Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The vomeronasal system (VNS) or accessory olfactory system is specialized in detecting chemical signals, primarily pheromones, kairomones, and molecules from the major histocompatibility complex.
- chemical communication
- olfactory systems
- vomeronasal organ
- accessory olfactory bulb
1. Introduction
Mammalian chemical communication, a significant and complex domain of study, fundamentally hinges upon the vomeronasal system’s ability to sense pheromone-mediated interactions. This system, beyond its basal functions, profoundly influences various social and sexual behaviors such as reproduction, hierarchical dynamics, maternal bonding, and species-specific recognition. What becomes particularly compelling is the pronounced evolutionary variability the vomeronasal system displays; more so when juxtaposed against the variations observed within the olfactory system. Such adaptive diversities present both challenges and opportunities in research, amplifying the call for an in-depth examination of its neuroanatomy and the nuances of its functional morphology.
2. Vomeronasal System
The vomeronasal system (VNS) or accessory olfactory system is specialized in detecting chemical signals, primarily pheromones, kairomones, and molecules from the major histocompatibility complex. It consists of a set of anatomically and histologically distinguishable structures from the main olfactory system. It is present in most reptiles [1][2] and amphibians [3], but it is particularly developed in mammals, in which this chemosensory system comprises three main components: the vomeronasal organ (VNO) (Figure 1), which acts as the peripheral chemoreceptor organ detecting chemical signals; the vomeronasal nerve, transmitting information to the brain; and the accessory olfactory bulb (AOB), the first neural center where vomeronasal afferent information is processed and integrated before heading to specific areas of the CNS [4].
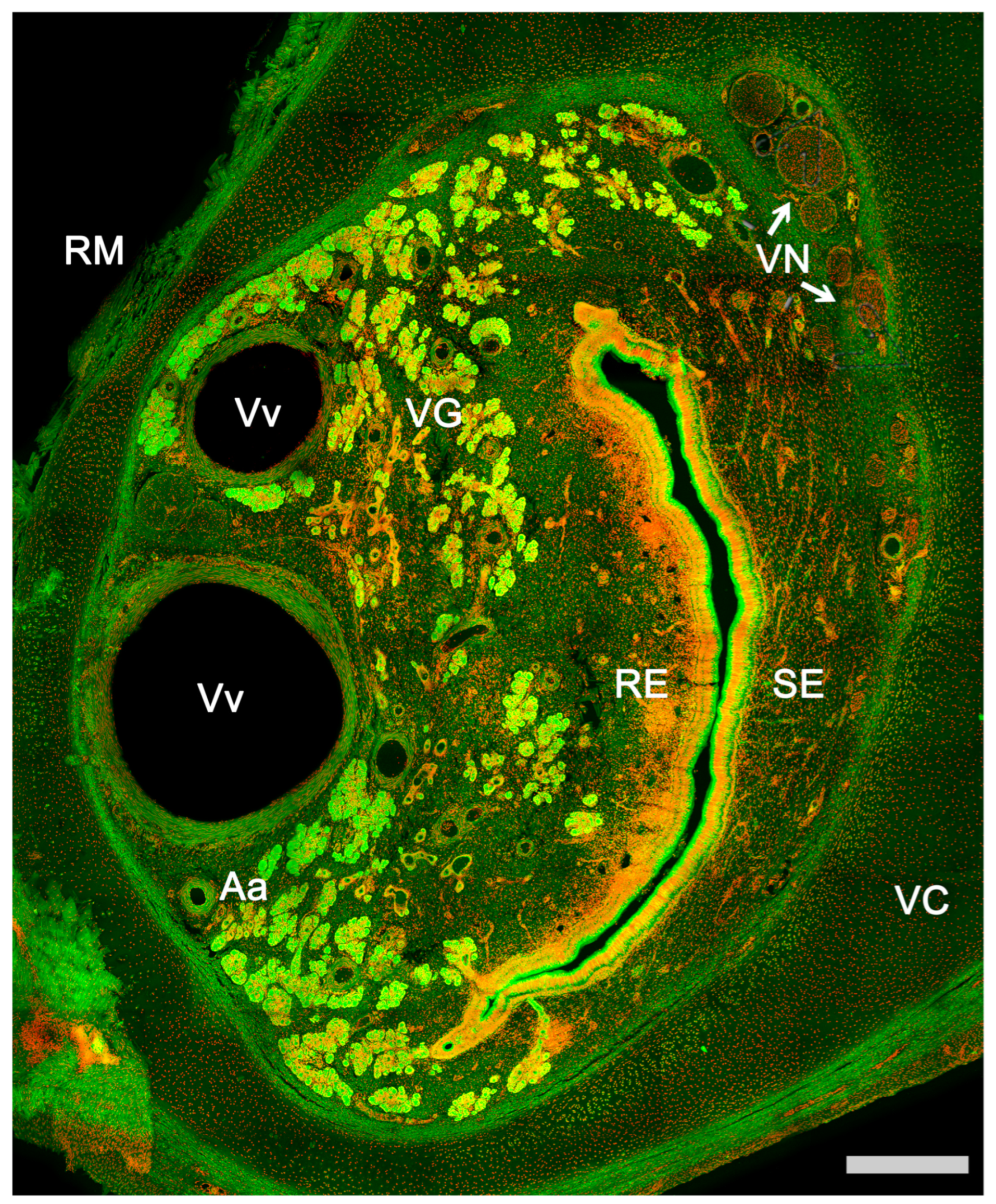
Figure 1. Vomeronasal organ of a horse. Autofluorescence transversal section of the VNO in which all components of the organ are identified. Aa, Artery; RE, Respiratory epithelium; RM, Respiratory mucosa; SE, Sensory epithelium; VC, Vomeronasal cartilage; VG, Vomeronasal glands; VN, Vomeronasal nerves; Vv, Veins. Nuclear contrast: TOPRO-3 iodide. Scale bar: 250 μm.
The significant functional diversity of the vomeronasal system is expressed in the existence of three distinct subpopulations of vomeronasal sensory neurons. Each is associated with a specific family of chemosensory receptors: vomeronasal type 1 receptors (V1R), vomeronasal type 2 receptors (V2R), and formyl peptide receptors (FPR) [5]. However, it is crucial to note that not all mammalian species have functional receptors in all three families, indicating a diversity in chemical signal detection and specialized adaptation based on the biological and ecological needs of each species. This phenomenon underscores the richness and versatility of the VNS in detecting and processing chemical signals, highlighting the importance of chemical communication in mammalian life.
Identifying these receptor families was essential, and the study of G-proteins was crucial. Initially, by analyzing the expression of Gαo and Gαi2 proteins in rats, it was observed that the nerve endings of the vomeronasal neuroreceptor cells of the AOB were organized into two complementary regions [6]. Subsequently, both G-proteins’ involvement in the transduction chain of vomeronasal neurons was determined, and the V1R receptor family in mice was discovered [7]. At that time, the expression pattern of the V1R receptors matched that of the Gαi2 protein. However, the second family of vomeronasal receptors was not identified until two years later, when three separate studies examining the Gαo protein expression in the VNO simultaneously demonstrated the existence of the second vomeronasal receptor family, V2R [8][9][10]. Finally, the third family of vomeronasal receptors, formylated peptide receptors (FPR), which coexpress with both G-proteins (Gαi2 and Gαo), was identified [11]. On the other hand, each neuronal population maintains a specific projection pattern to the AOB. Despite the fewer types of vomeronasal receptors compared to olfactory receptors, the vomeronasal receptor neurons’ projection pattern to multiple AOB glomeruli seems to be more complex than those in the primary olfactory system [12].
There is a general consensus that the VNS is primarily responsible for perceiving pheromones, although it also perceives other non-pheromonal chemical signals, such as kairomones, which mediate defensive behavior [13], and other types of chemical signals vital for tracking prey and attack behavior, as seen in reptiles [14] and urodele amphibians [15]. In certain mammals, such as the gray short-tailed opossum, the VNS also influences food preference [16].
Chemical stimuli found in urine deposits, vaginal secretions, odorous gland secretions, or saliva can be investigated by direct contact. However, specific behaviors are also used to facilitate the entry of non-volatile substances into the VNO, such as facial grooming and “flehmen”. The “flehmen” behavior is seen in ungulates and felines and consists of adopting a specific facial posture with the head tilted back, the mouth slightly open, the upper lip everted, and the neck extended for a few seconds [17][18]. It usually occurs after contact with biological secretions from conspecifics, and males exhibit this behavior more frequently [19][20].
Historical interest in the vomeronasal system began with the discovery of the VNO by the Danish anatomist Ludvig Levis Jacobson in the early 19th century, who described its key macroscopic features in a broad range of non-human mammals [21]. While previous illustrations showed the supposed location of the VNO in human nasal septum drawings [22], Jacobson reported this structure absence in Homo sapiens. However, the human VNO was later discovered in embryos [23] and a detailed histological description was then carried out both in fetuses and adults [24]. Although Jacobson also contemplated the hypothesis of a possible sensory function of the organ, he mainly suggested a secretory role. The histological contributions of Balogh [25], Klein [26], and Piana [27] revived the hypothesis of the sensory function, but only by the end of the 19th century did the availability of the Golgi technique definitively show the morphological similarity of the neurons of the olfactory and vomeronasal epithelium of the snake, thus establishing the sensory function of the VNO [28]. On the other hand, approximately half a century after the discovery of the VNO, the AOB was identified in sheep by Balogh [25] using traceability and dissection of the vomeronasal nerve. However, the term AOB was coined by Von Gudden [29] following his studies on the vomeronasal nerves in rabbits. Later on, Santiago Ramón y Cajal provided a detailed, accurate, and specific description of the AOB in various mammals, revealing its laminar architecture and the presence of different cell types [30]. It was the North American neuroanatomist Rollo McCotter [31] who established, in a broad range of species, the different nature of the olfactory and vomeronasal nerves and their respective destinations in the MOB and AOB. Finally, in the second half of the 20th century, a clear relationship between the VNS and reproductive behavior was established [32][33] leading to the seminal work of Powers and Winans [34] which convincingly demonstrated the critical role that the VNO plays in rodent reproduction.
Evolutionarily, the vomeronasal system has been linked to the transition of vertebrates to terrestrial environments; however, recent evidence suggests that a precursor VNS exists in teleost fish and its evolutionary origin predates the divergence between teleosts and tetrapods [35][36]. Added to this is the unique case of several species of lungfish that have a vomeronasal system homologous to mammals, showcasing a defined vomeronasal organ and an accessory olfactory bulb [37][38][39][40]. Hence, the significance of chemical communication has been a constant throughout the evolutionary history of vertebrates, resulting in significant morphofunctional variations among the chemosensory systems of different species. Specifically, the shift from aquatic to terrestrial life led to changes that significantly impacted pheromonal communication in vertebrates. This arose from the transformation of its key chemical property from solubility to volatility, a process that altered pheromone release mechanisms, accompanied by morphological and physiological changes in the sensory organs [41].
Subsequently, some terrestrial animal species returned to aquatic environments, such as cetaceans, which underwent drastic changes in their olfactory morphology during this migration. While their terrestrial relatives, including hippos, exhibit a defined VNO [42][43], no VNO has been found in any cetacean [44]. In contrast, toothed whales also lost their main olfactory system [45]. However, sea snakes, which evolved from terrestrial tetrapod reptiles, feature a functional and well-developed underwater VNS, while losing their main olfactory system [46]. In snakes, the vomeronasal system is predominantly considered to be the major chemosensory system [47][48][49][50]. The failure of their olfactory system to adapt to aquatic life, in contrast to the successful adaptation of their vomeronasal system, underscores the importance and development of the VNS in these reptiles. Other primarily aquatic reptiles, such as sea turtles, have a well-developed VNS [51]. However, while some alligator and crocodile embryos show a VNS, it regresses to be absent in adulthood [52][53]. Yet, most reptiles [54] and amphibians [55] have a functional VNS.
Regarding airborne vertebrates, there is no evidence of pheromonal communication in most birds due to the absence of their VNS [56]. The same is observed in many bats, though certain species possess a particularly well-developed VNS [57]. Finally, the vast majority of terrestrial mammals have a functional VNS, and some, like rodents, lagomorphs, or marsupials, have an especially developed VNS. Semi-aquatic mammals like the capybara [58], hippopotamus [59], beaver [60], and platypus also exhibit a VNS [61].
Among primates, it is believed that the last common ancestor with a functional vomeronasal system might have been small, arboreal, and nocturnal [62][63]. Without adequate light, vision is limited, heightening the reliance on olfactory signals [64]. Presently, primates can be classified into strepsirrhines and haplorhines, based on the presence or absence of a rhinarium: a moist, hairless skin area around the nostrils seen in some mammals. Strepsirrhines, which include lemurs and lorises, possess a rhinarium and are nocturnal, in addition to having a highly developed VNS [65]. On the other hand, haplorhines lack a rhinarium and are mostly diurnal. They include New World monkeys or platyrrhines, and Old World monkeys or catarrhines, among which are the great apes and humans. Regarding the vomeronasal system, platyrrhine monkeys have a well-developed VNO [66]. In parallel, the VNO of catarrhine primates has been generally considered absent; however, a rudimentary VNO in the postnatal stage has been observed in certain chimpanzee and human individuals [67]. Postnatal chimpanzees possess bilateral, ciliated epithelial tubes in the anteroinferior portion of the nasal septum. Both species are similar in possessing a relatively superiorly positioned VNO, which lacks the clear sensory epithelium seen in prosimians and New World primates [68]. It is of utmost importance that further investigations ascertain whether the VNO is retained in other apes. In the meantime, the presence of the VNO in adult Old World primates continues to pose a phylogenetic challenge.
In humans, the VNO undergoes significant development in the early gestational stages, exhibiting a pronounced neural projection from the organ to the olfactory bulb. A salient marker expressed during this period is the luteinizing hormone-releasing hormone (LHRH) [69]. However, a continuous projection from the vomeronasal nerve to the olfactory bulb beyond the 14–28 week period has not been found [70], rendering the function of the human VNO ambiguous. In fact, an accessory olfactory bulb has not been clearly identified since the description by Tryphena Humphrey in 1940 [71], which has not been replicated.
In adult humans, the proper VNO structure is retained in the majority of individuals. The bipolar cells within the human VNO exhibit structural similarities to olfactory receptor cells. Still, there is limited information concerning the histological configuration of its epithelium (VNE). Electron microscopic observations suggest that sections of the duct have a highly specialized epithelium similar to a chemoreceptive organ [72][73]. Yet, the epithelium does not appear to express neuronal features like OMP or PGP 9.5 reactivity, leading to concerns about the functionality of the human VNO. Conversely, the vomeroranasal epithelium displays a distinct arrangement of cell adhesion molecules, distinct from the adjacent nasal epithelia, which could indicate specific chemosensory roles [74]. Consequently, the VNE emerges as a specialized structure with an enigmatic function. Finally, the presence of a nerve connection from the VNO to a presumptive accessory olfactory bulb in adult humans has not been detected, so it remains a major issue in this debate [75][76][77].
2.1. Anatomy of the Vomeronasal Organ
The vomeronasal organ comprises two tubular structures located bilaterally at the base of the anterior nasal septum. Both organs have a single point of communication with the exterior which, depending on the species, can either be located in the nasopalatine or incisive duct—a conduit that connects the oral and nasal cavities through the palatine fissure—or directly in the nasal cavity [78].
Each organ consists of two clearly differentiated elements: the vomeronasal duct, which forms the lumen of the organ and is lined by a pseudostratified columnar epithelium, and the vomeronasal capsule, a rigid and protective envelope of either bony or cartilaginous nature, depending on the species [79]. Associated with the vomeronasal duct is the parenchyma: a tissue responsible for the organ function, consisting of an accumulation of soft tissue associated with the duct, rich in glands, vessels, nerves, and connective tissue [80].
Upon making a transverse cut in the central part of the organ, the typical crescent shape of the duct lumen at that level is observed. Internally, the duct is lined throughout its surface by two distinguishable epithelia. On its lateral side, it has a pseudostratified and ciliated respiratory epithelium, while on its medial part, the vomeronasal sensory epithelium (Figure 2) is located [81]. This sensory epithelium consists of a thin layer of basal cells, a broad central layer of bipolar neuroreceptor cells, and an outer layer of supporting cells that sustain the dendritic processes of the neuroreceptor cells (Figure 3). These dendritic processes project towards the lumen. At this level, they form microvilli that contain the vomeronasal receptors, which are responsible for recognizing the molecules involved in chemocommunication [82][83].
