bax, Puma, Caspase-3,9 Mcl-1, Bcl-x |
Thymidine incorporation to DNA G2/M arrest |
GLUT-1, MYC, LDHA, MCT-1 |
|||
|
IND |
P53, Caspase-3,7, Bcl-2 |
Ki67, AKR1C3 |
||||
|
MFA |
[Ca2+] intracellular, Caspase-3 PARP-1 |
|||||
|
CXB |
P21, P27, Bax. Survivin, Erk1/2, PARP-1, Bcl-2 |
Cyclin D1, PCNA |
COX-2 |
VEGF, HIF-1, TGF-β |
3. Adverse Effects of NSAIDs
This entry is adapted from the peer-reviewed paper 10.3390/cancers15225435
References
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics. 2020 CA Cancer J. Clin. Am. Cancer Soc. 2020, 70, 7–30.
- Virtanen, V.; Paunu, K.; Ahlskog, J.K.; Varnai, R.; Sipeky, C.; Sundvall, M. PARP inhibitors in prostate cancer–the preclinical rationale and current clinical development. Genes 2019, 10, 565.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249.
- Giri, V.N.; Beebe-Dimmer, J.L. (Eds.) Familial Prostate Cancer. Seminars in Oncology; Elsevier: Amsterdam, The Netherlands, 2016.
- De Carlo, F.; Celestino, F.; Verri, C.; Masedu, F.; Liberati, E.; Di Stasi, S.M. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: Surgical, oncological, and functional outcomes: A systematic review. Urol. Int. 2014, 93, 373–383.
- Amin, N.P.; Sher, D.J.; Konski, A.A. Systematic review of the cost effectiveness of radiation therapy for prostate cancer from 2003 to 2013. Appl. Health Econ. Health Policy 2014, 12, 391–408.
- Mitsuzuka, K.; Arai, Y. Metabolic changes in patients with prostate cancer during androgen deprivation therapy. Int. J. Urol. 2018, 25, 45–53.
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269.
- Nakai, Y.; Nonomura, N. Inflammation and prostate carcinogenesis. Int. J. Urol. 2013, 20, 150–160.
- Taverna, G.; Pedretti, E.; Di Caro, G.; Borroni, E.M.; Marchesi, F.; Grizzi, F. Inflammation and prostate cancer: Friends or foe? Inflamm. Res. 2015, 64, 275–286.
- Schillaci, O.; Scimeca, M.; Trivigno, D.; Chiaravalloti, A.; Facchetti, S.; Anemona, L.; Bonfiglio, R.; Santeusanio, G.; Tancredi, V.; Bonanno, E.; et al. Prostate cancer and inflammation: A new molecular imaging challenge in the era of personalized medicine. Nucl. Med. Biol. 2019, 68, 66–79.
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545.
- Koul, H.; Kumar, B.; Koul, S.; Deb, A.; Hwa, J.; Maroni, P.; van Bokhoven, A.; Lucia, M.; Kim, F.; Meacham, R. The role of inflammation and infection in prostate cancer: Importance in prevention, diagnosis and treatment. Drugs Today 2010, 46, 929–943.
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24.
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, inflammation, and prostate cancer. J. Clin. Med. 2019, 8, 201.
- Narita, S.; Nara, T.; Sato, H.; Koizumi, A.; Huang, M.; Inoue, T.; Habuchi, T. Research evidence on high-fat diet-induced prostate cancer development and progression. J. Clin. Med. 2019, 8, 597.
- Kashfi, K. Anti-inflammatory agents as cancer therapeutics. Adv. Pharmacol. 2009, 57, 31–89.
- Zhang, Z.; Chen, F.; Shang, L. Advances in antitumor effects of NSAIDs. Cancer Manag. Res. 2018, 10, 4631.
- Choe, K.S.; Cowan, J.E.; Chan, J.M.; Carroll, P.R.; D’Amico, A.V.; Liauw, S.L. Aspirin use and the risk of prostate cancer mortality in men treated with prostatectomy or radiotherapy. J. Clin. Oncol. 2012, 30, 3540.
