Solid state batteries could potentially improve the characteristics of the conventional Li-ion batteries (capacity, charge/discharge rate, safety and sustainability) by replacing the organic electrolyte of the standard battery with a solid (crystalline, but also polymer and hybrid) electrolyte. One of the most promising solid electrolytes is Li3xLa2/3−xTiO3 (LLTO). A number of synthesis techniques have been employed for the preparation of the LLTO compounds. These can be divided on two subcategories: bulk material synthesis and nanostructured material synthesis. The first category contains primarily two methods: sol-gel method and the solid-state reaction method. Nanostructured materials are obtained by thin film deposition techniques and by electrospinning.
- solid state electrolyte
- lithium batteries
- structure–properties correlation
- lithium lanthanum titanates
1. Sol-Gel Synthesis
| Final Product | Reagents | Gel Formation | Calcination | Sintering | Ref. |
|---|---|---|---|---|---|
| pristine LLTO and Sr doped LLTO Li0.35La0.55TiO3 Li0.35La0.35Sr0.03TiO3 | Sr(NO3)2, La2O3, LiNO3, C6H8O7×H2O | 80 °C for 2 h combustion at 250 °C | 650 °C for 6 h | 1250 °C for 4 h | [9] |
| Li0.35La0.55TiO3 | Ti, HCl, C4H6O6 (tartaric acid), LiNO3, La2O3 | 90 to 120 °C | 800, 900, 1000 and 1100 °C | 1250 °C | [1] |
| Eu doped Li0.5La0.5TiO3 | Ti[OCH(CH3)2]4, LiNO3, La(NO3)3×6H2O, Eu2O3, C6H8O7 (citric acid), (CH2OH)2 (ethylene glycol) |
120 °C for one hour | 350 °C for 2 h | 800 °C for 3 h | [3] |
| Al doped LLTO (Li0.33La0.56)1.005Ti0.99Al0.01O3 |
LiNO3, La2(NO3)3×6H2O, Ti[OCH(CH3)2]4, Al(NO3)3×9H2O, (CH2)2(OH)2, C6H8O7, Li2O |
70 °C for 12 h to form a gel then heating to 100 °C to form a resin | 350 °C for 6 h + 750 °C for 3 h |
1350 °C for 6 h | [4] |
| Li0.35La0.55TiO3 | LiNO3, La(NO3)3×6H2O, Ti(OC4H9)4 |
80 °C for gel formation and drying at 150 °C | combustion at 350 °C for 4 h + calcination at 900 °C for 2 h | no sintering | [5] |
| Li0.33La0.56TiO3 | La(NO3)3×4H2O, LiNO3, Ti(OC3H7)4 |
95 °C for 2 h and 100 °C for 12 h |
combustion at 450 °C for 30 min + calcination at 800–1200 °C for 12 h |
1150 °C for 10 h | [7] |
2. Solid State Reaction
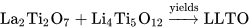
| Final Product | Reagents | Calcination | Sintering | Ref. |
|---|---|---|---|---|
| Li0.34La0.51TiO2.94 | La2O3, Li2CO3, TiO2 | 800 °C for 4 h + two heat treatments with intermediary grinding at 1150 °C for 12 h |
1350 °C for 6 h | [18] |
| Li0.33La0.56TiO3 | Li2CO3, La2O3, TiO2 | 800 °C for 8 h | 1250 °C + 1350 °C for 12 h followed by quenching |
[11] |
| Li0.33La0.56−yTiO3−3yF3y (y = 0.017, 0.05) Li0.33+3yLa0.56−yTiO3 (y = 0, 0.01, 0.02, 0.04) |
Li2CO3, La2O3, TiO2, LiF | 650 °C for 12 h | 1350 °C for 1.5 h followed by quenching | [17] |
| Li0.5−xLa0.5NaxTiO3 | Li2CO3, Na2CO3, La2O3, TiO2 | 1100 °C for 4 h | 1300–1330 °C for 6 h | [26] |
| LLTO doped with rare earths | La2O3, Li2CO3, Na2CO3, TiO2, SrCO3, BaCO3, MgO | 800 °C for 4 h, 1150–1200 °C for 6 to 12 h with intermediary grinding |
