BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) derivatives have attracted attention as probes in applications like imaging and sensing due to their unique properties like (1) strong absorption and emission in the visible and near-infrared regions of the electromagnetic spectrum, (2) strong fluorescence and (3) supreme photostability. They have also been employed in areas like photodynamic therapy. Over the last decade, BODIPY-based molecules have even emerged as candidates for cancer treatments. Cancer remains a significant health issue world-wide, necessitating a continuing search for novel therapeutic options. BODIPY is a flexible fluorophore with distinct photophysical characteristics and is a fascinating drug development platform.
- BODIPY
- BODIPY dyes
- fluorescent probe
- BODIPY-based functional materials
1. Introduction
BODIPY derivatives have been used not only in biological applications but also in other fields [1]. Due to their high fluorescence quantum yields and exceptional thermal and chemical durability, they have been used as fluorescent dyes in optoelectronic devices, including organic light-emitting diodes (OLEDs) [2] and organic photovoltaics (OPVs) [3]. Additionally, BODIPY-based sensors have also been utilized for environmental monitoring, the detection of pollutants, and food quality control [4]. Derivatives of BODIPY are frequently used in biological imaging [5]. They are excellent for observing cellular structures and processes because of their fluorescent characteristics. They can be used to identify certain biomolecules, like proteins or nucleic acids, and monitor their dynamics and localization inside cells [6]. BODIPY derivatives can also be used as sensors for different analytes, including ions, pH, reactive oxygen species, and enzymatic activities, providing real-time monitoring of biochemical processes in living systems [7]. Researchers have investigated BODIPY-based molecules for imaging and diagnostic uses, but their usage as pharmaceuticals for cancer treatment is still in its infancy [8]. BODIPY-based chemicals in photodynamic therapy (PDT) for cancer have shown potential improvement. Reactive oxygen species (ROS), capable of specifically killing cancer cells, are created by activating light-sensitive substances/photosensitizers in PDT [9]. BODIPY dyes have potent photostability and their beneficial photophysical characteristics render them effective photosensitizers in PDT [10]. Researchers have developed substances based on BODIPY that accumulate specifically in cancer cells and cause lethal effects when triggered by light [11]. These substances can be coupled with molecules that target cancer cells to increase their selectivity towards those cells while minimizing damage to healthy tissues [12][13]. Additionally, BODIPY dyes can be engineered to emit fluorescence, allowing for real-time monitoring of their distribution and therapeutic effects [14]. Overall, because of their wide range of uses, adjustable fluorescence characteristics, and photostability, BODIPY derivatives have gained a lot of attention as fluorescent probes.
2. Properties of BODIPY-Based Compounds
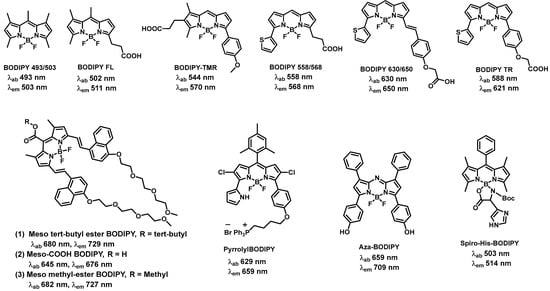
2.1. BODIPY-Based Fluorescent Compounds
2.2. BODIPY Probes Capable of Detecting and Tracking Amyloid-β Aggregates and Structural Changes
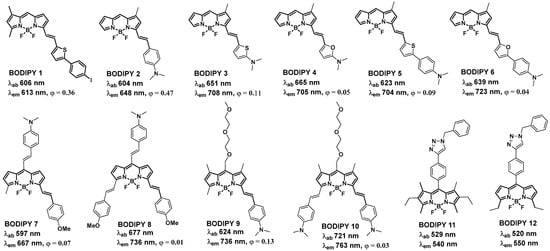
BODIPY Probes for Monitoring Aggregation and Conformational Changes of Amyloids
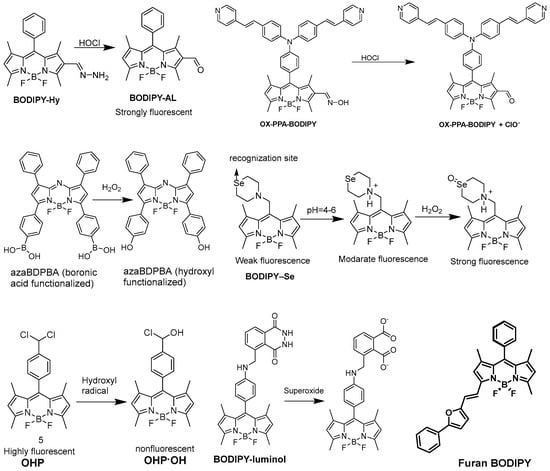
2.3. Detection of Reactive Oxygen Species
2.4. BODIPY-Based Probes in Cancer Detection
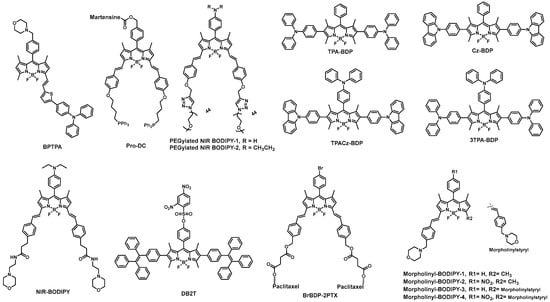
2.5. BODIPY Derivatives for PDT and PTT Applications
3. Summary
This entry is adapted from the peer-reviewed paper 10.3390/biom13121723
References
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Synthesis, optoelectronic, and transistor properties of BODIPY-and cyclopenta thiophene-containing π-conjugated copolymers. J. Phys. Chem. C 2015, 119, 15859–15867.