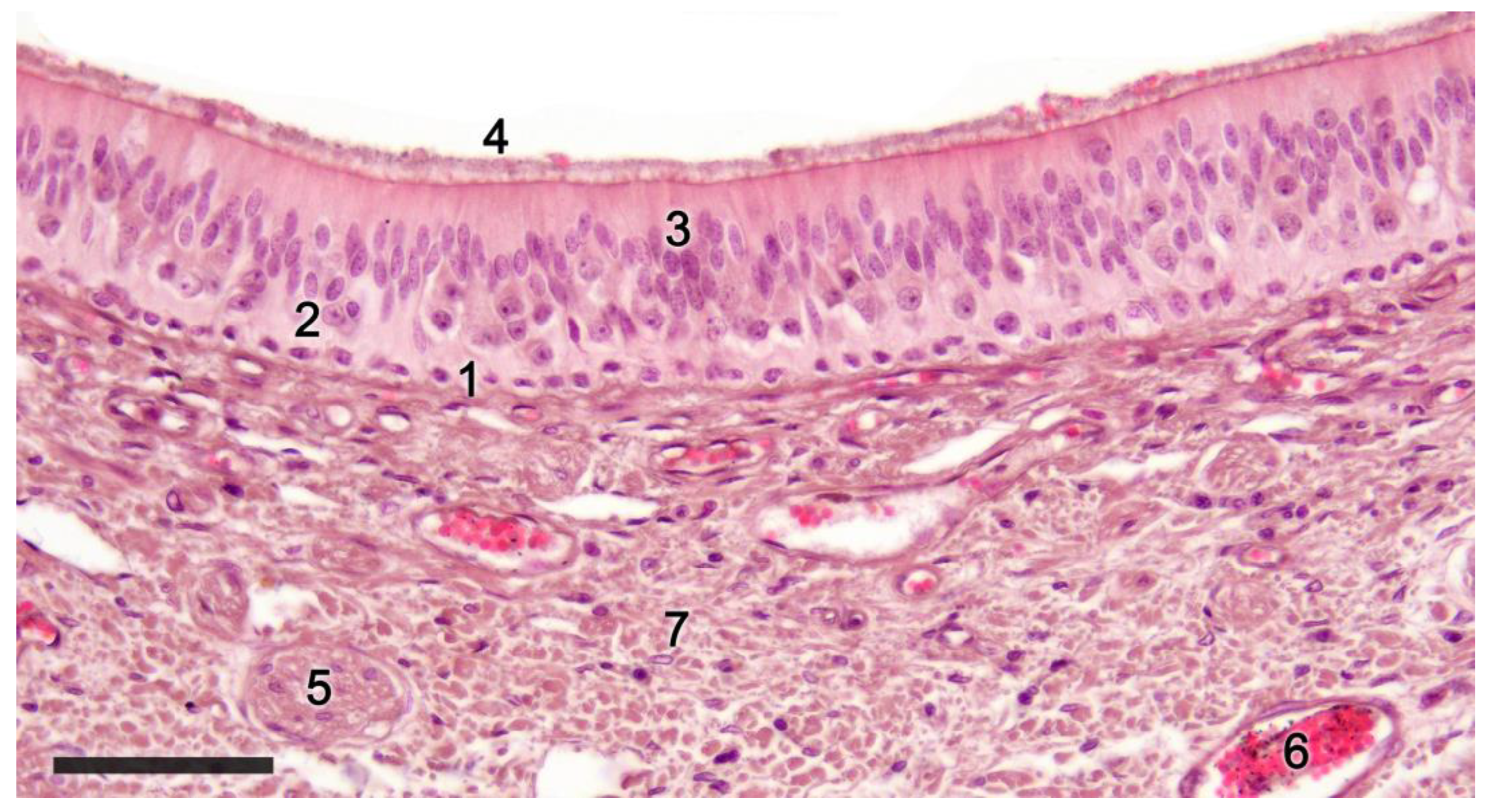
Figure 2. Sensory epithelium of the dama gazelle vomeronasal organ stained with hematoxylin-eosin. 1. Basal cells; 2. Neuroreceptor cells; 3. Sustentacular cells; 4. Mucomicrovillar complex; 5. Vomeronasal nerve axons; 6. Veins; 7. Collagen fibers. Scale bar: 50 μm.

Figure 3. Vomeronasal organ of a horse. Autofluorescence confocal microscopy of the sensory epithelium (SE) allows for the clear differentiation of the zones corresponding to the main strata: Basal (1); Neuroreceptor (2); Sustentacular (3); Mucomicrovillar (4). RE, respiratory epithelium; Red nuclear contrast: TOPRO-3 iodide. Scale bar: 50 μm.
The nerve fascicles of the parenchyma are constituted by the coalescence of the axonal processes of the neuroreceptor cells into bundles, which converge from the vomeronasal epithelium. These nerve bundles are located in the dorsal and medial areas of the VNO, although occasionally they can also run on the ventral or lateral side of the duct. In turn, these bundles come together to form the vomeronasal nerve, which courses dorsocaudally in the submucosa of the nasal septum [84]. After passing through the cribriform plate of the ethmoid on its medial part, the vomeronasal nerve projects towards the anterior area of the telencephalon to synapse in the accessory olfactory bulb [85].
In the central region of the VNO, both the glandular and vascular components of the parenchyma are predominantly situated in the lateral portion of the parenchyma. Meanwhile, the glandular tissue is also concentrated in the caudal part of the VNO. The vomeronasal glands (Figure 4) are responsible for secreting mucus that is discharged into the lumen of the duct, either at its commissures or in the central part of the respiratory epithelium [86]. The mucus facilitates the entry and exit of molecules within the duct, but it also plays a crucial role in vomeronasal perireceptor processes. Histologically, these glands are distinguished by their acinar or tubular morphology, and by their serous or mucoid secretions, respectively [87][88]. Concerning the vascular tissue of the VNO, only arteries of significant caliber can be found in its posterior region; however, it has large veins that run parallel to the vomeronasal duct on the lateral flank [89]. This distinct morphological organization is important as it is involved in the vascular pumping mechanism that is activated to allow the entry of chemical stimuli into the organ. Specifically, when the vessels of the parenchyma contract, the lumen of the duct expands, creating a vacuum effect that draws chemical molecules into the duct. Similarly, when the vessels dilate, a constriction of the duct occurs, expelling the content of the duct outward. This vascular pumping mechanism is known as the vomeronasal pump [90][91][92].

Figure 4. Vomeronasal gland of a cow stained with PAS-Alcian blue-hematoxylin staining. Both the glandular acini and the duct are stained. Scale bar: 150 μm.
The vomeronasal capsule provides structural support for the proper functioning of the vascular pump, counteracting the potential collapse of the parenchyma that the increase in negative pressure might induce, while simultaneously safeguarding the encapsulated structures. Its morphology not only varies along the VNO, but there are also notable differences among various species, both in its nature and its configuration [79]. More typically, towards the front, the capsule is shaped like an incomplete ring with a lateral opening associated with the outlet of the vomeronasal duct. In the central region, the ring becomes complete, but posteriorly the capsule separates again, adopting a “J” shape, with a dorsolateral slit allowing the passage of the vomeronasal nerves. As we progress caudally, on occasion, the ring closes entirely. Its nature can be bony, cartilaginous, or a combination of both in different patterns, making it one of the VNO components with the most diverse design from a comparative anatomy standpoint.
Regarding the sensory transduction of the VNO, in the most studied model, the murine, the neuroreceptor cells are organized into different subpopulations throughout the vomeronasal epithelium, following a clearly defined zoning. In this way, the V1R receptors, linked to the Gαi2 protein, are confined to the neurons whose cell bodies are located in the apical part of the epithelium, while the neurons expressing the V2R receptors, linked to the Gαo protein, locate their cell bodies in the basal layer of the vomeronasal sensory epithelium [93]. In both cases, each neuroreceptor cell exclusively expresses a single receptor among the total population of vomeronasal receptors [9].
Formyl peptide receptors (FPRs) co-express with both G proteins in the two neural layers of the VNO. FPRs react to products from viruses and bacteria, and they are found in various tissues and cell types, mainly associated with immune functions [94]. Within mammals, its expression has only been confirmed in the Muroidea superfamily of rodents and in Lagomorpha [95]. Seven distinct genes encoding FPRs have been identified in mice VNOs [11][96]. Some of these genes, like FPR-rs3, FPR-rs4, FPR-rs6, and FPR-rs7, are specific to the VNO and co-express with Gαi2. However, FPRrs1, recently renamed as FPR3, co-expresses with Gαo in vomeronasal sensory neurons and is also transcribed in immune cells. As there is no evidence that FPRs co-express with V1Rs or V2Rs, it is likely that certain vomeronasal sensory neurons express only FPRs. FPR3 reacts to a limited set of bacterial peptides crucial for managing infection virulence. This links the behavioral and immunological functions of the VNO [97].
On the other hand, it has been demonstrated that the peptides of the major histocompatibility complex (MHC) can perceive non-volatile molecules, both in the olfactory and vomeronasal epithelium of mammals. The MHC was first identified as a primary component in the adaptive immune response, recognizing and presenting antigens to T cells [98]. MHC is encoded in the highly polymorphic H2 and HLA genes for mice and humans, respectively. Current research estimates that over 109 distinct MHC antigenic phenotypes exist in outbreeding mammals, which suggests that MHC alleles might be crucial for determining genetic uniqueness [99]. In a lab setting, numerous studies indicate that MHC and its peptide ligands get secreted in animal urine and function as markers for self-identity during individual recognition [100][101]. Both genders of mice display a natural inclination to select mates with varied MHC haplotypes [102]. Additionally, when female mice that have recently mated are introduced to the scent of an unfamiliar male with different MHC peptides, it can lead to a pregnancy block [103]. In human contexts, there are various studies suggesting a connection between MHC differences and preferences in smell and mate selection. Women seem to be drawn to the scent of shirts worn by men who have different MHC genotypes [102].
Mates with specific MHC variations are viewed favorably because of their increased resistance to parasites, potentially leading to enhanced reproductive outcomes for their progeny [104]. Female sticklebacks show a preference for mating with males that exhibit a broad MHC diversity and have a certain MHC haplotype that wards off infections from prevalent parasites [105]. While MHC peptides can be found in both VNO and MOE neurons, they serve different behavioral functions. Specifically, the MOE ability to recognize and prefer urine containing unique MHC peptides contrasts with the VNO-driven Bruce effect, activated with dissimilar MHC peptides. All together suggests a general role of MHC peptides in chemical communication, even in species lacking a functional VNO [106][107].
The transduction cascade of virtually all vomeronasal neurons converges in most species on the activation of a member of the transient receptor potential (TRP) channel family, the TRPC2, which is expressed in both neural layers of the VNO [108]. It was observed that the protein resides in the microvilli of the sensory neurons and coincides with the expression of both Gai2 and Gao [83]. In fact, the genomic sequence of the intact TRPC2 gene is considered to be a predictive marker of the functionality of the vomeronasal organ [56]. When the Trpc2 gene was knocked out in mice, its pivotal role in vomeronasal-mediated behavior became evident. These modified mice exhibited reduced aggressive responses and did not differentiate between males and females, displaying sexual behaviors towards both. This suggests that these mice are unable to discern the gender of their counterparts due to a lack of olfactory signal transduction through VSNs [109][110]. The TRPC2 gene is ubiquitous among mammals, reptiles, amphibians, and fish species. Comprehensive genome studies indicate an absence of TRPC2 genes in avian species [111]. A noted absence of functional TRPC2 in certain primates, such as Old World monkeys, correlates with the lack of VNO [36][112]; although, orangutans and rhesus macaques possess three TRPC2 gene copies, which have been suggested to be involved in mediating the acrosomal response during fertilization [113]. In humans, the loss of a functional TRPC2 gene has been confirmed; however, the presence of functional V1R receptors has been observed in the olfactory mucosa, embryonic cerebral tissues, and non-neuronal cells [114]. It should also be noted that chemical communication in vertebrates originated long before the development of the VNO, and pheromone detection can also be mediated by other olfactory organs [115]. This brings forth questions about the specific impacts of Trpc2−/− on VNO activity. Mombaerts and colleagues integrated the lacZ gene into the Trpc2 site and mapped the pathways of Trpc2-active neurons. Their exploration revealed that two distinct MOE neuron types, which communicate with specific glomeruli on the ventral aspect of the primary olfactory bulb, expressed Trpc2 [116][117]. These observations indicate potential broader roles for Trpc2 within the primary olfactory framework and other cerebral regions. Thus, the behavioral patterns seen in Trpc2−/− mice might not be exclusively attributed to VNO disruption.
While initially, it was thought that all mammals expressed both families of vomeronasal receptors, the absence of the V2R family in the vomeronasal system of various species was subsequently described. Therefore, based on the presence or absence of the two neuronal subpopulations linked to the V1R and V2R vomeronasal receptors, two vomeronasal transduction models were identified: the segregated model and the uniform model. In the segregated model, both types of vomeronasal receptors coexist, while in the uniform model the V2R receptor is absent, and only V1R type neurons are expressed. Recent research on the Tammar wallaby (Notamacropus eugenii) concluded that macropods could present a third vomeronasal transduction model, consisting only of V2R neurons, as they did not find expression of the Gαi2 protein in the VNO or in the AOB [118].
A segregated-type vomeronasal system has been characterized in certain species of rodents [119], lagomorphs [120], and marsupials [121], while the rest of the studied mammals, such as ungulates, carnivores, or primates, fall within the uniform model [122][123][124].
The neurochemical study of the organ allows to obtain characteristics about its functionality using G proteins, but other markers are also used that provide specific information about this structure. The calcium-binding proteins calbindin (CB) and calretinin (CR) are used to identify neuroactive substances, but also to differentiate cell populations or to define the morphology of vomeronasal neuroreceptor cells [125]. On the other hand, the GAP-43 marker shows neuronal growth and is expressed in the nerve bundles present in the organ [126]. These markers are complemented with routine stains (hematoxylin-eosin) or specific stains (Gallego trichrome), which display the structural characteristics of the VNO. Likewise, using PAS (Periodic acid Schiff) and Alcian blue stains, the nature of the vomeronasal glands can be identified, staining their secretions with neutral and acid mucopolysaccharides, respectively [127].
Likewise, in the study of the VNO, lectins such as Ulex europaeus agglutinin (UEA), Bandeiraea simplicifolia isolectin B4 (BSI-B4), or Lycopersicon esculentum agglutinin (LEA) have been frequently employed, as some are specific to the vomeronasal and/or olfactory system in various mammals [128]. Thus, it is possible to differentiate neuronal populations and study the morphology of the neuroreceptor cells in the sensory epithelium of the VNO. On the other hand, when expressed in the neuronal axons of these cells, it becomes easier to identify the nerve bundles of the VNO and track the vomeronasal nerve by analyzing its topography and anatomical relationships.
Over the past decade, genomic studies have paved the way for groundbreaking insights into the VNS. To date, only a limited number of links between chemical ligands, vomeronasal receptors, and behavior have been clarified. This is primarily due to the challenges encountered when dealing with expansive, homologous gene families that have a high degree of sequence similarity. Nonetheless, when examining mouse strains with mutations in genes linked to ligand-VR signal transduction, the role played by the VNO in various social behaviors can be studied in a more specific way. These behaviors include male-to-male and maternal aggression, sexual allure, lordosis, selective pregnancy termination, and interspecies reactions such as aversion and defensive behaviors [129].
One significant discovery has been the broad expansion and diversification of the vomeronasal receptor gene families, V1Rs and V2Rs [112][130]. High-throughput sequencing methodologies, such as RNA-Seq, have enabled researchers to identify and catalog an extensive array of VNRs in various species, showcasing a vast diversity that arguably correlates with species-specific pheromonal communication [129][131][132]. Another groundbreaking revelation is the functional differentiation of V1R and V2R receptors, elucidated through transcriptomic analyses. While V1Rs primarily respond to small volatile molecules, V2Rs are more attuned to larger, peptide-based cues [133].
Comparative genomics indicates that the retention or loss of specific vomeronasal receptors often aligns with the ecological and social structures of the species in question. For instance, species with more intricate social hierarchies or mating systems tend to possess a richer repertoire of functional VNRs [134]. Moreover, the transcriptome analysis of the VNO in mice under different physiological and environmental conditions showed notable variations in the expression of vomeronasal receptors even among individuals of the same species [95][135][136]. For instance, during the final phase of pregnancy, the production of neural progenitors in the VNO of female mice is notably increased. Transcriptome analysis comparing pregnant and control female VNOs reveals differential expression of 101 genes, including 24 vomeronasal receptors and other genes related to cell proliferation and death [135]. Pregnancy or estrogen-driven increases in new chemosensory neurons have significant functional consequences, especially in maternal behaviors. For instance, the ability of the VNO to recognize social odors can induce varied behavioral responses based on the hormonal status, age, gender, or dominance of the individual. In humans, while the VNO appears non-functional, with VRs and transduction molecules being pseudogenized, there is still a sensory target for hormone modulation in the main olfactory epithelium. Further investigations are recommended to discern whether pregnancy also prompts an increase in neurogenesis in the main olfactory epithelium.
