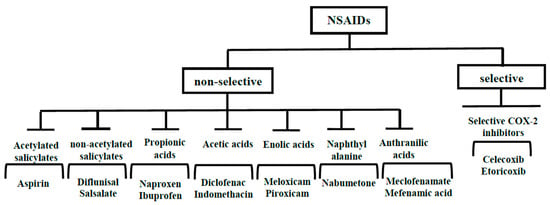
In clinical practice, aspirin (ASP) is widely used to reduce the risks of cardiovascular and cerebrovascular ischemia [
24]. Numerous pharmacological, clinical, and epidemiological studies have demonstrated the protective effect of ASP against several types of cancer [
25]. Cyclooxygenases (COX), the targets of NSAIDs, play a key role in homeostasis, inflammation, and immune modulation [
26]. Increased expression of COX-1 and COX-2 has been observed in PC [
27]. Additionally, increased production of thromboxane A2 (TXA2) and prostaglandins has been associated with the progression of PC [
28]. COX-1 induces platelet aggregation and facilitates the adhesion of cancer cells to endothelial cells during metastasis [
29,
30]. COX-2 responds to inflammatory cytokines such as IL-1α, IL-1β, TNF-α, and lipopolysaccharide and produces increased amounts of prostaglandins in inflamed tissue. COX-2 also causes an increase in the expression of Bcl-2 in PC [
18,
31]. The inhibition of COX-1 and COX-2 are among the primary mechanisms through which ASP is thought to prevent cell growth [
32]. ASP at low doses irreversibly inhibits COX-1 activity in platelets [
33]. Inhibition of the COX-1/TXA2 pathway in platelets reduces platelet aggregation in tumor cells, endothelial activation, adhesion of tumor cells to endothelium, recruitment of metastasis-promoting monocytes/macrophages, and formation of the pre-metastatic niche in prostate tissue. High doses of ASP could inhibit COX-2 [
30], resulting in the prevention of the production of prostanoids (TXA2, PGF2, PGE2, PGI2, and PGD2), which play a role in reducing apoptosis and increasing cell proliferation. It has been shown that PGE2 levels in human malignant PC tissues are 10 times higher than their levels in benign prostate tissues. PGE2 acts through receptors coupled with four G proteins named EP1, EP2, EP3, and EP4. EP3 has anti-tumor properties that can be induced by ASP. Therefore, it has been reported that ASP could exert its anti-PC properties through the activation of EP3 [
34]. In another study, it was reported that ASP increased tumor necrosis factor-related ligand in PC cells by decreasing the expression of survivin, a member of the family of apoptosis-inhibiting proteins [
32]. Studies have shown that ASP could inhibit the cell cycle by blocking cyclin-dependent kinases (CDKs) and causing cell cycle arrest in G0/G1 [
35,
36]. A retrospective case-control study demonstrated that treatment of PC cells with a combination of statin and ASP reduced both cyclin D1 expression and cell proliferation [
35]. Regulatory T cells (Treg) prevent T cells from generating an effective antitumor response through immune system suppression [
37,
38]. A study examined the use of aspirin and statins in relation to inflammation in benign prostate tissue and revealed that aspirin could lead to a decrease in regulatory T cells [
39]. COX-2/PGE2 inhibition has also been shown to reduce Treg cell activity in mouse lung cancer models [
40]. In a cohort study analyzing 6594 men, ASP (low-dose < 300 mg, regular-strength 300–499 mg, extra-strength ≥ 500 mg) use was inversely associated with PC mortality [
41]. Additionally, a prospective study reported that regular use of aspirin (325 mg, >3 d/wk for ≥1 yr) was associated with a reduction in the risk of lethal PC [
42].
Ibuprofen (IBN), the most common NSAID, is typically administered to treat mild to moderate pain associated with a variety of conditions such as dysmenorrhea, headache, migraine, dental pain, and more [
43]. IBN has also been reported to be effective in the prevention and treatment of certain cancers including colon, breast, lung, and gastric [
44]. Several studies have been conducted to investigate the effects of IBN on PC. An in vitro study conducted in 2002 suggested that IBN had greater apoptotic and anti-proliferative effects on hormone-responsive cell lines (LNCaP and DU-145) compared to other NSAIDs including acetaminophen, ASP, naproxen, and NS-398 [
45]. It was also reported that the anti-cancer effect of IBN treatment on PC3 and PC3 p53 +/+ cells was through cell cycle arrest at the G1/S stage, apoptosis induction via upregulation of caspase-3 and caspase-9, and inhibition of metastasis by upregulating E-cadherin [
46].
2.3. Naproxen
Naproxen (NAP) has protective effects against various cancers such as bladder, breast, and colorectal [
54]. Its anti-cancerous activities are due to the upregulation of p21, p53, caspase-3, IL-10 and downregulation of PCNA, CDK4, cyclin D1, and inflammatory molecules such as iNOS, TNF-α, IL-1β, IL-4, IL-6, and IL-12 [
55,
56,
57]. Additionally, NAP suppressed PGE2, which is a key factor in tumor progression [
55]. P-glycoprotein is another target of NAP [
58]. NAP exerted apoptosis by initiating the cleavage of caspase-3/7, PARP-1, and inhibiting PI3K, and Bcl-2 [
57].
