Worldwide, the number of revisions to total knee arthroplasty procedures is increasing. Revision surgery is a challenging procedure, required for the management of bone loss after removal of the first implant. Although further long-term follow-up studies are needed, the use of cones in revisions of total knee arthroplasty yields reliability in fixation and stability to restore joint lines, especially in challenging surgeries with poor bone stock. The introduction of 3D-printed cones in revision surgery seems to be advantageous for AORI type III bone defects, especially in reducing intraoperative complications and procedure times.
- revision surgery
- total knee arthroplasty
- bone loss
1. Introduction
2. Bone Defects: Classification System
| AORI Classification | Type of Lesion | Treatment Options |
|---|---|---|
| Type I | Limited to cancellous bone Metaphysis intact |
Cement, cement with screws, autograft or allografts |
| Type II | Metaphysis damaged | Allograft, modular metal augments, porous metallic cones, sleeves |
| Type IIa | Involves one femoral or tibial condyle | |
| Type IIb | Involves both femoral or tibial condyles | |
| Type III | Metaphysis severely deficient; bone loss that comprises a major portion of the condyle or plateau |
Allograft, metallic cones and sleeves, mega-prosthesis, modular stems |
3. Treatment Options

4. Metallic Cones and Materials
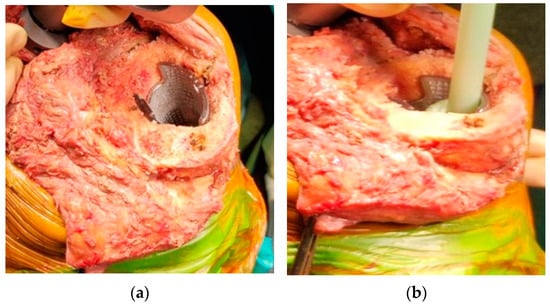
5. Surgical Technique
This entry is adapted from the peer-reviewed paper 10.3390/prosthesis5040082
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330.
- Romanini, E.; Decarolis, F.; Luzi, I.; Zanoli, G.; Venosa, M.; Laricchiuta, P.; Carrani, E.; Torre, M. Total knee arthroplasty in Italy: Reflections from the last fifteen years and projections for the next thirty. Int. Orthop. (SICOT) 2019, 43, 133–138.
- Evans, J.T.; Walker, R.W.; Evans, J.P.; Blom, A.W.; Sayers, A.; Whitehouse, M.R. How long does a knee replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 655–663.
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010, 468, 57–63.
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51.
- Rosso, F.; Cottino, U.; Dettoni, F.; Bruzzone, M.; Bonasia, D.E.; Rossi, R. Revision total knee arthroplasty (TKA): Mid-term outcomes and bone loss/quality evaluation and treatment. J. Orthop. Surg. Res. 2019, 14, 280.
- Gonzalez, M.H.; Mekhail, A.O. The Failed Total Knee Arthroplasty: Evaluation and Etiology. J. Am. Acad. Orthop. Surg. 2004, 12, 436–446.
- Mancuso, F.; Beltrame, A.; Colombo, E.; Miani, E.; Bassini, F. Management of metaphyseal bone loss in revision knee arthroplasty. Acta Biomed. 2017, 88, 98–111.
- Engh, G.A. Classification of Bone Defects Femur and Tibia. In Knee Arthroplasty Handbook; Scuderi, G.R., Tria, A.J., Eds.; Springer: New York, NY, USA, 2006; pp. 116–132.
- Mulhall, K.J.; Ghomrawi, H.M.; Engh, G.A.; Clark, C.R.; Lotke, P.; Saleh, K.J. Radiographic Prediction of Intraoperative Bone Loss in Knee Arthroplasty Revision. Clin. Orthop. Relat. Res. 2006, 446, 51–58.
- Belt, M.; Smulders, K.; van Houten, A.; Wymenga, A.; Heesterbeek, P.; van Hellemondt, G. What Is the Reliability of a New Classification for Bone Defects in Revision TKA Based on Preoperative Radiographs? Clin. Orthop. Relat. Res. 2020, 478, 2057–2064.
- Stambough, J.B.; Haynes, J.A.; Barrack, R.L.; Nunley, R.M. Acetabular wedge augments for uncontained tibial plateau defects in revision total knee arthroplasty. Arthroplast. Today 2018, 4, 313–318.
- Morgan-Jones, R.; Oussedik, S.I.; Graichen, H.; Haddad, F.S. Zonal fixation in revision total knee arthroplasty. Bone Jt. J. 2015, 97-B, 147–149.
- Jang, S.J.; Flevas, D.A.; Kunze, K.N.; Anderson, C.G.; Fontana, M.A.; Boettner, F.; Sculco, T.P.; Baldini, A.; Sculco, P.K. Standardized Fixation Zones and Cone Assessments for Revision Total Knee Arthroplasty Using Deep Learning. J. Arthroplast. 2023, 38, S259–S265.
- Khan, Y.; Arora, S.; Kashyap, A.; Patralekh, M.K.; Maini, L. Bone defect classifications in revision total knee arthroplasty, their reliability and utility: A systematic review. Arch. Orthop. Trauma. Surg. 2023, 143, 453–468.