The application of transcriptomic techniques has also made it possible to compare the expression of VRs in rabbits of different ages. Some VRs are more expressed in juveniles than in adults [95]. This difference might be attributed to increased exposure to new stimuli during early life stages. The difference in expression between young and adult animals could be crucial for innate or unconditioned responses to specific chemical signals. Likewise, it has been observed that sex-separation induces sex- and stage-specific gene expression differences in the VNO of male and female rabbits, both in adults and juveniles [136].
The presence of sexual dimorphism in the vomeronasal system is an issue of particular interest. Although no qualitative histological and immunohistochemical differences between sexes in both the VNO and the AOB have been identified, morphometric analyses have detected subtle yet significant disparities. In the 1980s, Guillamón and Segovia initiated a series of studies establishing that, in rats, the VNO and the AOB were larger in males than in females [137][138]. Specifically, male rats exhibited a greater numerical density of principal cells in the AOB compared to females [138]. Interestingly, in the AOB of rabbits, the trend was reversed, with females demonstrating higher morphological values [139]. These species-specific manifestations of sexual dimorphism likely reflect the reproductive, behavioral, and physiological characteristics unique to each species. For instance, while female rabbits are reflex ovulators, female rats are spontaneous ovulators. Additionally, there are distinct differences in sexual and maternal behaviors between these species. The contrasting patterns observed in closely related species like rats and rabbits underscore the caution needed when extrapolating findings on brain sexual dimorphism from one species to another.
Sexual dimorphism has also been examined in other mammalian groups. In a study involving wild voles (Microtus pennsylvanicus and M. ochrogaster), it was found that M. pennsylvanicus, which exhibits more pronounced differences in parental behaviors, had larger VNOs in females than in males [140]. A comparison between the VNO and AOB of the monogamous mandarin vole, Microtus mandarinus, and the polygamous reed vole, M. fortis, revealed significant differences only in the reed voles, suggesting that the degree of sexual dimorphism might be linked to the mating system [141]. Furthermore, a discernable sexual dimorphism in the rate of neuronal generation in the AOB of rats has been identified, with a higher proliferation rate in the anterior AOB in male rats compared to female rats [142].The morphological diversity of the VNO among different orders of mammals is also notable, and indeed, since the advent of Darwinian theory of evolution, the morphological configuration of the VNO has been used as a phylogenetic classification method [143][144][145]. A significant portion of the studies on the VNS have been conducted in rodents, specifically in myomorphic species like the laboratory rat (Rattus norvegicus domestica) or the mouse (Mus musculus), which have been used as general VNS models in rodents (Figure 5). Both species display a highly developed vomeronasal organ, with a large neuroreceptor epithelium, of considerable thickness and cell density [146]. The vascular pump consists mainly of a large central vein, which is surrounded by other smaller-diameter veins [147]. The vomeronasal capsule is made up of a thin bone layer in adults; however, in newborn rats, this covering is cartilaginous [80]. A thoroughly investigated aspect of the rodent VNO is the presence of postnatal proliferation in the vomeronasal epithelium. The distribution of these proliferating cells varies with age. In neonatal rats, such cells are distributed fairly evenly across the sensory epithelium. However, starting from P21, the majority of these cells tend to cluster near the boundaries with the non-sensory epithelium [148]. These findings have been more recently corroborated in mice using bromodeoxyuridine (BrdU) immunohistochemistry [149].
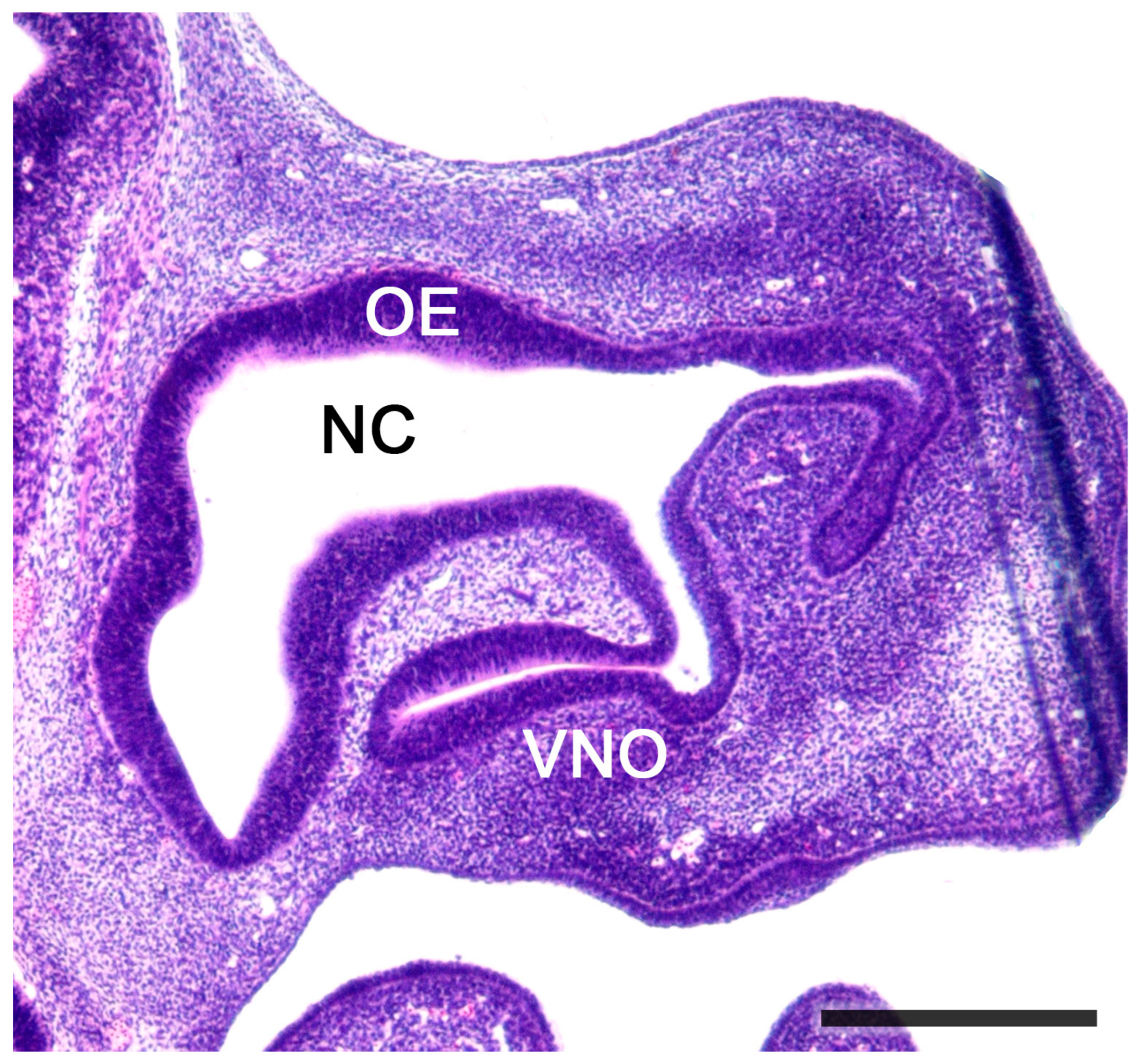
Figure 5. Sagittal section of the nose of a fetal mouse. The vomeronasal organ is located in the base of the nasal septum. A functional opening to the nasal cavity (NC) can be observed. The NC is lined by olfactory epithelium (OE). Hematoxylin-eosin staining. Scale bar: 500 μm.
Other myomorphic rodents display specific morphological features in their VNO, but in general, they all show a comparable level of development. For instance, the Gambian rat (Cricetomys gambianus) displays certain ossification areas in its cartilaginous vomeronasal capsule [150]. Among the sciuromorphic rodents, the Daurian ground squirrel has an exceptionally voluminous vomeronasal vein, which, together with other smaller-caliber veins, constitutes the vascular pump. It also displays a sensory epithelium with significant thickness, and its vomeronasal capsule is exclusively cartilaginous [151]. In hystricomorphic rodents, the long-tailed chinchilla (Chinchilla lanigera) showed considerable variation in the primary features of its VNO. Its sensory epithelium shifts from a medial location at the front of the VNO to an entirely dorsal position at the back. Similarly, the vomeronasal capsule in this species consists of cartilaginous tissue at its anterior level, but this shifts dorsally as we move caudally, eventually being completely replaced by a bone layer that encases the VNO at its back [152].
Concerning the vascular pump, the VNO of the chinchilla has several significant-diameter veins, but features a dominant central vein [153]. Similarly, within the hystricomorphs, African mole rats displayed unique characteristics concerning their vomeronasal organ. In these eusocial rodents, there is hardly any postnatal growth in their vomeronasal neuroepithelium [154]; however, all the components of their VNO exhibit significant and functional development. Thus, it has a sensory epithelium of substantial thickness, and laterally displays large venous sinuses. Transversely, the vomeronasal duct takes on the typical “J” or crescent shape. The parenchyma is protected by a cartilaginous capsule and is also reinforced by an external bone layer that occupies a ventral and/or lateral position depending on the species [155].
As with rodents, lagomorphs have an extensively developed vomeronasal organ (Figure 6). The rabbit (Oryctolagus cuniculus) has a double vomeronasal capsule, made up of an external bone covering and an internal cartilaginous lining [156][157]. Its sensory epithelium is thick compared to that of rodents, but its vascular pump comprises multiple large-caliber veins that provide a powerful suction capability to the VNO in this species [120][158].
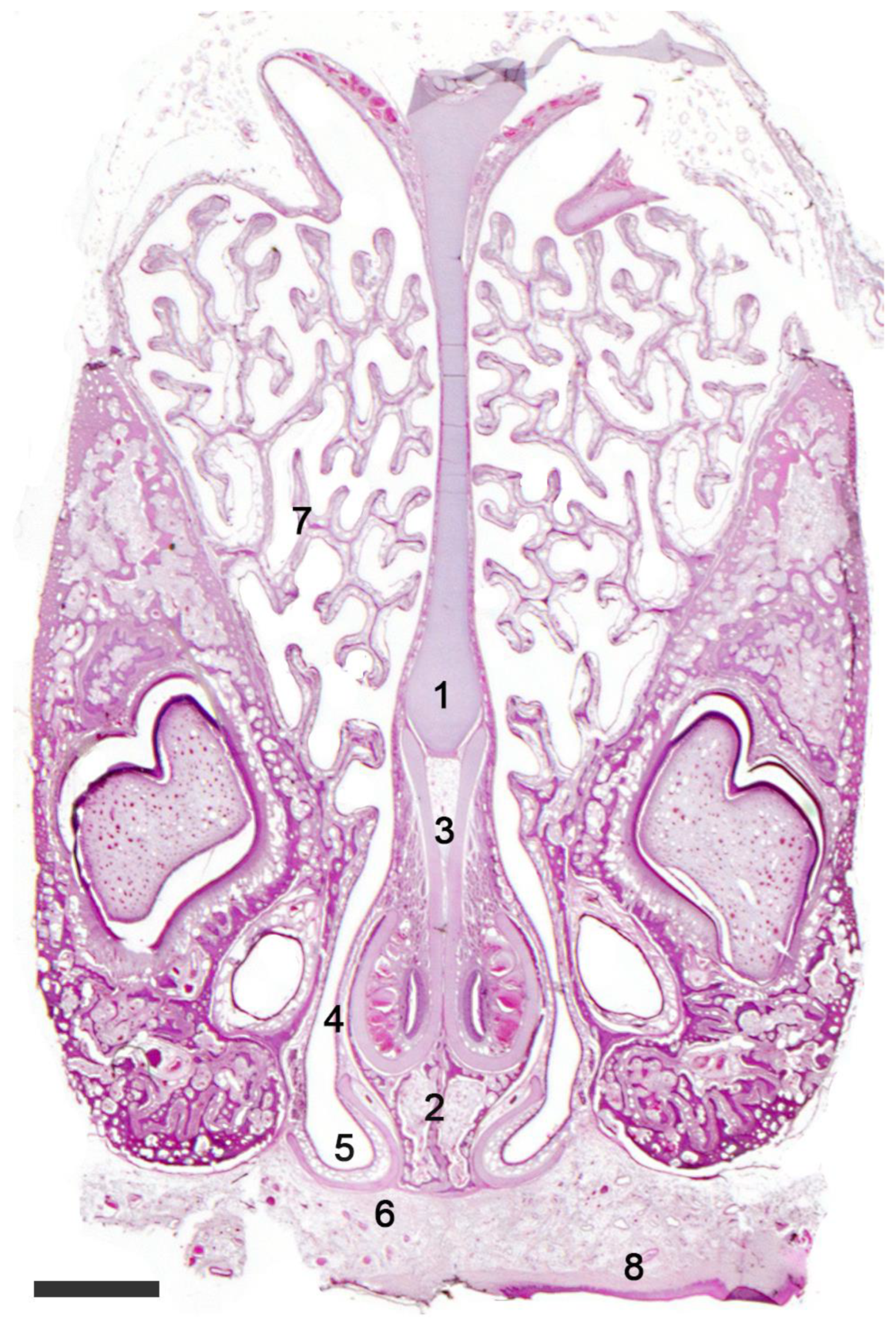
Figure 6. The vomeronasal organ of a rabbit. Hematoxylin-eosin staining. Transverse section of the head showing the nasal septum (1) with the VNOs located over the palatine processes of the incisive bone (2). The J-shaped cartilaginous envelope of the VNO (3) is covered by a thin bony layer (4). The ventral recess of the nasal cavity (5) is shaped by the cartilage of the incisive duct (6). (7). Ventral turbinate; (8). Palate. Scale bar: 500 μm.
Marsupials also display a remarkable development of the vomeronasal organ, suggesting that, as in rodents and lagomorphs, pheromonal communication plays an essential role in these mammals. The Tammar wallaby (Notamacropus eugenii), a macropodid of the order Diprotodontia, sports a complete cartilaginous capsule and a densely populated and extensive neuroreceptor epithelium. The vascular tissue enabling the pumping mechanism consists of numerous large-caliber veins, which are mostly distributed on the medial side, but voluminous veins also appear on the medial face of the vomeronasal duct [159]. In the order Didelphimorphia, the gray short-tailed opossum (Monodelphis domestica) presents certain unique features. Its vomeronasal capsule is cartilaginous, although at the caudal level, it is replaced by a bony capsule, which encircles a parenchyma very rich in glandular tissue. Its vascular pump is made up of a large main vessel which is located laterally to the vomeronasal duct, and other smaller vessels in the ventromedial and lateral areas. Its sensory epithelium is very thick and is characterized by a rosette-shaped structure that divides said epithelium in half at the caudal part of the organ. This configuration corresponds to the fusion of the main vomeronasal glandular secretion duct with the neuroepithelium, through its opening into the lumen of the vomeronasal duct [160].
In species of the order Monotremata, the VNO shows notable development. Studies on newborn individuals of the platypus and echidna determined that both possess a thick sensory epithelium and a cartilaginous capsule. In contrast, both showed few veins of modest caliber forming their vascular pump [161][162].
Within the order Eulipotyphla, the studied species display a highly developed VNO. The African pygmy hedgehog (Atelerix albiventris) has a cartilaginous vomeronasal capsule and a neuroreceptor epithelium of moderate thickness, which extends beyond the usual boundaries, occupying almost the entirety of the lumen in front of the respiratory epithelium. The parenchyma contains mucous and serous glands, and a network of venous sinuses that extend around the entire vomeronasal duct. Through a cross-section, the lumen of its VNO shows a circular shape at the front, which becomes more oval as we move caudally [163]. On the other hand, the VNO of the dasyurid marsupial Antechinus subtropicus displayed significant differences compared to the African pygmy hedgehog. In this species, the capsule is also cartilaginous, but it features a primary vein lateral to the vomeronasal duct, along with other secondary veins located both laterally and medially to it, forming its vascular pump. Additionally, its vomeronasal neuroepithelium has a notably greater thickness compared to the African pygmy hedgehog; however, its extension in front of the respiratory epithelium corresponds with the usual pattern, and its lumen shape, when observed in cross-section, is the typical “J” or crescent shape [164].
Mammals of the order Carnivora, both felines and canines, have shown moderate development of their VNO (Figure 7). Studies on the dog [123][165], domestic cat (Felis silvestris catus) [166], brown bear (Ursus arctos) [167], and European ferret (Mustela putorius) [168] revealed that all share the main structural characteristics of the VNO, such as a cartilaginous capsule, the distribution of vessels around the vomeronasal duct forming the vascular pump, and a broad sensory neuroepithelium, albeit thinner than mammals of the orders previously described. However, the red fox (Vulpes vulpes) represents an exception among domestic canines. In this species, the vomeronasal epithelium is not only more developed but also displays the expression of G proteins associated with both V1R and V2R receptors [169].