- Chapran, M.; Angioni, E.; Findlay, N.J.; Breig, B.; Cherpak, V.; Stakhira, P.; Tuttle, T.; Volyniuk, D.; Grazulevicius, J.V.; Nastishin, Y.A. An ambipolar BODIPY derivative for a white exciplex OLED and cholesteric liquid crystal laser toward multifunctional devices. ACS Appl. Mater. Interfaces 2017, 9, 4750–4757.
- Ho, D.; Ozdemir, R.; Kim, H.; Earmme, T.; Usta, H.; Kim, C. BODIPY—based semiconducting materials for organic bulk heterojunction photovoltaics and thin—film transistors. ChemPlusChem 2019, 84, 18–37.
- Wang, F.; Zhang, X.; Huangfu, C.; Zhu, M.; Li, C.; Feng, L. The BODIPY-based chemosensor for the fluorometric determination of organochlorine pesticide dicofol. Food Chem. 2022, 370, 131033.
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366.
- Li, F.-Z.; Wu, Z.; Lin, C.; Wang, Q.; Kuang, G.-C. Photophysical properties regulation and applications of BODIPY-based derivatives with electron donor-acceptor system. Results Chem. 2022, 4, 100384.
- Dai, J.; Ma, C.; Zhang, P.; Fu, Y.; Shen, B. Recent progress in the development of fluorescent probes for detection of biothiols. Dye. Pigment. 2020, 177, 108321.
- Gao, D.; Zhang, B.; Liu, Y.; Hu, D.; Sheng, Z.; Zhang, X.; Yuan, Z. Molecular engineering of near-infrared light-responsive BODIPY-based nanoparticles with enhanced photothermal and photoacoustic efficiencies for cancer theranostics. Theranostics 2019, 9, 5315.
- Li, M.; Tian, R.; Fan, J.; Du, J.; Long, S.; Peng, X. A lysosome-targeted BODIPY as potential NIR photosensitizer for photodynamic therapy. Dye. Pigment. 2017, 147, 99–105.
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. Int. J. Mol. Sci. 2022, 23, 10198.
- Bertrand, B.; Passador, K.; Goze, C.; Denat, F.; Bodio, E.; Salmain, M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coord. Chem. Rev. 2018, 358, 108–124.
- Debnath, S.; Hao, G.; Guan, B.; Thapa, P.; Hao, J.; Hammers, H.; Sun, X. Theranostic small-molecule prodrug conjugates for targeted delivery and controlled release of toll-like receptor 7 agonists. Int. J. Mol. Sci. 2022, 23, 7160.
- Amendoeira, A.F.; Luz, A.; Valente, R.; Roma-Rodrigues, C.; Ali, H.; van Lier, J.E.; Marques, F.; Baptista, P.V.; Fernandes, A.R. Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes. Int. J. Mol. Sci. 2023, 24, 3600.
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396.
- Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Piskorz, J.; Bocian, S.; Ziegler-Borowska, M.; Kędziera, D.; Kaczmarek-Kędziera, A. Photochemical properties and stability of BODIPY dyes. Int. J. Mol. Sci. 2021, 22, 6735.
- Squeo, B.M.; Ganzer, L.; Virgili, T.; Pasini, M. BODIPY-based molecules, a platform for photonic and solar cells. Molecules 2020, 26, 153.
- Kumar, N.R.; Agrawal, A.R.; Debnath, S.; Choudhury, A.; Zade, S.S. Effect of connectivity variation in azulene-BODIPY triads and their optoelectronic properties. New J. Chem. 2023, 47, 2456–2463.