- Brooks, P.J.; Walker, P.S.; Scott, R.D. Tibial Component Fixation in Deficient Tibial Bone Stock. Clin. Orthop. Relat. Res. 1984, 184, 302–308.
- Engh, G.A.; Ammeen, D.J. Use of Structural Allograft in Revision Total Knee Arthroplasty in Knees with Severe Tibial Bone Loss. J. Bone Jt. Surg.-Am. Vol. 2007, 89, 2640–2647.
- Stevenson, S.; Li, X.Q.; Davy, D.T.; Klein, L.; Goldberg, V.M. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. Quantification of a complex process and structure. J. Bone Jt. Surg. Am. 1997, 79, 1–16.
- Sheth, N.P.; Bonadio, M.B.; Demange, M.K. Bone Loss in Revision Total Knee Arthroplasty: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2017, 25, 348–357.
- Zanirato, A.; Formica, M.; Cavagnaro, L.; Divano, S.; Burastero, G.; Felli, L. Metaphyseal cones and sleeves in revision total knee arthroplasty: Two sides of the same coin? Complications, clinical and radiological results—A systematic review of the literature. Musculoskelet. Surg. 2020, 104, 25–35.
- Matar, H.E.; Bloch, B.V.; James, P.J. Role of metaphyseal sleeves in revision total knee arthroplasty: Rationale, indications and long-term outcomes. J. Orthop. 2021, 23, 107–112.
- Christie, M.J. Clinical applications of Trabecular Metal. Am. J. Orthop. 2002, 31, 219–220.
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J. Bone Jt. Surg. Br. Vol. 1999, 81-B, 907–914.
- Lachiewicz, P.F.; Watters, T.S. Porous metal metaphyseal cones for severe bone loss: When only metal will do. Bone Jt. J. 2014, 96-B, 118–121.
- Levine, B.; Sporer, S.; Valle, C.; Jacobs, J.; Paprosky, W. Porous Tantalum in Reconstructive Surgery of the Knee—A Review. J. Knee Surg. 2010, 20, 185–194.
- Cohen, R. A porous tantalum trabecular metal: Basic science. Am. J. Orthop. 2002, 31, 216–217.
- Zardiackas, L.D.; Parsell, D.E.; Dillon, L.D.; Mitchell, D.W.; Nunnery, L.A.; Poggie, R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J. Biomed. Mater. Res. 2001, 58, 180–187.
- Balla, V.K.; Bodhak, S.; Bose, S.; Bandyopadhyay, A. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010, 6, 3349–3359.
- Schildhauer, T.A.; Peter, E.; Muhr, G.; Köller, M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J. Biomed. Mater. Res. 2009, 88A, 332–341.
- Schildhauer, T.A.; Robie, B.; Muhr, G.; Koller, M. Bacterial Adherence to Tantalum Versus Commonly Used Orthopedic Metallic Implant Materials. J. Orthop. Trauma 2006, 20, 476–484.
- Denehy, K.M.; Abhari, S.; Krebs, V.E.; Higuera-Rueda, C.A.; Samuel, L.T.; Sultan, A.A.; Mont, M.A.; Malkani, A.L. Metaphyseal Fixation Using Highly Porous Cones in Revision Total Knee Arthroplasty: Minimum Two Year Follow Up Study. J. Arthroplast. 2019, 34, 2439–2443.
- Black, J. Biologic performance of tantalum. Clin. Mater. 1994, 16, 167–173.
- Ohlmeier, M.; Lausmann, C.; Wolff, M.; Abdelaziz, H.; Gehrke, T.; Citak, M. Preliminary clinical results of coated porous tibia cones in septic and aseptic revision knee arthroplasty. Arch. Orthop. Trauma. Surg. 2021, 141, 555–560.
- Rambani, R.; Nayak, M.; Aziz, M.S.; Almeida, K. Tantalum Versus Titanium Acetabular Cups in Primary Total Hip Arthroplasty: Current Concept and a Review of the Current Literature. Arch. Bone Jt. Surg. 2022, 10, 385–394.
- Ryan, G.; Pandit, A.; Apatsidis, D. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670.
- Cherny, A.A.; Kovalenko, A.N.; Kulyaba, T.A.; Kornilov, N.N. A prospective study on outcome of patient-specific cones in revision knee arthroplasty. Arch. Orthop. Trauma. Surg. 2021, 141, 2277–2286.
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D printing metal implants in orthopedic surgery: Methods, applications and future prospects. J. Orthop. Translat. 2023, 42, 94–112.
- Meneghini, R.M.; Lewallen, D.G.; Hanssen, A.D. Use of Porous Tantalum Metaphyseal Cones for Severe Tibial Bone Loss During Revision Total Knee Replacement. J. Bone Jt. Surg.-Am. Vol. 2008, 90, 78–84.
- Radnay, C.S.; Scuderi, G.R. Management of Bone Loss: Augments, Cones, Offset Stems. Clin. Orthop. Relat. Res. 2006, 446, 83–92.
- Chalmers, B.P.; Malfer, C.M.; Mayman, D.J.; Westrich, G.H.; Sculco, P.K.; Bostrom, M.P.; Jerabek, S.A. Early Survivorship of Newly Designed Highly Porous Metaphyseal Tibial Cones in Revision Total Knee Arthroplasty. Arthroplast. Today 2021, 8, 5–10.