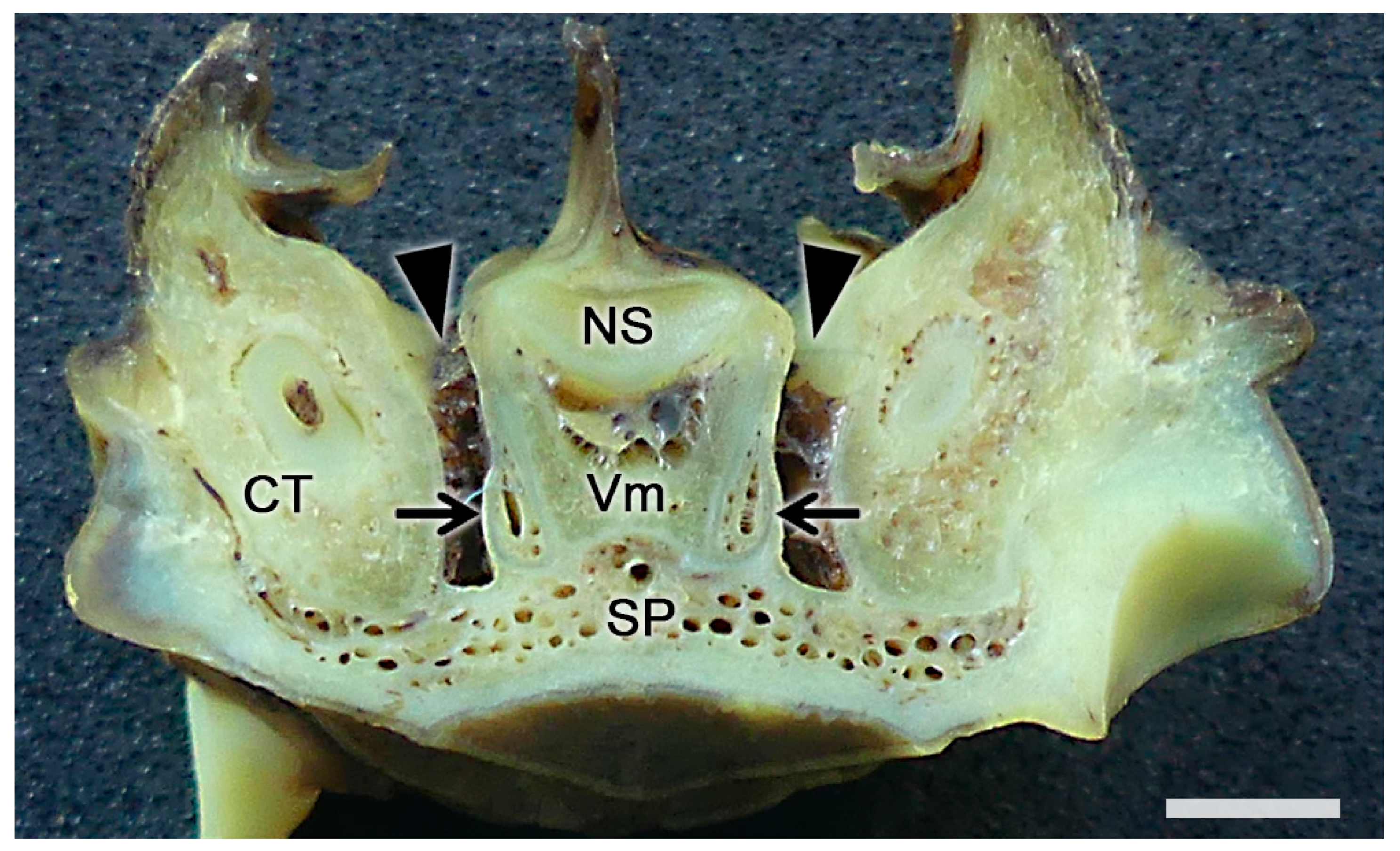
Figure 7. The vomeronasal organ of a fox. Transverse section of the base of the nasal septum (NS) showing the topographic relationships of the vomeronasal organs (arrows) with the ventral recesses of the nasal cavity (arrowheads). CT, canine tooth; SP, soft palate; Vm, vomer bone. Scale bar: 2 mm.
Artiodactyls exhibit a high level of VNO development. Their cartilaginous capsule is complex and developed, displaying numerous morphological differences between species. The length of the sensory vomeronasal epithelium tends to be longer compared to species of the aforementioned orders; however, its thickness is notably inferior, as seen in carnivores. Still, it maintains the three cellular layers that make up the neuroepithelium, which are organized with a clearly defined boundary. Vessels in the vomeronasal parenchyma are numerous, of medium caliber, and are uniformly arranged around the vomeronasal duct [170]. Regarding the morphology that the vomeronasal duct exhibits in cross-section, artiodactyls usually have an oval shape, unlike the usual crescent or “J” shape. Variations also exist between different species like cows [171][172], sheep [173], goats [174][175], deer [176], moose [177], giraffes [178], and duikers [150].
Strepsirrhine primates, which are mostly nocturnal, have a highly developed VNO compared to haplorhine primates, who tend to be diurnal and have a modestly developed VNO. Both have a cartilaginous capsule surrounding the vomeronasal parenchyma; however, the thickness of the vomeronasal epithelium is significantly greater in strepsirrhines. Similarly, the vascular pump in strepsirrhines has numerous large-caliber vessels around the entire vomeronasal duct, in contrast to the few vessels typically displayed by the VNO of haplorhine primates [179].
Among the various bats, the development of the VNO is extremely variable. From species that show a total absence of their VNO even in the embryonic phase, to other species that demonstrate astonishing development of this sensitive structure. Likewise, many other bat groups represent intermediate levels of development [57].
2.2. Neuroanatomy of the Accessory Olfactory Bulb
The accessory olfactory bulb (AOB), the first neuronal integration center of the vomeronasal sensory system, is located in the olfactory areas of the forebrain of certain vertebrate species; what we recognize as the basal rhinencephalon. In mammals, it is usually located in a dorsocaudal position relative to the main olfactory bulb (MOB) [180].
Macroscopically, in most species, the AOB is a relatively difficult structure to discern. Although in most cases it gives rise to a convex prominence in the dorsal transition between the main olfactory bulb and the olfactory tract, its boundaries with these structures are very diffuse. However, histologically, it is easily recognizable due to its laminar morphology. Although this laminar pattern is essentially analogous to that of the MOB, there are significant variations between different groups of mammals. Some species have clearly defined lamination and high cellularity, while others have much less defined layer boundaries (Figure 8 and Figure 9). Thus, from superficial to deep, the AOB layers are:
1. Vomeronasal nerve layer: It consists of bundles of unmyelinated fibers, originating from vomeronasal receptor neurons, surrounded by numerous glial cells, both astrocytic and ensheathing glia [181][182]. Whereas, in the MOB of mammals, each olfactory axon projects to a single glomerulus [183][184], in the AOB, fibers branch out and distribute to more than one glomerulus [185].
2. Glomerular layer: Comprised of spherical structures called glomeruli, these result from synaptic contact between axonal terminations of vomeronasal nerve fibers and the apical dendrites of mitral cells. These glomeruli show a relatively acellular texture rich in neuropil but are bounded by a narrow band of periglomerular cells, especially in their deepest part. Their degree of definition is lower than that observed in the main olfactory bulb.
3. External plexiform/Mitral/Internal plexiform: It is at the level of the three central layers of the accessory olfactory bulb where the greatest differences lie, both compared to the MOB and in the interspecific comparisons that can be made at the level of the AOB itself. The differentiation of an external plexiform layer—formed by the dendrites of the mitral cells and granular cells, as well as by tufted cells and other neuronal types —a mitral layer—formed by a linear band that includes the somas of the mitral cells, the second neuron of the olfactory pathway—and an internal plexiform layer—containing the axons of the mitral cells—which is constant in the organization of the MOB in mammals. However, in the case of the AOB, the existence of plexiform layers has only been proposed in those species where the degree of lamination is highest, mainly rodents, but the issue is controversial. Thus, Cajal [30] concluded that the plexiform layers were absent; however, classic comparative studies of the olfactory system [186][187] included the external plexiform in their description of the AOB but not the internal plexiform. Meisami and Bhatnagar [180] in their exhaustive bibliographic review state that it is inappropriate to extrapolate data from one species to another, indicating that, in mammals with large and well-developed AOBs, both plexiform layers exist; although, it should be recognized that the boundaries of these layers are not recognizable with Nissl staining, requiring more specific stains such as cytochrome oxidase or Galyas staining. However, Salazar et al. [84], in their exhaustive study of the mouse AOB, concluded the impossibility of determining the presence of plexiform layers, as well as differentiating mitral cells from tufted cells, coining the term mitral/tufted layer. In their subsequent study of the cat AOB, Salazar and Sánchez-Quinteiro [188] opted for the term mitral/tufted/plexiform layer which more accurately encompasses the fusion of the three AOB structures equivalent to the three inner layers of the MOB. In later works, this group leaned towards a simpler name: the mitral/plexiform layer, which the researchers have adopted throughout this research. It is important to clarify that the morphology of the main cells of this mitral/plexiform layer rarely corresponds to the typically mitral morphology observed in the mitral layer of the MOB. This has led certain authors to prefer to avoid the term mitral when describing this layer, as is the case with Larriva-Sahd [189] and Villamayor et al. [139] in their respective studies of the AOB in rats and rabbits. In both cases, they opted for the terms “outer cell layer” to designate the set of the mitral/plexiform layer and “principal cells” to designate the projection neurons that constitute this layer. While undoubtedly a more accurate name in terms of morphological reality, the direct functional equivalence between the mitral cells of the MOB and the principal cells of the AOB (projection neurons in both cases) advises maintaining the term mitral cells to designate the principal cells of the AOB. Regarding the use of the term plexiform, Larriva-Sahd [189] considers that, in contrast to the plexus arrangement of the proximal processes of the MOB mitral cells, in the case of the AOB these processes adopt a radial arrangement, resulting in a dense and convoluted dendritic frame, very different from the parallel fibrous texture that forms the plexiform layer of the MOB. The set of radial dendritic arborizations, together with the proximal processes of granular cells and other interneurons, make up a neuropil rather than a plexiform layer. Another interesting aspect that affects the stratification of the AOB is the topography of the lateral olfactory tract (LOT), which can be located either through or below the accessory bulb. Thus, in broad groups of mammals including rodents, insectivores, and primates, the broad axonal bundles that make up the dorsal component of the LOT run through the innermost area of the mitral/plexiform layer or outer cell layer [190].
4. Granule cell layer: It is composed of granular neurons, which lack axons and accumulate compactly. These cells are GABAergic and presumably inhibitory [191]. However, they are not a homogeneous population in terms of the expression of peptidic neuromodulators [192]. Their basal dendrites receive centrifugal impulses from the amygdala, whereas their apical dendrites interact with the basal dendrites of the mitral cells [193]. On the other hand, it has been observed that the number of granular cells is lower in domestic animals and animals kept in zoological facilities [194]. Furthermore, neuronal proliferation has been found in the AOB of adult rats, with most of these new cells being located in the granular layer [195]. Contrary to what one might assume, most of the proliferating neurons present in the OB do not derive from the subventricular zone of the olfactory bulb but from slow-dividing cells that might correspond to the population of resident neural stem cells. These could generate neurons that are incorporated into the OB circuits in vivo [196].
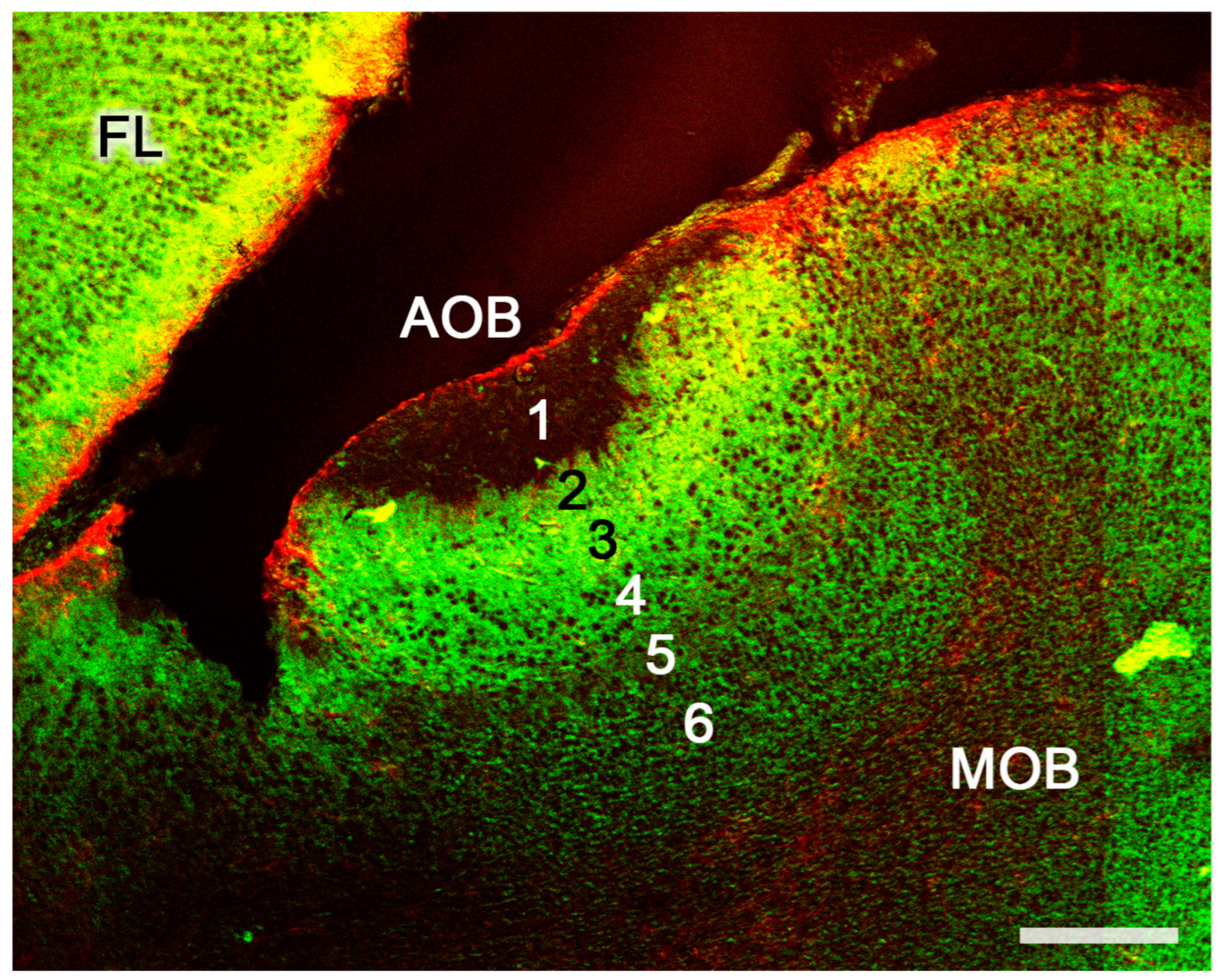
Figure 8. The accessory olfactory bulb of a postnatal mouse. Confocal double immunofluorescence of a sagittal section stained with anti-MAP2 (green) and GFAP (red) showing the lamination of the AOB. 1. Vomeronasal nerve layer; 2. Glomerular layer; 3. Mitral plexiform layer; 4. Somas of mitral cells appear as unstained circular structures; 5. Lateral olfactory tract; 6. Granular cells layer. FL, Frontal lobe. Scale bar: 250 μm.