- Cardwell, C.R.; Flahavan, E.M.; Hughes, C.M.; Coleman, H.G.; O’sullivan, J.M.; Powe, D.G.; Murray, L.J. Low-dose aspirin and survival in men with prostate cancer: A study using the UK Clinical Practice Research Datalink. Cancer Causes Control. 2014, 25, 33–43.
- Flahavan, E.; Bennett, K.; Sharp, L.; Barron, T. A cohort study investigating aspirin use and survival in men with prostate cancer. Ann. Oncol. 2014, 25, 154–159.
- Ashok, V.; Dash, C.; Rohan, T.E.; Sprafka, J.M.; Terry, P.D. Selective cyclooxygenase-2 (COX-2) inhibitors and breast cancer risk. Breast 2011, 20, 66–70.
- Ghlichloo, I.; Gerriets, V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs); StatPearls Publishing: Treasure Island, FL, USA, 2019.
- Di Bella, S.; Luzzati, R.; Principe, L.; Zerbato, V.; Meroni, E.; Giuffrè, M.; Crocè, L.S.; Merlo, M.; Perotto, M.; Dolso, E.; et al. Aspirin and Infection: A Narrative Review. Biomedicines 2022, 10, 263.
- Menter, D.G.; Bresalier, R.S. An Aspirin a Day: New Pharmacological Developments and Cancer Chemoprevention. Annu. Rev. Pharmacol. Toxicol. 2022, 63, 165–186.
- Malkowski, M.G. The Cyclooxygenases. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–18.
- Kirschenbaum, A.; Klausner, A.P.; Lee, R.; Unger, P.; Yao, S.; Liu, X.H.; Levine, A.C. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology 2000, 56, 671–676.
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386.
- Morita, I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002, 68, 165–175.
- Lucotti, S.; Cerutti, C.; Soyer, M.; Gil-Bernabé, A.M.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C.; et al. Aspirin blocks formation of metastatic intravascular niches by inhibiting platelet-derived COX-1/thromboxane A 2. J. Clin. Investig. 2019, 129, 1845–1862.
- Fujita, H.; Koshida, K.; Keller, E.T.; Takahashi, Y.; Yoshimito, T.; Namiki, M.; Mizokami, A. Cyclooxygenase-2 promotes prostate cancer progression. Prostate 2002, 53, 232–240.
- Bilani, N.; Bahmad, H.; Abou-Kheir, W. Prostate cancer and aspirin use: Synopsis of the proposed molecular mechanisms. Front. Pharmacol. 2017, 8, 145.
- Rauzi, F.; Kirkby, N.S.; Edin, M.L.; Whiteford, J.; Zeldin, D.C.; Mitchell, J.A.; Warner, T.D. Aspirin inhibits the production of proangiogenic 15 (S)-HETE by platelet cyclooxygenase-1. FASEB J. 2016, 30, 4256–4266.
- Kashiwagi, E.; Shiota, M.; Yokomizo, A.; Itsumi, M.; Inokuchi, J.; Uchiumi, T.; Naito, S. Prostaglandin receptor EP3 mediates growth inhibitory effect of aspirin through androgen receptor and contributes to castration resistance in prostate cancer cells. Endocr. Relat. Cancer 2013, 20, 431–441.
- Olivan, M.; Rigau, M.; Colás, E.; Garcia, M.; Montes, M.; Sequeiros, T.; Regis, L.; Celma, A.; Planas, J.; Placer, J.; et al. Simultaneous treatment with statins and aspirin reduces the risk of prostate cancer detection and tumorigenic properties in prostate cancer cell lines. BioMed Res. Int. 2015, 2015, 762178.
- Shiff, S.J.; Koutsos, M.I.; Qiao, L.; Rigas, B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: Effects on cell cycle and apoptosis. Exp. Cell Res. 1996, 222, 179–188.
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28.
- Sutmuller, R.; Garritsen, A.; Adema, G.J. Regulatory T cells and toll-like receptors: Regulating the regulators. Ann. Rheum. Dis. 2007, 66 (Suppl. S3), iii91–iii95.
- Hurwitz, L.M.; Kulac, I.; Gumuskaya, B.; Valle, J.A.B.D.; Benedetti, I.; Pan, F.; Liu, J.O.; Marrone, M.T.; Arnold, K.B.; Goodman, P.J.; et al. Use of Aspirin and Statins in Relation to Inflammation in Benign Prostate Tissue in the Placebo Arm of the Prostate Cancer Prevention TrialAspirin and Statin Use and Intraprostatic Inflammation. Cancer Prev. Res. 2020, 13, 853–862.