1350–1400 °C for 3 to 10 h | [30] |
| Li0.33La0.56TiO3−yFy 0 ≤ y ≤ 0.183 |
La2O3, TiO2, Li2CO3, LiF | 800 °C for 2 h | 1200 °C for 10 h | [31] |
3. Thin Films
| Method | Parameters | Observations | Ref. |
|---|---|---|---|
| e-beam evaporation | LLTO target chamber pressure 7 × 10−2 Pa O2/Ar ratio 1:2 target to substrate distance 30 cm beam power 300–600 W |
[24] | |
| PLD KrF laser 248 nm wavelength, aimed at 45° on rotating target |
LixLa2/3−xTiO3 (x = 0.1, 0.2, 0.3, 0.4, 0.5) target chamber pressure: 1 × 10−6 to 5 × 10−6 torr target substrate distance 59 mm substrate temperature: RT pulse frequency: 10 Hz laser power: 180 mJ/pulse |
amorphous film | [21] |
| RF magnetron sputtering | chamber pressure: 1 Pa O2/Ar ratio 30% O2, 70% Ar magnetron power 80 W |
[36] |
4. Electrospinning
This entry is adapted from the peer-reviewed paper 10.3390/ma16227088
References
- Diktanaitė, A.; Gaidamavičienė, G.; Kazakevičius, E.; Kežionis, A.; Žalga, A. Aqueous sol-gel synthesis, structural, thermoanalytical studies, and conductivity properties of lithium lanthanum titanate. Thermochim. Acta 2022, 715, 179268.
- Kežionis, A.; Kazakevičius, E.; Kazlauskas, S.; Žalga, A. Metal-like temperature dependent conductivity in fast Li+ ionic conductor Lithium Lanthanum Titanate. Solid State Ion. 2019, 342, 115060.
- Fernandes, S.L.; Gasparotto, G.; Teixeira, G.F.; Cebim, M.A.; Longo, E.; Zaghete, M.A. Lithium lanthanum titanate perovskite ionic conductor: Influence of europium doping on structural and optical properties. Ceram. Int. 2018, 44, 21578–21584.
- Le, H.T.; Kalubarme, R.S.; Ngo, D.T.; Jang, S.Y.; Jung, K.N.; Shin, K.H.; Park, C.J. Citrate gel synthesis of aluminum-doped lithium lanthanum titanate solid electrolyte for application in organic-type lithi-um-oxygen batteries. J. Power Sources 2015, 274, 1188–1199.
- Liang, Y.; Ji, L.; Guo, B.; Lin, Z.; Yao, Y.; Li, Y.; Alcoutlabi, M.; Qiu, Y.; Zhang, X. Preparation and electrochemical characterization of ionic-conducting lithium lanthanum titanate oxide/polyacrylonitrile submicron composite fiber-based lithium-ion battery separators. J. Power Sources 2011, 196, 436–441.
- Abhilash, K.; Selvin, P.C.; Nalini, B.; Somasundaram, K.; Sivaraj, P.; Bose, A.C. Study of the temperature dependent transport properties in nanocrystalline lithium lanthanum titanate for lithium ion batteries. J. Phys. Chem. Solids 2016, 91, 114–121.
- Bohnke, C.; Regrag, B.; Leberre, F.; Fourquet, J.-L.; Randrianantoandro, N. Comparison of pH sensitivity of lithium lanthanum titanate obtained by sol–gel synthesis and solid-state chemistry. Solid State Ion. 2005, 176, 73–80.
- Le, H.T.; Ngo, D.T.; Kim, Y.-J.; Park, C.-N.; Park, C.-J. A perovskite-structured aluminium-substituted lithium lanthanum titanate as a potential artificial solid-electrolyte interface for aqueous rechargeable lithium-metal-based batteries. Electrochim. Acta 2017, 248, 232–242.