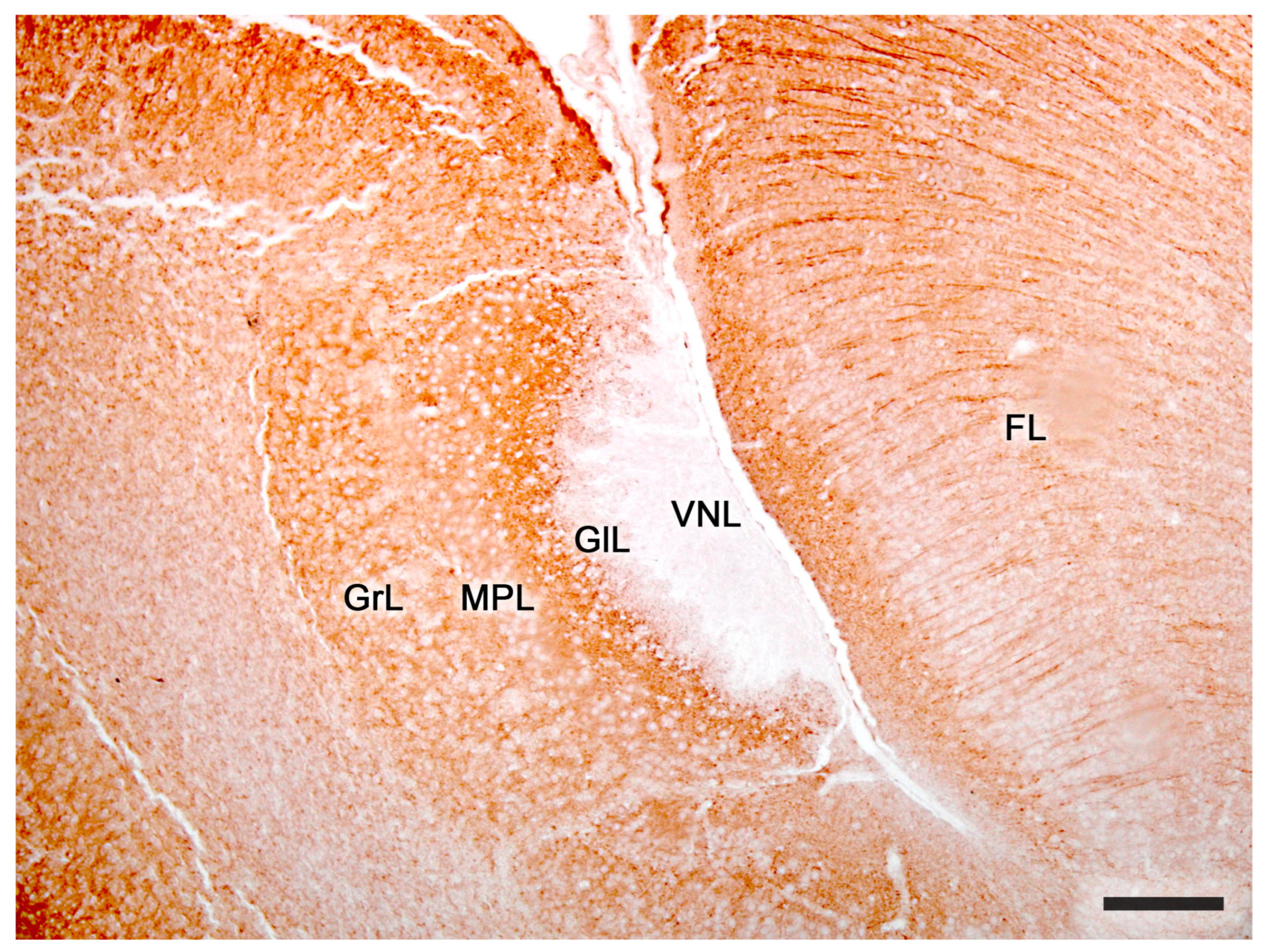
Figure 9. The accessory olfactory bulb of a mouse. Immunohistochemical staining against MAP2. FL. Frontal lobe; GlL, glomerular layer; GrL, granular layer; MPL, mitral-plexiform layer; VNL, vomeronasal nerve layer; 2. Glomerular layer; 3. Mitral plexiform layer; 4. Somas of mitral cells appear as unstained circular structures; 5. Lateral olfactory tract; 6. Granular cells layer. FL, Frontal lobe. Scale bar: 250 μm.
Projection neurons of the olfactory bulb send axons through the lateral olfactory tract (TOL), giving it a laminar structure. Thus, in embryonic stages, the TOL consists of three layers: superficial, middle, and deep. The axons from the BOA are the first to mature, so they are located in the deep layer, whereas the axons from the MOB constitute the middle and superficial strata. The middle layer is composed of mature axons, while the superficial layer consists of newly generated axons that are added, forming a series of stacked axonal laminae at different maturation stages. Initially, the superficial lamina is proportionally larger than the middle lamina, but throughout development, the middle lamina increases, while the superficial lamina drastically decreases [197]. This segregation of both pathways in the TOL, the vomeronasal and olfactory pathways, can be seen in adult animals like rabbits or rats [198][199].
Throughout all the layers that make up the BOA, different cell types coexist. From superficial to deep, we can find periglomerular cells, superficial short-axon neurons, mitral cells, round projection cells, tufted cells, external granular cells, dwarf cells, polygonal neurons, and internal granular cells.
Periglomerular cells (PGs) are small, short-axon neurons found at the base of the glomeruli in both the AOB and the BOP, although they are more numerous in the latter. In the AOB, these cells are primarily GABAergic and have inhibitory functions, possibly playing a modulatory role [200]. There are two classes of PG: amacrine cells, meaning they lack axons, and interneurons. Both have a small fusiform soma, from which they send one or two dendrites to one or several glomeruli. In them, their dendrites branch and originate a dense dendritic plexus. On the other hand, the axon of the interneurons distributes within the neuropil adjacent to the glomeruli.
Superficial short-axon cells are morphologically similar to PG but have some differences. They are larger in size and are located superficially in the external plexiform layer. They have a thick main dendrite that rises to enter a single glomerulus, where it establishes a dendritic network. They also emit other dendrites that distribute ventrally between the somas of PG and other superficial short-axon cells. Their axon interacts with the somas of adjacent PGs [181].
Mitral cells, also called principal cells, are the second neuron in the olfactory and vomeronasal sensory pathways. In the BOP, they are distributed linearly, forming a dense layer of mitral cells. In the AOB, this layer transforms into a diffuse mitral cell band integrated with both plexiform layers, constituting a single mitral-plexiform layer, also called the external cell layer. The cytoarchitecture of the accessory olfactory bulb has been addressed in depth in the rat through the study carried out with the Golgi technique by Larriva-Sahd [189]. This study shows that mitral cells have two types of dendrites: glomerular dendrites (thick) and accessory dendrites (thin). Glomerular dendrites are multiple and extend to the glomeruli located in their respective half of the AOB, according to the anteroposterior axis [201]. The shape of the soma of the mitral cells depends on the number of glomerular dendrites it comprises, so its morphology will be oval, triangular, or polyhedral, presenting two, three, or more primary dendrites, respectively. The axons of these neurons leave the AOB caudally [189].
Another cell type described in rats are the round projection cells, which have an oval shape and paired dendrites [189]. Their axon emerges from the base of a proximal dendrite and extends towards the lateral olfactory tract. In addition, from their axon emerge one or two extensions that flow into the soma or dendritic processes of a neighboring cell.
Tufted cells are triangular in shape, although they can have a fusiform morphology. Their size is slightly smaller compared to mitral cells, but they have a similar general organization of their dendrites. However, tufted cells have a single glomerular dendrite that attaches to a single glomerulus. In these cells, the axon usually arises from the base of a non-glomerular dendrite, although it occasionally originates directly from the soma. Then, the axon descends ventrally, traveling a broad, zigzag path to end near the dwarf cells, external granular cells, or mitral cells.
In the granular layer, closer to the surface, we find the external granular cells: neurons with round or elliptical somas that have two or three dendrites. The primary dendrites tend to be short, while the secondary branches are longer and branch at their ends. Additionally, their dendrites show the presence of numerous dendritic gemmules. These cells lack an axon, so they are included in the category of amacrine cells. They sometimes integrate into the LOT fibers [189].
In the deeper part of the granular layer are the internal granular cells, which have round or triangular somas and also lack an axon [202]. Their dendritic processes consist of a thick ascending dendrite and one or two sets of short, thin branches. Like the external granular cells, they have gemmules on their ascending dendrites. Internal granular cells communicate with the dendrites of tufted cells and mitral cells but also with the somas of certain periglomerular cells and short axon surface neurons [189][203].
In a more internal layer of the granular layer, we find the dwarf cells and the polygonal neurons. Dwarf cells are interneurons without axons that have a very small, spherical soma, and their dendritic tree lacks gemmules [189]. Polygonal neurons are small fusiform bipolar neurons that have paired dendrites. Their axons branch out, extending through the neuropil, interacting with external granular cells and dwarf cells.
Moreover, in the accessory olfactory bulb, different types of glial cells can be found. The radial glia cells, like the olfactory envelope glial cells, are the most common, but astrocytes and oligodendrocytes are also observed [204].
Regarding the basic circuit that sensory information follows in the AOB, there are functionally three main neuronal components that are activated sequentially through vomeronasal stimulation: the axons of the vomeronasal sensory neurons, the mitral cells, and the granular cells [205]. The axons of the sensory vomeronasal neurons, present in the apical layer of the vomeronasal epithelium (V1R), are distributed in the anterior zone of the AOB [206], while the sensory vomeronasal neurons of the basal layer (V2R) project their axons to the caudal half of it [8]. On the other hand, although mitral cells receive information from several glomeruli, they connect only with axons of sensory vomeronasal neurons of the same type (V1R or V2R). Thus, a convergence of specific sensory inputs occurs in a small population of mitral cells. In the MOB, a much stricter convergence pattern occurs since the information received by each mitral cell comes from a single glomerulus [185].
Morphologically, the AOB shows significant differences among the multiple species of mammals. In those animal groups where this structure is most developed, clear lamination can be identified. However, as the researchers have mentioned, this generally does not allow differentiation of the external plexiform, mitral, and internal plexiform layers, with these forming a broad layer (mitral plexiform/external cellular), consisting of projection cells (mitral/main) distributed within a neuropil. On the other hand, species with a less sophisticated AOB have a very reduced mitral/plexiform layer in which it even becomes difficult to discriminate the projection cells.
The case of the platypus (Ornithorhynchus anatinus) is unique among mammals, as in this species the AOB and MOB are of similar size. Likewise, both the platypus and the echidna (Tachyglossus aculeatus), both monotremes, have well-differentiated layers forming the laminar structure of the AOB [162].
Rodents also possess an AOB with a high degree of development; however, as has been described, both the rat [189][207] and the mouse [84][208] display poorly differentiated boundaries between the external plexiform, mitral, and internal plexiform layers. Nevertheless, both murine species have a thick mitral-plexiform layer containing a dense network of mitral and tufted cells. In the case of lagomorphs, the AOB has extensive development, and more specifically, the rabbit (Oryctolagus cuniculus) exhibits a laminar configuration highlighting a broad mitral-plexiform layer formed by three main types of cells: large, tufted, and rounded cells [139].
Among the different species of bats, there is significant variability in the development of their AOB. Generally, they present only four distinguishable layers; however, in some cases, such as Glossophaga soricina, Frahm and Bhatnagar [209] suggested the presence of the plexiform layers (internal and external) and the mitral cell layer. The rest of the studied mammalian orders show the plexiform layers and the mitral layer merged into a single mitral-plexiform layer. Marsupials possess a sophisticated AOB; however, their lamination is restricted to four layers. Despite this, their mitral-plexiform layer has a considerable thickness. These characteristics have been observed in the gray short-tailed opossum (Monodelphis domestica) [210] and in the Tammar wallaby (Notamacropus eugenii) [118].
Carnivores generally display a surprisingly moderate development of their AOB, with domestic ones being the most studied (Figure 10). There are notable differences between felids and canids in this regard. Although both exhibit four layers in their lamination, felids show a more pronounced development of the mitral-plexiform layer [123][188]. However, in the red fox (Vulpes vulpes), a higher degree of AOB differentiation than in dogs has been observed, exhibiting a thick glomerular layer. Additionally, in the latter species, the imaginary lines separating different strata follow irregular trajectories, to the extent that the mitral-plexiform layer shows prominent extensions into the glomerular layer [211]. This raises the hypothesis of a possible involution of the vomeronasal system as a consequence of selection pressure and crossbreeding associated with the domestication of dogs. Differences between domesticated and wild canids are not limited to the VNS but also extend to the olfactory system. In a detailed analysis of the cribriform plate (CP) morphology across 46 dog breeds, a coyote, and a gray wolf using high-tech CT scans, it was observed that all dog breeds, even those known for their olfactory prowess, possess a CP surface area relative to body size that is smaller than in both wild canids [212]. These researchers previously established a correlation between CP size and the number of OR genes in a species, proposing that the CP size might represent evolutionary trends in mammalian olfaction [213].The presence of differences between domesticated animals and their closely related non-domesticated counterparts is not exclusive to canids. Significant differences in the expression of vomeronasal receptors have been identified between Mus subspecies and species. This suggests that these receptors could have a role in guiding behavioral adaptations. Furthermore, commonly used, highly inbred laboratory strains exhibit a significantly diminished capacity for differential pheromone-mediated behaviors [214]. From a strictly anatomical perspective, it is noteworthy that the differences in the organization of the olfactory bulb between domestic and wild canids are not so pronounced at the level of the main olfactory bulb. However, recent neuroanatomical observations have identified subtle morphological and neurochemical differences between the MOB of wolves and foxes and the MOB of dogs [215].
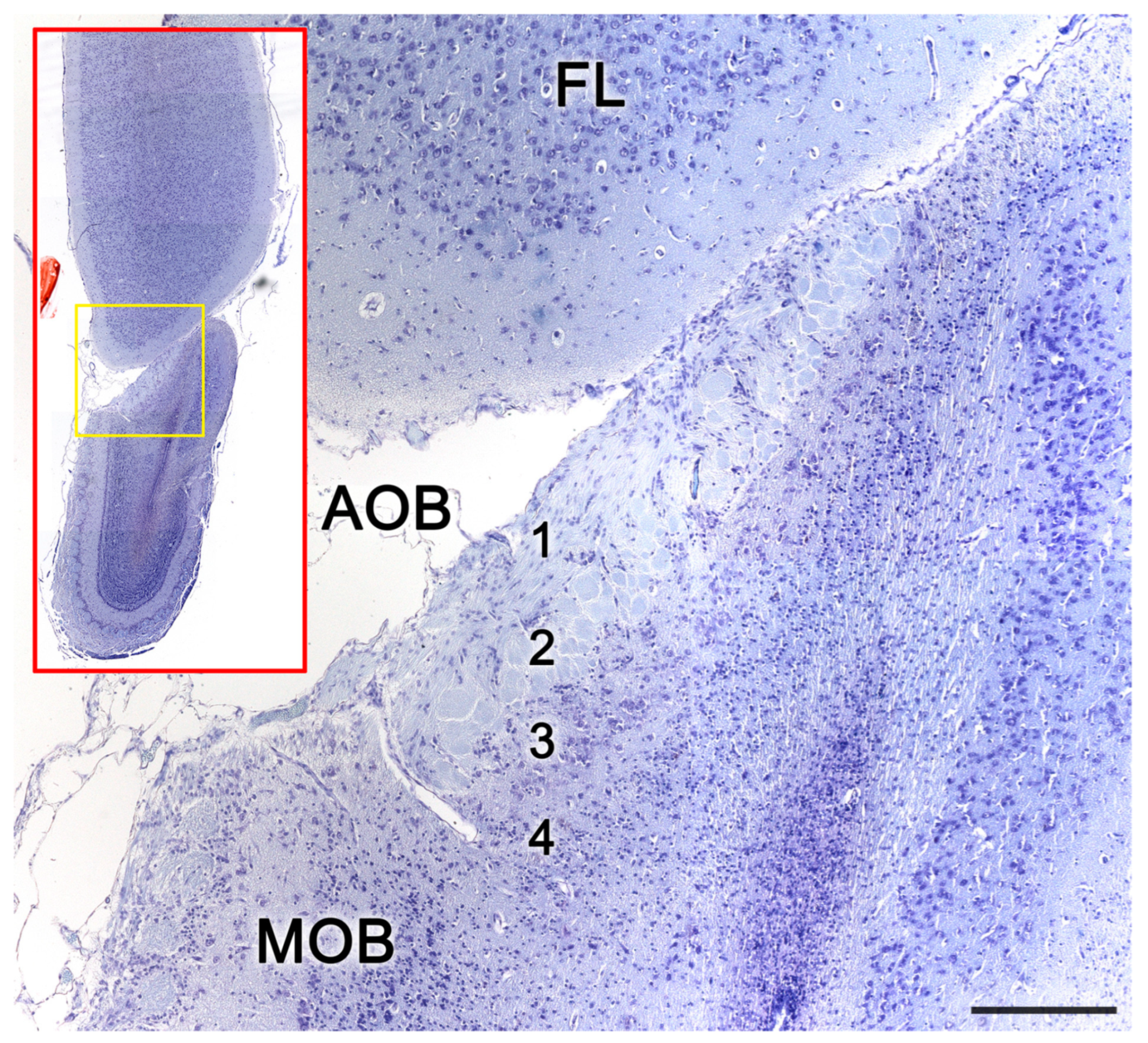
Figure 10. The accessory olfactory bulb of a cat. Nissl staining of a histological transverse section. The red box shows the topographic relationship of the olfactory bulb and the telencephalon frontal lobe. At higher magnification the yellow area containing the AOB allows to differentiate the lamination of the AOB. 1. Vomeronasal nerve layer; 2. Glomerular layer; 3. Mitral-plexiform layer; 4. Granular cell layer. Scale bar: 250 μm.