- Sharma, S.; Yang, S.C.; Zhu, L.; Reckamp, K.; Gardner, B.; Baratelli, F.; Huang, M.; Batra, R.K.; Dubinett, S.M. Tumor cyclooxygenase-2/prostaglandin E2–dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005, 65, 5211–5220.
- Hurwitz, L.M.; Joshu, C.E.; Barber, J.R.; Prizment, A.E.; Vitolins, M.Z.; Jones, M.R.; Folsom, A.R.; Han, M.; Platz, E.A. Aspirin and Non-Aspirin NSAID Use and Prostate Cancer Incidence, Mortality, and Case Fatality in the Atherosclerosis Risk in Communities StudyAspirin and Prostate Cancer Incidence and Mortality. Cancer Epidemiol. Biomark. Prev. 2019, 8, 563–569.
- Downer, M.K.; Allard, C.B.; Preston, M.A.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; Batista, J.L. Regular aspirin use and the risk of lethal prostate cancer in the physicians’ health study. Eur. Urol. 2017, 72, 821–827.
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of ibuprofen. Oman Med. J. 2010, 25, 155.
- Raegg, C.; Dormond, O. Suppression of tumor angiogenesis by nonsteroidal anti-inflammatory drugs: A new function for old drugs. Sci. World J. 2001, 1, 808–811.
- Andrews, J.; Djakiew, D.; Krygier, S.; Andrews, P. Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer Chemother. Pharmacol. 2002, 50, 277–284.
- Arisan, E.D.; Akar, R.O.; Rencuzogullari, O.; Yerlikaya, P.O.; Gurkan, A.C.; Akın, B.; Dener, E.; Kayhan, E.; Unsal, N.P. The molecular targets of diclofenac differs from ibuprofen to induce apoptosis and epithelial mesenchymal transition due to alternation on oxidative stress management p53 independently in PC3 prostate cancer cells. Prostate Int. 2019, 7, 156–165.
- Espinosa-Cano, E.; Huerta-Madronal, M.; Camara-Sanchez, P.; Seras-Franzoso, J.; Schwartz, S., Jr.; Abasolo, I.; San Román, J.; Aguilar, M.R. Hyaluronic acid (HA)-coated naproxen-nanoparticles selectively target breast cancer stem cells through COX-independent pathways. Mater. Sci. Eng. C 2021, 124, 112024.
- Mohammed, A.; Janakiram, N.B.; Madka, V.; Zhang, Y.; Singh, A.; Biddick, L.; Li, Q.; Lightfoot, S.; Steele, V.E.; Lubet, R.A.; et al. Intermittent Dosing Regimens of Aspirin and Naproxen Inhibit Azoxymethane-Induced Colon Adenoma Progression to Adenocarcinoma and Invasive CarcinomaAspirin and Naproxen Dosing Regimens for Prevention of CRC. Cancer Prev. Res. 2019, 12, 751–762.
- Suh, N.; Reddy, B.S.; DeCastro, A.; Paul, S.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Janakiram, N.B.; et al. Combination of Atorvastatin with Sulindac or Naproxen Profoundly Inhibits Colonic Adenocarcinomas by Suppressing the p65/β-Catenin/Cyclin D1 Signaling Pathway in RatsAtorvastatin, with Sulindac or Naproxen, Inhibits Colon Cancer. Cancer Prev. Res. 2011, 4, 1895–1902.
- Kim, M.S.; Kim, J.E.; Lim, D.Y.; Huang, Z.; Chen, H.; Langfald, A.; Lubet, R.A.; Grubbs, C.J.; Dong, Z.; Bode, A.M. Naproxen Induces Cell-Cycle Arrest and Apoptosis in Human Urinary Bladder Cancer Cell Lines and Chemically Induced Cancers by Targeting PI3KNaproxen Targets PI3K to Prevent Urinary Bladder Cancer. Cancer Prev. Res. 2014, 7, 236–245.
- Zrieki, A.; Farinotti, R.; Buyse, M. Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm. Res. 2008, 25, 1991–2001.