2.4. Diclofenac
Diclofenac (DCF) is widely used as an anti-inflammatory agent in degenerative joint disease and rheumatoid arthritis [
68]. Additionally, DCF is a potent analgesic drug used in physical injuries and post-surgery [
69]. Various in vitro and in vivo studies confirmed the anti-cancer effects of DCF in some cancer types including neuroblastoma [
70], colon [
71], ovarian [
72], and pancreatic [
73]. Regarding PC, one of the important studies about the anticancer effects of DCF was conducted by Arisan et al., reporting that DCF could elicit endothelial-mesenchymal transition in PC3 and PC3 p53 +/+ cells through ROS generation, upregulation of Snail, N-cadherin, and vimentin together with downregulation of E-cadherin without affecting p53. It could also arrest the cell cycle at G2/M, induce apoptosis through upregulating Fas, caspase-3, and caspase-9 as well as tumor suppressor genes (Bax, Bak, and Puma). DCF downregulated Bcl-x and Mcl-1 as well [
46]. Additionally, DCF as a PPARγ antagonist in combination with rosiglitazone showed an additive inhibitory effect on thymidine incorporation into DNA in PC3 cells, whereas DCF antagonized the inhibitory effect of rosiglitazone on DU-145 cells [
74,
75].
2.5. Indomethacin
Indomethacin (IND) currently has therapeutic efficacy against severe osteoarthritis, rheumatoid arthritis, gouty arthritis, or ankylosing spondylitis [
79].
AKR1C3 is an enzyme that is involved in the biosynthesis of potent androgens such as testosterone and dihydrotestosterone (DHT) and also catalyzes the conversion of PGH2 to PGF2α, which is crucial for PC cells to proliferate [
80,
81]. AKR1C3 has been reported to be elevated in CRPC patients and is considered as a therapeutic target in these patients [
82]. It is proposed that inhibition of this bifunctional enzyme may be useful in both androgen-sensitive and androgen-insensitive conditions [
83].
DU-145 overexpressing AKR1C3 cells have been shown to resist radiation therapy by enhancing the MAPK signaling pathway and inhibiting the PPARγ pathway. IND suppressed the resistance of these cells to irradiation by inhibiting AKR1C3 [
80]. IND in DuCaP cells promoted apoptosis by increasing activated caspases-3 and -7 [
82]. IND significantly decreased PSA mRNA and protein levels in VCaP cells [
84].
2.6. Mefenamic Acid
Mefenamic acid (MFA) is an NSAID that relieves dental and menstrual pain and is typically administered orally [
86]. This drug has a significant protective effect against increasing lipid peroxidation, protein oxidation, TNF-α and IL-1β levels, and ultimately reduces inflammation [
87,
88]. In addition, it can reduce cancer cell proliferation, progression, angiogenesis, and invasion [
89]. It has also been shown that MFA in combination with ionizing radiation increases apoptosis in tumor tissues and protects against DNA damage caused by ionizing radiation [
90,
91]. MFA is a class of NSAIDs that has high antiproliferative activity, whereas salicylates, the most common drugs used in clinical studies, show a relatively weak antiproliferative effect [
92]. MFA has been reported to act as a signaler for apoptosis by inhibiting calcium uptake in cells. On the other hand, it causes apoptosis by cleaving procaspase-3 and PARP-1 [
93]. MFA was found to be effective in the treatment of PC in in vitro and in vivo models [
94]. As mentioned earlier, inflammation is an essential component of PC, and it has been reported that MFA reduces its biochemical progression [
95].
2.7. Celecoxib
Celecoxib (CXB), a member of NSAIDs that specifically inhibits cyclooxygenase-2 (COX-2), has been proposed for the treatment of several neoplasms, including prostate, colorectal, breast, lung, stomach, head and neck cancers, as well as for the prevention of prostate, colorectal, breast, lung and skin cancers [
98,
99,
100,
101,
102,
103,
104,
105]. Studies have shown that CXB is effective in treating familial adenomatous polyposis, osteoarthritis, rheumatoid arthritis, primary dysmenorrhea and acute pain [
106,
107,
108,
109]. In comparison to other NSAIDs such as diclofenac, ibuprofen and naproxen, CXB has demonstrated greater absorption into the prostate in an animal study and is more suitable for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) [
64]. Additionally, CXB has shown greater cytotoxicity when compared to other cyclooxygenase inhibitors [
110].
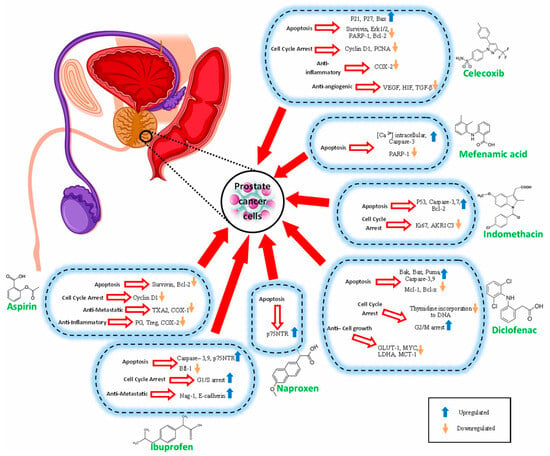
The possible anti-prostate cancer mechanisms of NSAIDs that have been discussed in various investigations are summarized in Table 1 and Figure 2.