- Zhang, S.; Zhao, H.; Guo, J.; Du, Z.; Wang, J.; Świerczek, K. Characterization of Sr-doped lithium lanthanum titanate with improved transport properties. Solid State Ion. 2019, 336, 39–46.
- Yang, T.; Li, Y.; Chan, C.K. Enhanced lithium ion conductivity in lithium lanthanum titanate solid electrolyte nanowires prepared by electrospinning. J. Power Sources 2015, 287, 164–169.
- Borštnar, P.; Žuntar, J.; Spreitzer, M.; Dražič, G.; Daneu, N. Exaggerated grain growth and the development of coarse-grained microstructures in lithium lanthanum titanate perovskite ceramics. J. Eur. Ceram. Soc. 2023, 43, 1017–1027.
- Hua, C.; Fang, X.; Wang, Z.; Chen, L. Lithium storage in perovskite lithium lanthanum titanate. Electrochem. Commun. 2013, 32, 5–8.
- Lalena, J.N.; Cleary, D.A.; Carpenter, E.E.; Dean, N.F. Solid-solid reactions. In Inorganic Materials Synthesis and Fabrication; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 183–209.
- Shui, F.; Li, Z.; Zhao, X. Effect of annealing temperature on the transparent lithium strontium lanthanum titanate thin films. Chem. Phys. Lett. 2020, 750, 137496.
- Schröckert, F.; Schiffmann, N.; Bucharsky, E.C.; Schell, K.G.; Hoffmann, M.J. Tape casted thin films of solid electrolyte Lithium-Lanthanum-Titanate. Solid State Ion. 2018, 328, 25–29.
- Takatori, K.; Saura, K.; Orum, A.; Kadoura, H.; Tani, T. Textured lithium lanthanum titanate polycrystals prepared by a reactive-templated grain growth method. J. Eur. Ceram. Soc. 2016, 36, 551–558.
- Okumura, T.; Yokoo, K.; Fukutsuka, T.; Uchimoto, Y.; Saito, M.; Amezawa, K. Improvement of Li-ion conductivity in A-site disordering lithium-lanthanum-titanate perovskite oxides by adding LiF in synthesis. J. Power Sources 2009, 189, 536–538.
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693.
- Zhou, X.; Gao, C.; Wang, D.; Peng, S.; Huang, L.; Yang, W.; Zhang, W.-H.; Gao, X. Revealing the dominant factor of domain boundary resistance on bulk conductivity in lanthanum lithium titanates. J. Energy Chem. 2022, 73, 354–359.
- Inaguma, Y.; Nakashima, M. A rechargeable lithium–air battery using a lithium ion-conducting lanthanum lithium titanate ceramics as an electrolyte separator. J. Power Sources 2013, 228, 250–255.
- Furusawa, S.-I.; Tabuchi, H.; Sugiyama, T.; Tao, S.; Irvine, J.T. Ionic conductivity of amorphous lithium lanthanum titanate thin film. Solid State Ion. 2005, 176, 553–558.
- Mori, K.; Tomihira, S.; Iwase, K.; Fukunaga, T. Visualization of conduction pathways in a lanthanum lithium titanate superionic conductor synthesized by rapid cooling. Solid State Ion. 2014, 268, 76–81.
- Tang, H.; Zan, L.; Zhu, J.; Ma, Y.; Zhao, N.; Tang, Z. High rate capacity nanocomposite lanthanum oxide coated lithium zinc titanate anode for rechargeable lithium-ion battery. J. Alloys Compd. 2016, 667, 82–90.
- Li, C.-L.; Zhang, B.; Fu, Z.-W. Physical and electrochemical characterization of amorphous lithium lanthanum titanate solid electrolyte thin-film fabricated by e-beam evaporation. Thin Solid Films 2006, 515, 1886–1892.