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Diclofenac as an anti-cancer agent. Ecancermedicalscience 2016, 10, 610.
- Barden, J.; Edwards, J.; Moore, R.; McQuay, H. Single dose oral diclofenac for postoperative pain. Cochrane Database Syst. Rev. 2004, CD004768.
- Johnsen, J.I.; Lindskog, M.; Ponthan, F.; Pettersen, I.; Elfman, L.; Orrego, A.; Sveinbjörnsson, B.; Kogner, P. NSAIDs in neuroblastoma therapy. Cancer Lett. 2005, 228, 195–201.
- Lanas, A. Nonsteroidal antiinflammatory drugs and cyclooxygenase inhibition in the gastrointestinal tract: A trip from peptic ulcer to colon cancer. Am. J. Med. Sci. 2009, 338, 96–106.
- Valle, B.L.; D’Souza, T.; Becker, K.G.; Wood, W.H., III; Zhang, Y.; Wersto, R.P.; Morin, P.J. Non-steroidal anti-inflammatory drugs decrease E2F1 expression and inhibit cell growth in ovarian cancer cells. PLoS ONE 2013, 8, e61836.
- Mayorek, N.; Naftali-Shani, N.; Grunewald, M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS ONE 2010, 5, e12715.
- Lea, M.A.; Sura, M.; Desbordes, C. Inhibition of cell proliferation by potential peroxisome proliferator-activated receptor (PPAR) gamma agonists and antagonists. Anticancer. Res. 2004, 24, 2765–2772.
- Adamson, D.J.; Frew, D.; Tatoud, R.; Wolf, C.R.; Palmer, C.N. Diclofenac antagonizes peroxisome proliferator-activated receptor-γ signaling. Mol. Pharmacol. 2002, 61, 7–12.
- Gebril, S.M.; Ito, Y.; Shibata, M.; Maemura, K.; Abu-Dief, E.E.; Hussein, M.R.A.; Abdelaal, U.M.; Elsayed, H.M.; Otsuki, Y.; Higuchi, K. Indomethacin can induce cell death in rat gastric parietal cells through alteration of some apoptosis-and autophagy-associated molecules. Int. J. Exp. Pathol. 2020, 101, 230–247.
- Sun, S.-Q.; Gu, X.; Gao, X.-S.; Li, Y.; Yu, H.; Xiong, W.; Yu, H.; Wang, W.; Li, Y.; Teng, Y.; et al. Overexpression of AKR1C3 significantly enhances human prostate cancer cells resistance to radiation. Oncotarget 2020, 7, 48050, Erratum in Oncotarget 2020, 11, 1575.
- Liu, C.; Lou, W.; Zhu, Y.; Yang, J.C.; Nadiminty, N.; Gaikwad, N.W.; Evans, C.P.; Gao, A.C. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015, 75, 1413–1422.
- Hamid, A.R.A.H.; Pfeiffer, M.J.; Verhaegh, G.W.; Schaafsma, E.; Brandt, A.; Sweep, F.C.G.J.; Sedelaar, J.P.M.; Schalken, J.A. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol. Med. 2012, 18, 1449–1455.
- Liedtke, A.J.; Adeniji, A.O.; Chen, M.; Byrns, M.C.; Jin, Y.; Christianson, D.W.; Marnett, L.J.; Penning, T.M. Development of potent and selective indomethacin analogues for the inhibition of AKR1C3 (type 5 17β-hydroxysteroid dehydrogenase/prostaglandin F synthase) in castrate-resistant prostate cancer. J. Med. Chem. 2013, 56, 2429–2446.
- Cai, C.; Chen, S.; Ng, P.; Bubley, G.J.; Nelson, P.S.; Mostaghel, E.A.; Marck, B.; Matsumoto, A.M.; Simon, N.I.; Wang, H.; et al. Intratumoral De Novo Steroid Synthesis Activates Androgen Receptor in Castration-Resistant Prostate Cancer and Is Upregulated by Treatment with CYP17A1 InhibitorsProstate Cancer Resistance to CYP17A1 Inhibitors. Cancer Res. 2011, 71, 6503–6513.
- Cimolai, N. The potential and promise of mefenamic acid. Expert Rev. Clin. Pharmacol. 2013, 6, 289–305.