Despite the presumed regression of the VNS in dogs compared to their wild relatives, the wolves, there is a growing interest in exploring the clinical implications of the VNO in domestic dogs. This has been bolstered by the optimization of an MRI protocol for in vivo visualization of the VNO [216], and the recent identification of a case of canine vomeronasal agenesis, which manifested significant behavioral disorders, such as an inability for sexual discrimination of conspecifics and reduced sexual behavior [217]. Moreover, the use of pheromonotherapy, like the application of dog-appeasing pheromone (DAP) to mitigate symptoms of separation anxiety, including house soiling, vocalizations, and damage [218][219], along with a deepening in the links between VNO inflammation and aggressive behaviors in canids, felids, and livestock [220][221][222][223], has further developed this interest in the dog VNS.
Within the carnivores, the Mustelidae family has been the subject of extensive study, as the characterization of its AOB remains unresolved. Whereas the mink (Mustela vison) shows a well-developed VNO [224], the characterization of its AOB has proved challenging. Despite efforts, a precise and consistent morphological definition has not been possible using traditional neuroanatomical techniques [225]. However, the AOB has been studied more successfully in the ferret using immunohistochemical markers and lectins, which have revealed a poorly developed AOB [168].
The studied artiodactyls species, such as the sheep (Ovis orientalis aries), the Siberian roe deer (Capreolus pygargus), or the common hippopotamus (Hippopotamus amphibius), have a moderate development of the AOB (Figure 11), higher than carnivores, as they also exhibit four distinct layers forming their laminar organization [167][176][226].

Figure 11. The accessory olfactory bulb of a sheep. Consecutive Nissl (A) and Tolivia (B) staining of a sagittal section. The red box shows the topographic relationship of the olfactory bulb and the telencephalon frontal lobe. At higher magnification the yellow area containing the AOB allows to differentiate the lamination of the AOB. 1. Duramater; 2. Vomeronasal nerve layer; 3. Glomerular layer; 4. Mitral-plexiform layer; 5. Lateral olfactory tract; 6. Granular cell layer. Scale bar: 500 μm.
Regarding the neurochemical study of the AOB, the use of Gαi2 and Gαo proteins has garnered much attention, as both proteins indicate the expression of vomeronasal receptor families V1R and V2R, respectively, establishing an anteroposterior zonation in species that exhibit both receptors. This delineates the AOB organization into two areas: an anterior region rich in Gαi2 and a posterior region rich in Gαo [5]. Thus, the analysis of both G proteins allows an assessment if the studied species fits within a uniform model, or conversely, exhibits a segregated pattern. For the Gαi2 marker, labeling is restricted to the neural and glomerular layers of the AOB, while the Gαo marker is expressed in both the neural and glomerular layers as well as the other strata of the AOB [227].
On the other hand, various immunohistochemical markers have been utilized in AOB studies. Calcium-binding proteins such as calbindin (CB), calretinin (CR), parvalbumin (PV), neurocalcin (NC), and secretagogin are typically expressed in certain layers and cell populations, both in the AOB and BOP [210][228][229]. Additionally, CR labels mitral cells and can be expressed in atypical BOP glomeruli, typically located near the AOB [230]. The glial fibrillary acidic protein (GFAP) is used to generally identify the glial component of the AOB, and specifically astrocytes and ensheathing cells [231]. The microtubule-associated protein 2 (MAP2) is primarily expressed in the dendrites of mitral cells [232], leading to intense labeling in the external plexiform and mitral layers, but can also be expressed in the internal plexiform and granular layers. The growth-associated protein 43 (GAP-43), also termed the plasticity protein, is expressed in neuronal growth cones during development [233]. In the AOB, its immunolabeling is restricted to the neural, glomerular, and granular layers [139]. The olfactory marker protein (OMP) is involved in signal transduction and is used to evaluate neuronal maturity [234] of the olfactory system. In the AOB the expression of this marker is confined to the vomeronasal nerve and glomerular layers. Various markers in the OB are also employed to identify neuronal populations expressing different types of neuronal receptors, such as GABA receptors, dopaminergic receptors [235], cholinergic receptors [236], adrenergic receptors [237], and serotonergic receptors [238]. Additionally, immunohistochemical studies of various neuropeptides in the AOB, such as substance P, cholecystokinin, or neurotensin [239], and neuronal markers like PGP9.5 [240] or doublecortin (DCX) [241], are commonly conducted.
Another technique commonly used in the characterization of the AOB is histochemical labeling with lectins. The labeling pattern produced by each lectin may vary between the different species studied, although the Ulex europaeus agglutinin (UEA), Bandeiraea simplicifolia isolectin B4 (BSI-B4), and Lycopersicon esculentum agglutinin (LEA) are regularly expressed in the nervous and glomerular layers. Specifically, UEA allows differentiation of the anteroposterior zonation of the AOB in species of the segregated model, as it is expressed more intensely in the anterior region (VR1) [242]. In contrast, Vicia villosa agglutinin (VVA) shows more intense labeling in the posterior part of the AOB [243]. In this way, different neuronal populations can be identified. Many other lectins are also used in the study of the AOB, such as soybean lectin (SBA), wheat germ lectin (WGA), and Dolichos biflorus agglutinin (DBA) [244][245].
2.3. Olfactory Pathways
Regarding the vomeronasal information flow, vomeronasal sensory neurons send the sensory information received from the vomeronasal receptor epithelium to the mitral cells of the AOB [246]. In contrast, olfactory information is captured in the main olfactory epithelium and later travels through the olfactory nerves to the MOB. Then, these stimuli are transmitted through various projections to different brain areas.
The application of neuronal tracer studies to the olfactory pathways allowed the discrimination of the existence of segregated and parallel projections from the main and accessory olfactory bulbs [247][248]. This was known as the dual olfactory system hypothesis, a formulation demonstrated for the first time using the Fink–Heimer technique in rabbits [249]. Although this hypothesis is still currently valid, it has been refined over the past decades, especially regarding the study of secondary and tertiary projections, proving the existence of neuronal communication between both pathways.
The efferences of the MOB project ipsilaterally to the anterior olfactory nucleus, tenia tecta, olfactory tubercle, piriform cortex, lateral and medial areas of the entorhinal cortex, and lateral amygdaloid nuclei [250][251]. Among the tertiary projections of the pathway, the communication of the entorhinal cortex with the hippocampus, and the piriform cortex and lateral amygdala with the hypothalamus and mediodorsal thalamic nucleus, stand out [252][253]. It has recently been shown that there is a direct projection of olfactory sensory information to the anterior subdivision of the medial amygdala, a structure traditionally involved in mediating pheromonal information. This supports the hypothesis that both olfactory systems act collaboratively and not differentially in the control of socio-sexual and anti-predator behaviors [254].
Unlike the main olfactory system, efferent projections from the AOB bypass the thalamocortical axis, distributing ipsilaterally to third-order limbic system nuclei, such as the accessory olfactory tract nucleus, the terminal stria nucleus, and the medial and posteromedial cortical amygdaloid nuclei (together forming the vomeronasal amygdala) [248][255][256][257][258]. The medial amygdala is strongly interconnected with other structures that receive vomeronasal information from the AOB. This suggests that the information detected by the vomeronasal system undergoes complex intrinsic processing before being transmitted to other structures [259]. The final processing center between vomeronasal information and effectors is the hypothalamus [260]. In this way, the VNS is directly involved in the activity of sex hormones and can facilitate the development of aggressive, defensive, or reproductive behaviors [261]. It is important to note that olfactory and vomeronasal projections show some degree of overlap in the amygdaloid cortex and medial amygdala, suggesting that there are anatomical pathways that allow extensive integration of olfactory and vomeronasal information [259][262][263].
Additionally, the MOB and AOB differ in their centrifugal afferent connections [264]. The MOB receives massive cholinergic and GABAergic projections from the basal forebrain, which mainly originate in the nucleus of the horizontal branch of Broca diagonal band and the magnocellular preoptic nucleus [265]. Cholinergic signaling within the OB modulates olfactory learning and memory, odor discrimination, odor habituation, and social interactions [266]. From a comparative neuroanatomy perspective, the study by Liberia et al. [267] indicates that the synaptic connectivity of the afferent cholinergic circuits is highly conserved in the OB of macrosmatic and microsmatic mammals. Regarding the GABAergic afferents of the basal forebrain, they innervate all the layers of the MOB at least as densely as cholinergic axons, but only a few studies have examined their function in odor processing [268].
The AOB receives significant afferents from a wide range of nerve centers. The bed nucleus of the stria terminalis and the VN amygdala project reciprocally to the AOB, thus forming a feedback circuit [269]. Tracer studies have revealed that both feedback projections to the AOB are topographically organized and use different neurotransmitters [270]. Specifically, GABAergic projections from the bed nucleus terminate in the outer cell layer, while glutamatergic projections from the amygdala are directed to the inner layer of granule cells. A significant number of these feedback neurons in both areas express estrogen receptors ER-α, linking the animal endocrine state with integration in the AOB. The relevance of this pathway was subsequently confirmed with different morphofunctional approaches [271][272].
Other important afferents for the function of the AOB come from noradrenergic structures in the brainstem, which play a crucial role in the formation of olfactory memory [273]. This comes into play, for example, during mating, in which stimulation of the vaginocervical zone leads to sustained increases in the levels of noradrenaline in the AOB, which persist for about 4 h [274]. This time span establishes a crucial phase in which noradrenaline generates plastic changes in the intensity of dendrodendritic synaptic connections [275]. Additionally, the diagonal band of Broca is the origin of numerous cholinergic fibers involved in increasing the excitability of granule cells [276], while from the raphe, abundant serotonergic fibers are sent to both olfactory bulbs that act similarly to the cholinergic ones [238].
In summary, the olfactory and vomeronasal systems represent two separate systems which are functionally interconnected and are in charge of processing olfactory and pheromonal stimuli, respectively. These systems differ in their afferent and efferent connections, morphology, physiology, and functional implications, but both contribute to the complex sensory processing of olfactory and pheromonal information that leads to behavior [277]. The increasing evidence suggests the existence of a more complex and intricate relationship between these systems than previously assumed. Recognizing and understanding the complexities of this interaction will provide a clearer picture of how odors and pheromones shape animal behavior.
This entry is adapted from the peer-reviewed paper 10.3390/anatomia2040031
References
- Filoramo, N.I.; Schwenk, K. The Mechanism of Chemical Delivery to the Vomeronasal Organs in Squamate Reptiles: A Comparative Morphological Approach. J. Exp. Zool. 2009, 311, 20–34.
- Kondoh, D.; Kaneoya, Y.; Tonomori, W.; Kitayama, C. Histological Features and Gα olf Expression Patterns in the Nasal Cavity of Sea Turtles. J. Anat. 2023, 243, 486–503.
- Reiss, J.O.; Eisthen, H.L. Comparative Anatomy and Physiology of Chemical Senses in Amphibians. In Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates; University of California Press: Berkeley, CA, USA, 2008; ISBN 978-0-520-25278-3.
- Monti-Bloch, L.; Jennings-White, C.; Berliner, D.L. The Human Vomeronasal System: A Review. Ann. N. Y. Acad. Sci. 1998, 855, 373–389.
- Tirindelli, R. Coding of Pheromones by Vomeronasal Receptors. Cell Tissue Res. 2021, 383, 367–386.
- Shinohara, H.; Asano, T.; Kato, K. Differential Localization of G-Proteins Gi and Go in the Accessory Olfactory Bulb of the Rat. J. Neurosci. 1992, 12, 1275–1279.
- Dulac, C.; Axel, R. A Novel Family of Genes Encoding Putative Pheromone Receptors in Mammals. Cell 1995, 83, 195–206.
- Herrada, G.; Dulac, C. A Novel Family of Putative Pheromone Receptors in Mammals with a Topographically Organized and Sexually Dimorphic Distribution. Cell 1997, 90, 763–773.
- Matsunami, H.; Buck, L.B. A Multigene Family Encoding a Diverse Array of Putative Pheromone Receptors in Mammals. Cell 1997, 90, 775–784.
- Ryba, N.J.P.; Tirindelli, R. A New Multigene Family of Putative Pheromone Receptors. Neuron 1997, 19, 371–379.
- Rivière, S.; Challet, L.; Fluegge, D.; Spehr, M.; Rodriguez, I. Formyl Peptide Receptor-like Proteins Are a Novel Family of Vomeronasal Chemosensors. Nature 2009, 459, 574–577.
- Brennan, P.A. The Vomeronasal System. Cell. Mol. Life Sci. 2001, 58, 546–555.
- Papes, F.; Logan, D.W.; Stowers, L. The Vomeronasal Organ Mediates Interspecies Defensive Behaviors through Detection of Protein Pheromone Homologs. Cell 2010, 141, 692–703.
- Halpern, M.; Kubie, J.L. The Role of the Ophidian Vomeronasal System in Species-Typical Behavior. Trends Neurosci. 1984, 7, 472–477.
- Placyk, J.S., Jr.; Graves, B.M. Prey Detection by Vomeronasal Chemoreception in a Plethodontid Salamander. J. Chem. Ecol. 2002, 28, 1017–1036.
- Halpern, M.; Daniels, Y.; Zuri, I. The Role of the Vomeronasal System in Food Preferences of the Gray Short-Tailed Opossum, Monodelphis Domestica. Nutr. Metab. 2005, 2, 6.
- Dagg, A.I.; Taub, A. Flehmen. Mammalia 1970, 34, 686–695.
- Estes, R.D. The Role of the Vomeronasal Organ in Mammalian Reproduction. Mammalia 1972, 36, 315–341.
- Hart, B.L. Flehmen Behavior and Vomeronasal Organ Function. In Chemical Signals in Vertebrates 3; Müller-Schwarze, D., Silverstein, R.M., Eds.; Springer: Boston, MA, USA, 1983; pp. 87–103. ISBN 978-1-4757-9652-0.
- Hart, L.A.; Hart, B.L. Flehmen, Osteophagia, and Other Behaviors of Giraffes (Giraffa giraffa angolensis): Vomeronasal Organ Adaptation. Animals 2023, 13, 354.
- Jacobson, L. Anatomisk Beskrivelse over et myt Organ i Huusdyrenes Naese. Vet. Selsk. Skr. 1813, 2, 209–246.
- Ruysch, F. Thesaurus Anatomicus Tertius; J. Wolters: Amsterdam, Netherlands, 1703; pp. 48–49.
- Dursy, E. Zur Entwicklungsgeschichte des Kopfes des Menschen und der höheren Wirbelthiere: Mit Holzschnitten und einem Atlas von neun Kupfertafeln mit erklärendem Texte; H. Laupp: Tübingen, Germany, 1869.
- Kölliker, A. Über Die Jacobson’schen Organe Des Menschen. In Festschrift zu dem 40 jährign Professoren-Jubiläum des Herrn Franz von Rinecker 31 März 1877; Wilhelm Engelmann: Leipzig, Germany, 1877; pp. 3–11.
- Balogh, C. Das Jacobson’sche Organ des Schafes, Sitzungsberichte Der Kais. Akad. Der Wiss. Wien. 1860, 42, 449–476.
- Klein, E. Memoirs: Contributions to the Minute Anatomy of the Nasal Mucous Membrane. J. Cell Sci. 1881, 2, 98–113.
- Piana, G. Contribuzioni alla conoscenza della strutture e della funzione dell’ organo di jacobson. Deusch. Zeitsch. f. Tiermedizin 1882, 7, 325.
- Retzius, G. Die riechzellen der ophidier in der riechschleimhaut und im jacobson’schen organ. Biol. Untersuch. Neue Folge 1894, 6, 48–51.
- Gudden, B.V. Experimentaluntersuchungen Über Das Peripherische Und Centrale Nervensystem. Arch. Psychiatr. Nervenkr. 1870, 2, 693–723.
- Ramón y Cajal, S.R. Textura Del Lobulo Olfativo Accesorio. Rev. Micros. 1902, 1, 141–150.
- McCotter, R.E. The Connection of the Vomeronasal Nerves with the Accessory Olfactory Bulb in the Opossum and Other Mammals. Anat. Rec. 1912, 6, 299–318.
- Vandenbergh, J.G. Male Odor Accelerates Female Sexual Maturation in Mice. Endocrinology 1969, 84, 658–660.
- Whitten, W.K. Modification of the Oestrous Cycle of the Mouse by External Stimuli Associated with the Male. J. Endocrinol. 1956, 13, 399–404.
- Powers, J.B.; Winans, S.S. Vomeronasal Organ: Critical Role in Mediating Sexual Behavior of the Male Hamster. Science 1975, 187, 961–963.
- Villamayor, P.R.; Arana, Á.J.; Coppel, C.; Ortiz-Leal, I.; Torres, M.V.; Sanchez-Quinteiro, P.; Sánchez, L. A Comprehensive Structural, Lectin and Immunohistochemical Characterization of the Zebrafish Olfactory System. Sci. Rep. 2021, 11, 8865.