- Reda, A. Effect of ZnO on sintering and microwave dielectric properties of 0.5CaTiO3-0.5 (Li0.5La0.5) TiO3 ceramics. J. Indian Chem. Soc. 2023, 100, 100901.
- V’yunov, O.; Plutenko, T.; Fedorchuk, O.; Belous, A.; Lobko, Y. Synthesis and dielectric properties in the lithium-ion conducting material La0.5Li0.5−xNaxTiO3. J. Alloys Compd. 2021, 889, 161556.
- Salami, T.J.; Imanieh, S.H.; Lawrence, J.G.; Martin, I. Amorphous glass-perovskite composite as solid electrolyte for lithium-ion battery. Mater. Lett. 2019, 254, 294–296.
- Maqueda, O.; Sauvage, F.; Laffont, L.; Martínez-Sarrión, M.; Mestres, L.; Baudrin, E. Structural, microstructural and transport properties study of lanthanum lithium titanium perovskite thin films grown by Pulsed Laser Deposition. Thin Solid Films 2008, 516, 1651–1655.
- Diaz-Moreno, C.A.; Ding, Y.; Portelles, J.; Heiras, J.; Macias, A.H.; Syeed, A.; Paez, A.; Li, C.; López, J.; Wicker, R. Optical properties of ferroelectric lanthanum lithium niobate. Ceram. Int. 2018, 44, 4727–4733.
- Inaguma, Y.; Chen, L.; Itoh, M.; Nakamura, T. Candidate Compounds with Perovskite Structure for High Lithium Ionic conductivity. Solid State Ion. 1994, 70–71, 196–202.
- Li, J.; Wen, Z.; Xu, X.; Zhu, X. Lithium-ion conduction in the anion substituted La2/3–xLi3x–yTiO3–yFy electrolyte with perovskite-type structure. Solid State Ion. 2005, 176, 2269–2273.
- Deng, Y.; Xu, X.; Zhang, L.; Du, F.; Liu, Q.; Chen, J.; Meng, K.; Wu, Y.; Yang, M.; Jiang, Y. Lithium incorporation enhanced resistive switching behaviors in lithium lanthanum titanium oxide-based heterostructure. J. Mater. Sci. Technol. 2022, 128, 142–147.
- Bohnke, O.; Lorant, S.; Roffat, M.; Berger, P. Fast H+/Li+ ion exchange in Li0.30La0.57TiO3 nanopowder and films in water and in ambient air. Solid State Ion. 2014, 262, 563–567.
- Abhilash, K.; Sivaraj, P.; Selvin, P.; Nalini, B.; Somasundaram, K. Investigation on spin coated LLTO thin film nano-electrolytes for rechargeable lithium ion batteries. Ceram. Int. 2015, 41, 13823–13829.
- Aguesse, F.; Roddatis, V.; Roqueta, J.; García, P.; Pergolesi, D.; Santiso, J.; Kilner, J.A. Microstructure and ionic conductivity of LLTO thin films: Influence of different substrates and excess lithium in the target. Solid State Ion. 2015, 272, 1–8.
- Xiong, Y.; Tao, H.; Zhao, J.; Cheng, H.; Zhao, X. Effects of annealing temperature on structure and opt-electric properties of ion-conducting LLTO thin films prepared by RF magnetron sputtering. J. Alloys Compd. 2011, 509, 1910–1914.
- Na Lee, Y.; Yoon, Y.S. Cycle stability increase by insertion of Li–La–Ta–O thin-film electrolyte between cathode and solid electrolyte for all-solid-state battery. Thin Solid Films 2015, 579, 75–80.
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings—Science, Applications and Technology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–902.
- Ohring, M. Materials Science of Thin Films, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1–781.
- Lv, L.; Zhang, X.; Wang, J.; Yuan, L.; Fan, J. Construction of Li0.5La0.5TiO3 (LLTO)-In2O3 n-n step scheme heterostructure nanorods for drastically heightening the sensing behavior in H2S gas. Mater. Chem. Phys. 2023, 295, 127085.