- Armagan, G.; Turunc, E.; Kanit, L.; Yalcin, A. Neuroprotection by mefenamic acid against D-serine: Involvement of oxidative stress, inflammation and apoptosis. Free. Radic. Res. 2012, 46, 726–739.
- Asanuma, M.; Nishibayashi-Asanuma, S.; Miyazaki, I.; Kohno, M.; Ogawa, N. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J. Neurochem. 2001, 76, 1895–1904.
- Patel, S.S.; Tripathi, R.; Chavda, V.K.; Savjani, J.K. Anticancer Potential of Mefenamic Acid Derivatives with Platelet-Derived Growth Factor Inhibitory Property. Anticancer Agents Med. Chem. 2020, 20, 998–1008.
- Hosseinimehr, S.J.; Nobakht, R.; Ghasemi, A.; Pourfallah, T.A. Radioprotective effect of mefenamic acid against radiation-induced genotoxicity in human lymphocytes. Radiat. Oncol. J. 2015, 33, 256.
- Seyyedi, R.; Amiri, F.T.; Farzipour, S.; Mihandoust, E.; Hosseinimehr, S.J. Mefenamic acid as a promising therapeutic medicine against colon cancer in tumor-bearing mice. Med. Oncol. 2022, 39, 18.
- Čeponytė, U.; Paškevičiūtė, M.; Petrikaitė, V. Comparison of NSAIDs activity in COX-2 expressing and non-expressing 2D and 3D pancreatic cancer cell cultures. Cancer Manag. Res. 2018, 10, 1543.
- Woo, D.H.; Han, I.-S.; Jung, G. Mefenamic acid-induced apoptosis in human liver cancer cell-lines through caspase-3 pathway. Life Sci. 2004, 75, 2439–2449.
- Soriano-Hernández, A.D.; Galvan-Salazar, H.R.; Montes-Galindo, D.A.; Rodriguez-Hernandez, A.; Martinez-Martinez, R.; Guzman-Esquivel, J.; Valdez-Velazquez, L.L.; Baltazar-Rodriguez, L.M.; Espinoza-Gómez, F.; Rojas-Martinez, A.; et al. Antitumor effect of meclofenamic acid on human androgen-independent prostate cancer: A preclinical evaluation. Int. Urol. Nephrol. 2012, 44, 471–477.
- Melnikov, V.; Tiburcio-Jimenez, D.; A Mendoza-Hernandez, M.; Delgado-Enciso, J.; De-Leon-Zaragoza, L.; Guzman-Esquivel, J.; Rodriguez-Sanchez, I.P.; Martinez-Fierro, M.L.; Lara-Esqueda, A.; Delgado-Enciso, O.G.; et al. Improve cognitive impairment using mefenamic acid non-steroidal anti-inflammatory therapy: Additional beneficial effect found in a controlled clinical trial for prostate cancer therapy. Am. J. Transl. Res. 2021, 13, 4535.
- Quiñones, O.G.; Pierre, M.B. Cutaneous application of celecoxib for inflammatory and cancer diseases. Curr. Cancer Drug Targets 2019, 19, 5–16.
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Nowaszewska, B.K.; Celińska-Janowicz, K.; Miltyk, W. Celecoxib in cancer therapy and prevention–review. Curr. Drug Targets 2019, 20, 302–315.
- Benson, P.; Yudd, M.; Sims, D.; Chang, V.; Srinivas, S.; Kasimis, B. Renal effects of high-dose celecoxib in elderly men with stage D2 prostate carcinoma. Clin. Nephrol. 2012, 78, 376–381.
- Atari-Hajipirloo, S.; Nikanfar, S.; Heydari, A.; Kheradmand, F. Imatinib and its combination with 2,5-dimethyl-celecoxibinduces apoptosis of human HT-29 colorectal cancer cells. Res. Pharm. Sci. 2017, 12, 67–73.
- Atari-Hajipirloo, S.; Nikanfar, S.; Heydari, A.; Noori, F.; Kheradmand, F. The effect of celecoxib and its combination with imatinib on human HT-29 colorectal cancer cells: Involvement of COX-2, Caspase-3, VEGF and NF-κB genes expression. Cell. Mol. Biol. 2016, 62, 68–74.