- Grus, W.E.; Zhang, J. Origin and Evolution of the Vertebrate Vomeronasal System Viewed through System-Specific Genes. Bioessays 2006, 28, 709–718.
- González, A. Lungfishes, like Tetrapods, Possess a Vomeronasal System. Front. Neuroanat. 2010, 4, 130.
- Nakamuta, S.; Nakamuta, N.; Taniguchi, K.; Taniguchi, K. Histological and Ultrastructural Characteristics of the Primordial Vomeronasal Organ in Lungfish. Anat. Rec. 2012, 295, 481–491.
- Nakamuta, S.; Yamamoto, Y.; Miyazaki, M.; Sakuma, A.; Nikaido, M.; Nakamuta, N. Type 1 Vomeronasal Receptors Expressed in the Olfactory Organs of Two African Lungfish, Protopterus annectens and Protopterus amphibius. J. Comp. Neurol. 2023, 531, 116–131.
- Wittmer, C.; Nowack, C. Epithelial Crypts: A Complex and Enigmatic Olfactory Organ in African and South American Lungfish (Lepidosireniformes, Dipnoi). J. Morphol. 2017, 278, 791–800.
- Swaney, W.T.; Keverne, E.B. The Evolution of Pheromonal Communication. Behav. Brain Res. 2009, 200, 239–247.
- Pihlström, H. Comparative Anatomy and Physiology of Chemical Senses in Aquatic Mammals. In Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates; Thewissen, J.G.M., Ed.; University of California Press: Berkeley, CA, USA, 2008; ISBN 978-0-520-25278-3.
- Suzuki, H.; Nishida, H.; Kondo, H.; Yoda, R.; Iwata, T.; Nakayama, K.; Enomoto, T.; Wu, J.; Moriya-Ito, K.; Miyazaki, M.; et al. A Single Pheromone Receptor Gene Conserved across 400 Million Years of Vertebrate Evolution. Mol. Biol. Evol. 2018, 35, 2928–2939.
- Breathnach, A.S. The Cetacean Central Nervous System. Biol. Rev. 1960, 35, 187–230.
- McGowen, M.R.; Clark, C.; Gatesy, J. The Vestigial Olfactory Receptor Subgenome of Odontocete Whales: Phylogenetic Congruence between Gene-Tree Reconciliation and Supermatrix Methods. Syst. Biol. 2008, 57, 574–590.
- Kishida, T. Olfaction of Aquatic Amniotes. Cell Tissue Res. 2021, 383, 353–365.
- Halpern, M. Nasal Chemical Senses in Reptiles: Structure and Function. In Hormones, Brain, and Behaviour. Biology of the Reptilia; University of Chicago Press: Chicago, IL, USA, 1992; Volume 18, pp. 423–523.
- Schwenk, K. Of Tongues and Noses: Chemoreception in Lizards and Snakes. Trends Ecol. Evol. 1995, 10, 7–12.
- Kondoh, D.; Yamamoto, Y.; Nakamuta, N.; Taniguchi, K.; Taniguchi, K. Lectin Histochemical Studies on the Olfactory Epithelium and Vomeronasal Organ in the Japanese Striped Snake, Elaphe Quadrivirgata. J. Morphol. 2010, 271, 1197–1203.
- Kondoh, D.; Yamamoto, Y.; Nakamuta, N.; Taniguchi, K.; Taniguchi, K. Seasonal Changes in the Histochemical Properties of the Olfactory Epithelium and Vomeronasal Organ in the Japanese Striped Snake, Elaphe Quadrivirgata: Seasonal Changes in Snake Olfactory Organs. Anat. Histol. Embryol. 2012, 41, 41–53.
- Hatanaka, T.; Matsuzaki, O. Odor Responses of the Vomeronasal System in Reeve’s Turtle, Geoclemys reevesii. Brain Behav. Evol. 1993, 41, 183–186.
- Parsons, T.S. Studies on the Comparative Embryology of the Reptilian Nose. Bull. Mus. Comp. Zool. Harv. Coll. 1959, 120, 261–275.
- Parsons, T.S. Evolution of the Nasal Structure in the Lower Tetrapods. Am. Zool. 1967, 7, 397–413.
- Houck, L.D. Pheromone Communication in Amphibians and Reptiles. Annu. Rev. Physiol. 2009, 71, 161–176.
- Eisthen, H.L. Presence of the Vomeronasal System in Aquatic Salamanders. Philos. Trans. R. Soc. Lond. B 2000, 355, 1209–1213.
- Silva, L.; Antunes, A. Vomeronasal Receptors in Vertebrates and the Evolution of Pheromone Detection. Annu. Rev. Anim. Biosci. 2017, 5, 353–370.
- Bhatnagar, K.P.; Meisami, E. Vomeronasal Organ in Bats and Primates: Extremes of Structural Variability and Its Phylogenetic Implications. Microsc. Res. Tech. 1998, 43, 465–475.
- Suárez, R.; Fernández-Aburto, P.; Manger, P.R.; Mpodozis, J. Deterioration of the Gαo Vomeronasal Pathway in Sexually Dimorphic Mammals. PLoS ONE 2011, 6, e26436.
- Kondoh, D.; Watanabe, K.; Nishihara, K.; Ono, Y.S.; Nakamura, K.G.; Yuhara, K.; Tomikawa, S.; Sugimoto, M.; Kobayashi, S.; Horiuchi, N.; et al. Histological Properties of Main and Accessory Olfactory Bulbs in the Common Hippopotamus. Brain Behav. Evol. 2017, 90, 224–231.
- Tomiyasu, J.; Korzekwa, A.; Kawai, Y.K.; Robstad, C.A.; Rosell, F.; Kondoh, D. The Vomeronasal System in Semiaquatic Beavers. J. Anat. 2022, 241, 809–819.
- Grus, W.E.; Shi, P.; Zhang, J. Largest Vertebrate Vomeronasal Type 1 Receptor Gene Repertoire in the Semiaquatic Platypus. Mol. Biol. Evol. 2007, 24, 2153–2157.
- Cartmill, M. Rethinking Primate Origins: The Characteristic Primate Traits Cannot Be Explained Simply as Adaptations to Arboreal Life. Science 1974, 184, 436–443.
- Heesy, C.P.; Ross, C.F. Evolution of Activity Patterns and Chromatic Vision in Primates: Morphometrics, Genetics and Cladistics. J. Hum. Evol. 2001, 40, 111–149.
- Wang, G.; Shi, P.; Zhu, Z.; Zhang, Y.-P. More Functional V1R Genes Occur in Nest-Living and Nocturnal Terricolous Mammals. Genom. Biol. Evol. 2010, 2, 277–283.
- Garrett, E.C.; Dennis, J.C.; Bhatnagar, K.P.; Durham, E.L.; Burrows, A.M.; Bonar, C.J.; Steckler, N.K.; Morrison, E.E.; Smith, T.D. The Vomeronasal Complex of Nocturnal Strepsirhines and Implications for the Ancestral Condition in Primates: Vomeronasal Complex of Nocturnal Strepsirhines. Anat. Rec. 2013, 296, 1881–1894.
- Smith, T.D.; Garrett, E.C.; Bhatnagar, K.P.; Bonar, C.J.; Bruening, A.E.; Dennis, J.C.; Kinznger, J.H.; Johnson, E.W.; Morrison, E.E. The Vomeronasal Organ of New World Monkeys (Platyrrhini). Anat. Rec. 2011, 294, 2158–2178.
- Smith, T.D.; Siegel, M.I.; Bonar, C.J.; Bhatnagar, K.P.; Mooney, M.P.; Burrows, A.M.; Smith, M.A.; Maico, L.M. The Existence of the Vomeronasal Organ in Postnatal Chimpanzees and Evidence for Its Homology with That of Humans. J. Anat. 2001, 198, 77–82.
- Smith, T.D.; Siegel, M.I.; Bhatnagar, K.P. Reappraisal of the Vomeronasal System of Catarrhine Primates: Ontogeny, Morphology, Functionality, and Persisting Questions. Anat. Rec. 2001, 265, 176–192.
- Boehm, N.; Roos, J.; Gasser, B. Luteinizing Hormone-Releasing Hormone (LHRH)-Expressing Cells in the Nasal Septum of Human Fetuses. Dev. Brain Res. 1994, 82, 175–180.
- Kjær, I.; Hansen, B.F. The Human Vomeronasal Organ: Prenatal Developmental Stages and Distribution of Luteinizing Hormone-Releasing Hormone. Eur. J. Oral Sci. 1996, 104, 34–40.
- Humphrey, T. The Development of the Olfactory and the Accessory Olfactory Formations in Human Embryos and Fetuses. J. Comp. Neurol. 1940, 73, 431–468.
- Moran, D.T.; Jafek, B.W.; Rowley, J.C. The Vomeronasal (Jacobson’s) Organ in Man: Ultrastructure and Frequency of Occurence. J. Steroid Biochem. Mol. Biol. 1991, 39, 545–552.
- Hazem, A.G.; Ahmed, A.T.; Tantawy, A.M.; Diaa, M.H.; Hesham, M.S. The Vomeronasal (Jacobson’s) Organ in Adult Humans: Frequency of Occurrence and Enzymatic Study. Acta Otolaryngol. 1998, 118, 409–412.
- Witt, M.; Hummel, T. Vomeronasal Versus Olfactory Epithelium: Is There a Cellular Basis for Human Vomeronasal Perception? Int. Rev. Cytol. 2006, 248, 209–259.
- Meredith, M. Human Vomeronasal Organ Function: A Critical Review of Best and Worst Cases. Chem. Senses 2001, 26, 433–445.
- Zhang, J.; Webb, D.M. Evolutionary Deterioration of the Vomeronasal Pheromone Transduction Pathway in Catarrhine Primates. Proc. Natl. Acad. Sci. USA 2003, 100, 8337–8341.
- Smith, T.D.; Laitman, J.T.; Bhatnagar, K.P. The Shrinking Anthropoid Nose, the Human Vomeronasal Organ, and the Language of Anatomical Reduction: The Shrinking Anthropoid Nose. Anat. Rec. 2014, 297, 2196–2204.
- Negus, V.E. The Organ of Jacobson. J. Anat. 1956, 90, 515–519.
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M. Comparative Anatomy of the Vomeronasal Cartilage in Mammals: Mink, Cat, Dog, Pig, Cow and Horse. Ann. Anat. 1995, 177, 475–481.
- Salazar, I.; Sanchez-Quinteiro, P. Supporting Tissue and Vasculature of the Mammalian Vomeronasal Organ: The Rat as a Model. Microsc. Res. Tech. 1998, 41, 492–505.
- Døving, K.B.; Trotier, D. Structure and Function of the Vomeronasal Organ. J. Exp. Biol. 1998, 201, 2913–2925.
- Menco, B.P.M. Ultrastructural Aspects of Olfactory Signaling. Chem. Senses 1997, 22, 295–311.
- Menco, B.P.M.; Carr, V.M.; Ezeh, P.I.; Liman, E.R.; Yankova, M.P. Ultrastructural Localization of G-Proteins and the Channel Protein TRP2 to Microvilli of Rat Vomeronasal Receptor Cells. J. Comp. Neurol. 2001, 438, 468–489.
- Salazar, I.; Sanchez-Quinteiro, P.; Cifuentes, J.M.; De Troconiz, P.F. General Organization of the Perinatal and Adult Accessory Olfactory Bulb in Mice. Anat. Rec. 2006, 288, 1009–1025.
- Jungblut, L.D.; Reiss, J.O.; Pozzi, A.G. Olfactory Subsystems in the Peripheral Olfactory Organ of Anuran Amphibians. Cell Tissue Res. 2021, 383, 289–299.
- Mendoza, A.S.; Kühnel, W. Morphological Evidence for a Direct Innervation of the Mouse Vomeronasal Glands. Cell Tissue Res. 1987, 247, 457–459.
- Takami, S.; Getchell, M.L.; Getchell, T.V. Resolution of Sensory and Mucoid Glycoconjugates with Terminal α-Galactose Residues in the Mucomicrovillar Complex of the Vomeronasal Sensory Epithelium by Dual Confocal Laser Scanning Microscopy. Cell Tissue Res. 1995, 280, 211–216.
- Kondoh, D.; Tomiyasu, J.; Itakura, R.; Sugahara, M.; Yanagawa, M.; Watanabe, K.; Alviola, P.A.; Yap, S.A.; Cosico, E.A.; Cruz, F.A.; et al. Comparative Histological Studies on Properties of Polysaccharides Secreted by Vomeronasal Glands of Eight Laurasiatheria Species. Acta Histochem. 2020, 122, 151515.
- Salazar, I.; Sánchez Quinteiro, P.; Cifuentes, J.M.; Fernández, P.; Lombardero, M. Distribution of the Arterial Supply to the Vomeronasal Organ in the Cat. Anat. Rec. 1997, 247, 129–136.
- Meredith, M.; O’Connell, R.J. Efferent Control of Stimulus Access to the Hamster Vomeronasal Organ. J. Physiol. 1979, 286, 301–316.
- Eccles, R. Autonomic Innervation of the Vomeronasal Organ of the Cat. Physiol. Behav. 1982, 28, 1011–1015.
- Meredith, M. Chronic Recording of Vomeronasal Pump Activation in Awake Behaving Hamsters. Physiol. Behav. 1994, 56, 345–354.
- Halpern, M.; Shapiro, L.S.; Jia, C. Heterogeneity in the Accessory Olfactory System. Chem. Senses 1998, 23, 477–481.
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829.
- Villamayor, P.R.; Robledo, D.; Fernández, C.; Gullón, J.; Quintela, L.; Sánchez-Quinteiro, P.; Martínez, P. Analysis of the Vomeronasal Organ Transcriptome Reveals Variable Gene Expression Depending on Age and Function in Rabbits. Genomics 2021, 113, 2240–2252.
- Liberles, S.D.; Horowitz, L.F.; Kuang, D.; Contos, J.J.; Wilson, K.L.; Siltberg-Liberles, J.; Liberles, D.A.; Buck, L.B. Formyl Peptide Receptors Are Candidate Chemosensory Receptors in the Vomeronasal Organ. Proc. Natl. Acad. Sci. USA 2009, 106, 9842–9847.
- Bufe, B.; Teuchert, Y.; Schmid, A.; Pyrski, M.; Pérez-Gómez, A.; Eisenbeis, J.; Timm, T.; Ishii, T.; Lochnit, G.; Bischoff, M.; et al. Bacterial MgrB Peptide Activates Chemoreceptor Fpr3 in Mouse Accessory Olfactory System and Drives Avoidance Behaviour. Nat. Commun. 2019, 10, 4889.
- Swain, S.L. T Cell Subsets and the Recognition of MHC Class. Immunol. Rev. 1983, 74, 129–142.
- Singh, P. Chemosensation and Genetic Individuality. Reproduction 2001, 121, 529–539.
- Brennan, P.A.; Kendrick, K.M. Mammalian Social Odours: Attraction and Individual Recognition. Philos. Trans. R. Soc. B 2006, 361, 2061–2078.
- Ruff, J.S.; Nelson, A.C.; Kubinak, J.L.; Potts, W.K. MHC Signaling during Social Communication. In Self and Nonself; Advances in Experimental Medicine and Biology; López-Larrea, C., Ed.; Springer: New York, NY, USA, 2012; Volume 738, pp. 290–313. ISBN 978-1-4614-1679-1.
- Yamazaki, K.; Beauchamp, G.K. Genetic Basis for MHC-Dependent Mate Choice. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 2007; Volume 59, pp. 129–145. ISBN 978-0-12-017660-1.
- Leinders-Zufall, T.; Brennan, P.; Widmayer, P.; Shivalingappa, P.C.; Maul-Pavicic, A.; Jäger, M.; Li, X.-H.; Breer, H.; Zufall, F.; Boehm, T. MHC Class I Peptides as Chemosensory Signals in the Vomeronasal Organ. Science 2004, 306, 1033–1037.
- Konjević, D.; Erman, V.; Bujanić, M.; Svetličić, I.; Arbanasić, H.; Lubura Strunjak, S.; Galov, A. Wild Boar (Sus scrofa)—Fascioloides Magna Interaction from the Perspective of the MHC Genes. Pathogens 2022, 11, 1359.
- Milinski, M.; Griffiths, S.; Wegner, K.M.; Reusch, T.B.H.; Haas-Assenbaum, A.; Boehm, T. Mate Choice Decisions of Stickleback Females Predictably Modified by MHC Peptide Ligands. Proc. Natl. Acad. Sci. USA 2005, 102, 4414–4418.