- Duan, X.; Li, P.; Li, P.; Xie, T.; Yu, F.; Tang, B. The synthesis of polarity-sensitive fluorescent dyes based on the BODIPY chromophore. Dye. Pigment. 2011, 89, 217–222.
- Glavaš, M.; Zlatić, K.; Jadreško, D.; Ljubić, I.; Basarić, N. Fluorescent pH sensors based on BODIPY structure sensitive in acidic media. Dye. Pigment. 2023, 220, 111660.
- Listenberger, L.L.; Brown, D.A. Fluorescent detection of lipid droplets and associated proteins. Curr. Protoc. Cell Biol. 2007, 35, 24.2.1–24.2.11.
- Kurata, S.; Kanagawa, T.; Yamada, K.; Torimura, M.; Yokomaku, T.; Kamagata, Y.; Kurane, R. Fluorescent quenching-based quantitative detection of specific DNA/RNA using a BODIPY® FL-labeled probe or primer. Nucleic Acids Res. 2001, 29, e34.
- Guseva, G.B.; Antina, E.V.; Berezin, M.B.; Pavelyev, R.S.; Kayumov, A.R.; Ostolopovskaya, O.V.; Gilfanov, I.R.; Frolova, L.L.; Kutchin, A.V.; Akhverdiev, R.F. Design, spectral characteristics, and possibilities for practical application of BODIPY FL-labeled monoterpenoid. ACS Applied Bio. Mater. 2021, 4, 6227–6235.
- Clardy, S.M.; Mohan, J.F.; Vinegoni, C.; Keliher, E.J.; Iwamoto, Y.; Benoist, C.; Mathis, D.; Weissleder, R. Rapid, high efficiency isolation of pancreatic ß-cells. Sci. Rep. 2015, 5, 13681.
- Brubaker, K.D.; Mao, F.; Gay, C.V. Localization of carbonic anhydrase in living osteoclasts with bodipy 558/568-modified acetazolamide, a thiadiazole carbonic anhydrase inhibitor. J. Histochem. Cytochem. 1999, 47, 545–550.
- Herbert, S.M.; Leung, T.L.; Bishop, P.J. Fluorescent probes as a tool for labelling and tracking the amphibian chytrid fungus Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2011, 96, 169–174.
- Rae, J.; Fontaine, F.; Salim, A.A.; Lo, H.P.; Capon, R.J.; Parton, R.G.; Martin, S. High-throughput screening of Australian marine organism extracts for bioactive molecules affecting the cellular storage of neutral lipids. PLoS ONE 2011, 6, e22868.
- Dale, C.L.; Hill, S.J.; Kellam, B. New potent, short-linker BODIPY-630/650™ labelled fluorescent adenosine receptor agonists. MedChemComm 2012, 3, 333–338.
- Kok, Z.Y.; Stoddart, L.A.; Mistry, S.J.; Mocking, T.A.; Vischer, H.F.; Leurs, R.; Hill, S.J.; Mistry, S.N.; Kellam, B. Optimization of Peptide Linker-Based Fluorescent Ligands for the Histamine H1 Receptor. J. Med. Chem. 2022, 65, 8258–8288.
- Ni, Y.; Zeng, L.; Kang, N.Y.; Huang, K.W.; Wang, L.; Zeng, Z.; Chang, Y.T.; Wu, J. meso—Ester and carboxylic acid substituted BODIPYs with far—red and near—infrared emission for bioimaging applications. Chem. A Eur. J. 2014, 20, 2301–2310.
- Miao, W.; Guo, X.; Yan, X.; Shang, Y.; Yu, C.; Dai, E.; Jiang, T.; Hao, E.; Jiao, L. Red-to-Near-Infrared Emitting PyrrolylBODIPY Dyes: Synthesis, Photophysical Properties and Bioimaging Application. Chem. A Eur. J. 2023, 29, e202203832.
- Pino, Y.C.; Aguilera, J.A.; Garcia-Gonzalez, V.; Alatorre-Meda, M.; Rodríguez-Velázquez, E.; Espinoza, K.A.; Frayde-Gomez, H.; Rivero, I.A. Synthesis of Aza-BODIPYs, Their Differential Binding for Cu (II), and Results of Bioimaging as Fluorescent Dyes of Langerhans β-Cells. ACS Omega 2022, 7, 42752–42762.
- Pavliukeviciene, B.; Zentelyte, A.; Jankunec, M.; Valiuliene, G.; Talaikis, M.; Navakauskiene, R.; Niaura, G.; Valincius, G. Amyloid β oligomers inhibit growth of human cancer cells. PLoS ONE 2019, 14, e0221563.