- Mohammadian, M.; Zeynali, S.; Azarbaijani, A.F.; Ansari, M.H.K.; Kheradmand, F. Cytotoxic effects of the newly-developed chemotherapeutic agents 17-AAG in combination with oxaliplatin and capecitabine in colorectal cancer cell lines. Res. Pharm. Sci. 2017, 12, 517.
- Nikanfar, S.; Atari-Hajipirloo, S.; Kheradmand, F.; Rashedi, J.; Heydari, A. Cytotoxic effect of 2, 5-dimethyl-celecoxib as a structural analog of celecoxib on human colorectal cancer (HT-29) cell line. Cell. Mol. Biol. 2018, 64, 8–13.
- Nikanfar, S.; ATARI-HAJIPIRLOO, S.; KHERADMAND, F.; HEYDARI, A. Imatinib Synergizes with 2, 5-Dimethylcelecoxib, a Close Derivative of Celecoxib, in HT-29 Colorectal Cancer Cells: Involvement of Vascular Endothelial Growth Factor. J. Res. Pharm. 2023, 27, 948–956.
- Zielinski, S.L. Despite positive studies, popularity of chemoprevention drugs increasing slowly. J. Natl. Cancer Inst. 2004, 96, 1410–1412.
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000, 342, 1946–1952.
- Henney, J.E. Celecoxib indicated for FAP. JAMA 2000, 283, 1131.
- Smith, M.R.; Manola, J.; Kaufman, D.S.; Oh, W.K.; Bubley, G.J.; Kantoff, P.W. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J. Clin. Oncol. 2006, 24, 2723–2728.
- Yellepeddi, V.K.; Radhakrishnan, J.; Radhakrishnan, R. Penetration and pharmacokinetics of non-steroidal anti-inflammatory drugs in rat prostate tissue. Prostate 2018, 78, 80–85.
- Brizzolara, A.; Benelli, R.; Venè, R.; Barboro, P.; Poggi, A.; Tosetti, F.; Ferrari, N. The ErbB family and androgen receptor signaling are targets of Celecoxib in prostate cancer. Cancer Lett. 2017, 400, 9–17.
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847.
- Fernandes, D.C.; Norman, A.J. Drug-induced gastrointestinal disorders. Medicine 2019, 47, 301–308.
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131.
- Masso Gonzalez, E.L.; Patrignani, P.; Tacconelli, S.; Rodríguez, L.A.G. Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum. 2010, 62, 1592–1601.
- Antonucci, R.; Cuzzolin, L.; Arceri, A.; Dessì, A.; Fanos, V. Changes in urinary PGE 2 after ibuprofen treatment in preterm infants with patent ductus arteriosus. Eur. J. Clin. Pharmacol. 2009, 65, 223–230.
- Horbach, S.J.; Lopes, R.D.; Guaragna, J.C.D.C.; Martini, F.; Mehta, R.H.; Petracco, J.B.; Bodanese, L.C.; Adauto Filho, C.; Cirenza, C.; de Paola, A.A.; et al. Naproxen as prophylaxis against atrial fibrillation after cardiac surgery: The NAFARM randomized trial. Am. J. Med. 2011, 124, 1036–1042.
- Varga, Z.; rafay ali Sabzwari, S.; Vargova, V.; Sabzwari, S.R.A. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: An under-recognized public health issue. Cureus 2017, 9, e1144.
- Fanelli, A.; Ghisi, D.; Aprile, P.L.; Lapi, F. Cardiovascular and cerebrovascular risk with nonsteroidal anti-inflammatory drugs and cyclooxygenase 2 inhibitors: Latest evidence and clinical implications. Ther. Adv. Drug Saf. 2017, 8, 173–182.
- Vonkeman, H.E.; van de Laar, M.A. (Eds.) Nonsteroidal Anti-Inflammatory Drugs: Adverse Effects and Their Prevention; Seminars in arthritis and rheumatism; Elsevier: Amsterdam, The Netherlands, 2010.
- Lanas, A.; Hunt, R. Prevention of anti-inflammatory drug-induced gastrointestinal damage: Benefits and risks of therapeutic strategies. Ann. Med. 2006, 38, 415–428.