- Jin, W.-S.; Bu, X.-L.; Liu, Y.-H.; Shen, L.-L.; Zhuang, Z.-Q.; Jiao, S.-S.; Zhu, C.; Wang, Q.-H.; Zhou, H.-D.; Zhang, T. Plasma amyloid-beta levels in patients with different types of cancer. Neurotox. Res. 2017, 31, 283–288.
- Qin, H.; Cui, T.; Liu, Z.; Zhou, Y.; Niu, J.; Ren, J.; Qu, X. Engineering amyloid aggregation as a new way to eliminate cancer stem cells by the disruption of iron homeostasis. Nano Lett. 2021, 21, 7379–7387.
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56.
- Smith, N.W.; Alonso, A.; Brown, C.M.; Dzyuba, S.V. Triazole-containing BODIPY dyes as novel fluorescent probes for soluble oligomers of amyloid Aβ1–42 peptide. Biochem. Biophys. Res. Commun. 2010, 391, 1455–1458.
- Sozmen, F.; Kolemen, S.; Kumada, H.-O.; Ono, M.; Saji, H.; Akkaya, E.U. Designing BODIPY-based probes for fluorescence imaging of β-amyloid plaques. RSC Adv. 2014, 4, 51032–51037.
- Ono, M.; Ishikawa, M.; Kimura, H.; Hayashi, S.; Matsumura, K.; Watanabe, H.; Shimizu, Y.; Cheng, Y.; Cui, M.; Kawashima, H. Development of dual functional SPECT/fluorescent probes for imaging cerebral β-amyloid plaques. Bioorganic Med. Chem. Lett. 2010, 20, 3885–3888.
- Ono, M.; Watanabe, H.; Kimura, H.; Saji, H. BODIPY-based molecular probe for imaging of cerebral β-amyloid plaques. ACS Chem. Neurosci. 2012, 3, 319–324.
- Sutharsan, J.; Dakanali, M.; Capule, C.C.; Haidekker, M.A.; Yang, J.; Theodorakis, E.A. Rational design of amyloid binding agents based on the molecular rotor motif. ChemMedChem Chem. Enabling Drug Discov. 2010, 5, 56–60.
- Jameson, L.P.; Smith, N.W.; Dzyuba, S.V. Dye-binding assays for evaluation of the effects of small molecule inhibitors on amyloid (Aβ) self-assembly. ACS Chem. Neurosci. 2012, 3, 807–819.
- Smith, N.W.; Annunziata, O.; Dzyuba, S.V. Amphotericin B interactions with soluble oligomers of amyloid Aβ1-42 peptide. Bioorganic Med. Chem. 2009, 17, 2366–2370.
- Tonali, N.; Dodero, V.I.; Kaffy, J.; Hericks, L.; Ongeri, S.; Sewald, N. Real—Time BODIPY—Binding Assay To Screen Inhibitors of the Early Oligomerization Process of Aβ1-42 Peptide. ChemBioChem 2020, 21, 1129–1135.
- Wang, E.; Qiao, H.; Zhou, Y.; Pang, L.; Yu, F.; Zhang, J.; Ma, T. A novel “turn-on” fluorogenic probe for sensing hypochlorous acid based on BODIPY. RSC Adv. 2015, 5, 73040–73045.
- Xu, J.; Zhai, J.; Xu, Y.; Zhu, J.; Qin, Y.; Jiang, D. A near-infrared fluorescent aza-bodipy probe for dual-wavelength detection of hydrogen peroxide in living cells. Analyst 2016, 141, 2380–2383.
- Xu, C.; Qian, Y. A selenamorpholine-based redox-responsive fluorescent probe for targeting lysosome and visualizing exogenous/endogenous hydrogen peroxide in living cells and zebrafish. J. Mater. Chem. B 2019, 7, 2714–2721.
- Zhang, P.-L.; Wang, Z.-K.; Chen, Q.-Y.; Du, X.; Gao, J. Biocompatible G-Quadruplex/BODIPY assembly for cancer cell imaging and the attenuation of mitochondria. Bioorganic Med. Chem. Lett. 2019, 29, 1943–1947.
- Quan, Y.-Y.; Fan, L.; Shen, H.; Wu, B.; Kong, S.; Luo, Y.; Huang, Z.-S.; Ye, X. A multifunctional BODIPY based fluorescent probe for hydrogen sulfide detection and photodynamic anticancer therapy in HCT116 colon cancer cell. Dye. Pigment. 2022, 197, 109897.
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-BODIPY-based nanomedicines in cancer phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925.