- Leinders-Zufall, T.; Lane, A.P.; Puche, A.C.; Ma, W.; Novotny, M.V.; Shipley, M.T.; Zufall, F. Ultrasensitive Pheromone Detection by Mammalian Vomeronasal Neurons. Nature 2000, 405, 792–796.
- Spehr, M.; Kelliher, K.R.; Li, X.-H.; Boehm, T.; Leinders-Zufall, T.; Zufall, F. Essential Role of the Main Olfactory System in Social Recognition of Major Histocompatibility Complex Peptide Ligands. J. Neurosci. 2006, 26, 1961–1970.
- Liman, E.R.; Corey, D.P.; Dulac, C. TRP2: A Candidate Transduction Channel for Mammalian Pheromone Sensory Signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 5791–5796.
- Leypold, B.G.; Yu, C.R.; Leinders-Zufall, T.; Kim, M.M.; Zufall, F.; Axel, R. Altered Sexual and Social Behaviors in Trp2 Mutant Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 6376–6381.
- Stowers, L.; Holy, T.E.; Meister, M.; Dulac, C.; Koentges, G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science 2002, 295, 1493–1500.
- Zhang, G.; Li, C.; Li, Q.; Li, B.; Larkin, D.M.; Lee, C.; Storz, J.F.; Antunes, A.; Greenwold, M.J.; Meredith, R.W.; et al. Comparative Genomics Reveals Insights into Avian Genome Evolution and Adaptation. Science 2014, 346, 1311–1320.
- Shi, P.; Zhang, J. Comparative Genomic Analysis Identifies an Evolutionary Shift of Vomeronasal Receptor Gene Repertoires in the Vertebrate Transition from Water to Land. Genome Res. 2007, 17, 166–174.
- Young, J.M.; Massa, H.F.; Hsu, L.; Trask, B.J. Extreme Variability among Mammalian V1R Gene Families. Genome Res. 2010, 20, 10–18.
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A Putative Pheromone Receptor Gene Expressed in Human Olfactory Mucosa. Nat. Genet. 2000, 26, 18–19.
- Baum, M.J.; Kelliher, K.R. Complementary Roles of the Main and Accessory Olfactory Systems in Mammalian Mate Recognition. Annu. Rev. Physiol. 2009, 71, 141–160.
- Omura, M.; Mombaerts, P. Trpc2-Expressing Sensory Neurons in the Main Olfactory Epithelium of the Mouse. Cell Rep. 2014, 8, 583–595.
- Omura, M.; Mombaerts, P. Trpc2-Expressing Sensory Neurons in the Mouse Main Olfactory Epithelium of Type B Express the Soluble Guanylate Cyclase Gucy1b2. Mol. Cell. Neurosci. 2015, 65, 114–124.
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. Goα Expression in the Vomeronasal Organ and Olfactory Bulb of the Tammar Wallaby. Chem. Senses 2012, 37, 567–577.
- Suárez, R.; Mpodozis, J. Heterogeneities of Size and Sexual Dimorphism between the Subdomains of the Lateral-Innervated Accessory Olfactory Bulb (AOB) of Octodon degus (Rodentia: Hystricognathi). Behav. Brain Res. 2009, 198, 306–312.
- Villamayor, P.R.; Cifuentes, J.M.; Fdz-de-Troconiz, P.; Sanchez-Quinteiro, P. Morphological and Immunohistochemical Study of the Rabbit Vomeronasal Organ. J. Anat. 2018, 233, 814–827.
- Halpern, M.; Shapiro, L.S.; Jia, C. Differential Localization of G Proteins in the Opossum Vomeronasal System. Brain Res. 1995, 677, 157–161.
- Young, J.M.; Trask, B.J. V2R Gene Families Degenerated in Primates, Dog and Cow, but Expanded in Opossum. Trends Genet. 2007, 23, 212–215.
- Salazar, I.; Cifuentes, J.M.; Sánchez-Quinteiro, P. Morphological and Immunohistochemical Features of the Vomeronasal System in Dogs. Anat. Rec. 2013, 296, 146–155.
- Moriya-Ito, K.; Hayakawa, T.; Suzuki, H.; Hagino-Yamagishi, K.; Nikaido, M. Evolution of Vomeronasal Receptor 1 (V1R) Genes in the Common Marmoset (Callithrix jacchus). Gene 2018, 642, 343–353.
- Kishimoto, J.; Keverne, E.B.; Emson, P.C. Calretinin, Calbindin-D28k and Parvalbumin-like Immunoreactivity in Mouse Chemoreceptor Neurons. Brain Res. 1993, 610, 325–329.
- Verhaagen, J.; Oestreicher, A.; Gispen, W.; Margolis, F. The Expression of the Growth Associated Protein B50/GAP43 in the Olfactory System of Neonatal and Adult Rats. J. Neurosci. 1989, 9, 683–691.
- Salazar, I.; Sánchez Quinteiro, P. Lectin Binding Patterns in the Vomeronasal Organ and Accessory Olfactory Bulb of the Rat. Anat. Embryol. 1998, 198, 331–339.
- Ichikawa, M.; Osada, T.; Ikai, A. Bandeiraea Simplicifolia Lectin I and Vicia Villosa Agglutinin Bind Specifically to the Vomeronasal Axons in the Accessory Olfactory Bulb of the Rat. Neurosci. Res. 1992, 13, 73–79.
- Ibarra-Soria, X.; Levitin, M.O.; Saraiva, L.R.; Logan, D.W. The Olfactory Transcriptomes of Mice. PLoS Genet. 2014, 10, e1004593.
- Francia, S.; Silvotti, L.; Ghirardi, F.; Catzeflis, F.; Percudani, R.; Tirindelli, R. Evolution of Spatially Coexpressed Families of Type-2 Vomeronasal Receptors in Rodents. Genom. Biol. Evol. 2015, 7, 272–285.
- Saraiva, L.R.; Ahuja, G.; Ivandic, I.; Syed, A.S.; Marioni, J.C.; Korsching, S.I.; Logan, D.W. Molecular and Neuronal Homology between the Olfactory Systems of Zebrafish and Mouse. Sci. Rep. 2015, 5, 11487.
- Yohe, L.R.; Davies, K.T.J.; Rossiter, S.J.; Dávalos, L.M. Expressed Vomeronasal Type-1 Receptors (V1rs) in Bats Uncover Conserved Sequences Underlying Social Chemical Signaling. Genom. Biol. Evol. 2019, 11, 2741–2749.
- Haga, S.; Hattori, T.; Sato, T.; Sato, K.; Matsuda, S.; Kobayakawa, R.; Sakano, H.; Yoshihara, Y.; Kikusui, T.; Touhara, K. The Male Mouse Pheromone ESP1 Enhances Female Sexual Receptive Behaviour through a Specific Vomeronasal Receptor. Nature 2010, 466, 118–122.
- Silva, L.; Mendes, T.; Antunes, A. Acquisition of Social Behavior in Mammalian Lineages Is Related with Duplication Events of FPR Genes. Genomics 2020, 112, 2778–2783.
- Oboti, L.; Ibarra-Soria, X.; Pérez-Gómez, A.; Schmid, A.; Pyrski, M.; Paschek, N.; Kircher, S.; Logan, D.W.; Leinders-Zufall, T.; Zufall, F.; et al. Pregnancy and Estrogen Enhance Neural Progenitor-Cell Proliferation in the Vomeronasal Sensory Epithelium. BMC Biol. 2015, 13, 104.
- Villamayor, P.R.; Gullón, J.; Quintela, L.; Sánchez-Quinteiro, P.; Martínez, P.; Robledo, D. Sex Separation Unveils the Functional Plasticity of the Vomeronasal Organ in Rabbits. Front. Mol. Neurosci. 2022, 15, 1034254.
- Segovia, S.; Guillamón, A. Effects of Sex Steroids on the Development of the Vomeronasal Organ in the Rat. Dev. Brain Res. 1982, 5, 209–212.
- Guillamón, A.; Segovia, S. Sex Differences in the Vomeronasal System. Brain Res. Bull. 1997, 44, 377–382.
- Villamayor, P.R.; Cifuentes, J.M.; Quintela, L.; Barcia, R.; Sanchez-Quinteiro, P. Structural, Morphometric and Immunohistochemical Study of the Rabbit Accessory Olfactory Bulb. Brain Struct. Funct. 2020, 225, 203–226.
- Maico, L.M.; Burrows, A.M.; Mooney, M.P.; Siegel, M.I.; Bhatnagar, K.P.; Smith, T.D. Size of the Vomeronasal Organ in Wild Microtus with Different Mating Strategies. Acta Biol. Hung. 2003, 54, 263–274.
- Tai, F.D.; Wang, T.Z.; Zhang, Y.H.; Sun, R.Y. Sexual Dimorphism of the Vomeronasal Organ and the Accessory Olfactory Bulb of the Mandarin Vole Microtus mandarinus and the Reed Vole M. fortis. Acta Theriol. 2004, 49, 33–42.
- Peretto, P.; Giachino, C.; Panzica, G.; Fasolo, A. Sexually Dimorphic Neurogenesis Is Topographically Matched with the Anterior Accessory Olfactory Bulb of the Adult Rat. Cell Tissue Res. 2001, 306, 385–389.
- Broom, R. A Contribution to the Comparative Anatomy of the Mammalian Organ of Jacobson. Trans. R. Soc. Edinb. 1900, 39, 231–255.
- Wöhrmann-Repenning, A. Phylogenetic aspects of the Jacobson’s organ and nasopalatine duct topography in insectivores, primates, Tupaia and Didelphis. Anat. Anz. 1984, 157, 137–149.
- Sánchez-Villagra, M.R. Ontogenetic and Phylogenetic Transformations of the Vomeronasal Complex and Nasal Floor Elements in Marsupial Mammals. Zool. J. Linn. Soc. 2001, 131, 459–479.
- Vaccarezza, O.L.; Sepich, L.N.; Tramezzani, J.H. The Vomeronasal Organ of the Rat. J. Anat. 1981, 132, 167–185.
- Mechin, V.; Pageat, P.; Teruel, E.; Asproni, P. Histological and Immunohistochemical Characterization of Vomeronasal Organ Aging in Mice. Animals 2021, 11, 1211.
- Weiler, E.; McCulloch, M.A.; Farbman, A.I. Proliferation in the Vomeronasal Organ of the Rat during Postnatal Development. Eur. J. Neurosci. 1999, 11, 700–711.
- Giacobini, P.; Benedetto, A.; Tirindelli, R.; Fasolo, A. Proliferation and Migration of Receptor Neurons in the Vomeronasal Organ of the Adult Mouse. Dev. Brain Res. 2000, 123, 33–40.
- Ibokwe, C.; Okpe, G.C. Morphological Studies Of Vomeronasal Organ In The Wild Juvenile Red-Flanked Duiker Cephalophus Rufilatus (GRAY 1864). Anim. Res. Int. 2009, 6, 932–937.
- Wang, H.; Wang, J.; Yang, C.; He, Y. Histological Structure of the Vomeronasal Organ and Accessory Olfactory Bulb and the Seasonal Changes of Olfactory Bulb C-Fos Expression in Spermophilus dauricus. Acta Theriol. Sin. 2021, 41, 685.
- Oikawa, T.; Shimamura, K.; Saito, T.R.; Taniguchi, K. Fine Structure of the Vomeronasal Organ in the Chinchilla (Chinchilla laniger). Exp. Anim. 1994, 43, 487–497.
- Jurcisek, J.A.; Durbin, J.E.; Kusewitt, D.F.; Bakaletz, L.O. Anatomy of the Nasal Cavity in the Chinchilla. Cells Tissues Organs 2003, 174, 136–152.
- Smith, T.D.; Alport, L.J.; Burrows, A.M.; Bhatnagar, K.P.; Dennis, J.C.; Tuladhar, P.; Morrison, E.E. Perinatal Size and Maturation of the Olfactory and Vomeronasal Neuroepithelia in Lorisoids and Lemuroids. Am. J. Primatol. 2007, 69, 74–85.
- Dennis, J.C.; Stilwell, N.K.; Smith, T.D.; Park, T.J.; Bhatnagar, K.P.; Morrison, E.E. Is the Mole Rat Vomeronasal Organ Functional? Anat. Rec. 2020, 303, 318–329.
- Luckhaus, G. Light and electron microscopic findings in the epithelial lamina of the vomeronasal organ of the rabbit. Anat. Anz. 1969, 124, 477–489.
- Mahdy, E.; El Behery, E.; Mohamed, S. Comparative Morpho-Histological Analysis on the Vomeronasal Organ and the Accessory Olfactory Bulb in Balady Dogs (Canis familiaris) and New Zealand Rabbits (Oryctolagus cuniculus). J. Adv. Vet. Res. 2019, 6, 506.
- Elgayar, S.A.M.; Eltony, S.A.; Othman, M.A. Morphology of Non-Sensory Epithelium during Post-Natal Development of the Rabbit Vomeronasal Organ. Anat. Histol. Embryol. 2014, 43, 282–293.
- Schneider, N.Y.; Fletcher, T.P.; Shaw, G.; Renfree, M.B. The Vomeronasal Organ of the Tammar Wallaby. J. Anat. 2008, 213, 93–105.
- Poran, N.S. Vomeronasal Organ and Its Associated Structures in the opossum Monodelphis domestica. Microsc. Res. Tech. 1998, 43, 500–510.
- Schneider, N.Y. The Development of the Olfactory Organs in Newly Hatched Monotremes and Neonate Marsupials: Olfaction in Monotremes and Marsupials. J. Anat. 2011, 219, 229–242.
- Ashwell, K.W.S. Development of the Olfactory Pathways in Platypus and Echidna. Brain Behav. Evol. 2012, 79, 45–56.
- Kondoh, D.; Tanaka, Y.; Kawai, Y.K.; Mineshige, T.; Watanabe, K.; Kobayashi, Y. Morphological and Histological Features of the Vomeronasal Organ in African Pygmy Hedgehog (Atelerix albiventris). Animals 2021, 11, 1462.
- Aland, R.C.; Gosden, E.; Bradley, A.J. Seasonal Morphometry of the Vomeronasal Organ in the Marsupial Mouse, Antechinus subtropicus. J. Morphol. 2016, 277, 1517–1530.
- Dennis, J.C.; Allgier, J.G.; Desouza, L.S.; Eward, W.C.; Morrison, E.E. Immunohistochemistry of the Canine Vomeronasal Organ. J. Anat. 2003, 203, 329–338.
- Salazar, I.; Sanchez Quinteiro, P.; Cifuentes, J.M.; Garcia Caballero, T. The Vomeronasal Organ of the Cat. J. Anat. 1996, 188 Pt 2, 445–454.
- Tomiyasu, J.; Kondoh, D.; Sakamoto, H.; Matsumoto, N.; Sasaki, M.; Kitamura, N.; Haneda, S.; Matsui, M. Morphological and Histological Features of the Vomeronasal Organ in the Brown Bear. J. Anat. 2017, 231, 749–757.
- Kelliher, K.R.; Baum, M.J.; Meredith, M. The Ferret’s Vomeronasal Organ and Accessory Olfactory Bulb: Effect of Hormone Manipulation in Adult Males and Females. Anat. Rec. 2001, 263, 280–288.
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; López-Beceiro, A.; Sanchez-Quinteiro, P. The Vomeronasal Organ of Wild Canids: The Fox (Vulpes vulpes) as a Model. J. Anat. 2020, 237, 890–906.
- Salazar, I.; Lombardero, M.; Sánchez-Quinteiro, P.; Roel, P.; Cifuentes, J.M. Origin and Regional Distribution of the Arterial Vessels of the Vomeronasal Organ in the Sheep. A Methodological Investigation with Scanning Electron Microscopy and Cutting-Grinding Technique. Ann. Anat. 1998, 180, 181–187.
- Salazar, I.; Sánchez-Quinteiro, P.; Alemañ, N.; Prieto, D. Anatomical, Immnunohistochemical and Physiological Characteristics of the Vomeronasal Vessels in Cows and Their Possible Role in Vomeronasal Reception. J. Anat. 2008, 212, 686–696.
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M. The Soft-Tissue Components of the Vomeronasal Organ in Pigs, Cows and Horses. Anat. Histol. Embryol. 1997, 26, 179–186.
- Salazar, I.; Quinteiro, P.S.; Alemañ, N.; Cifuentes, J.M.; Troconiz, P.F. Diversity of the Vomeronasal System in Mammals: The Singularities of the Sheep Model. Microsc. Res. Tech. 2007, 70, 752–762.
- Ichikawa, M.; Shin, T.; Kang, M.S. Fine Structure of the Vomeronasal Sensory Epithelium of Korean Goats (Capra Hircus). J. Rep. Dev. 1999, 45, 81–89.
- Yang, W.; Choi, Y.; Park, C.; Lee, K.-H.; Ahn, M.; Kang, W.; Heo, S.-D.; Kim, J.; Shin, T. Histological and Lectin Histochemical Studies in the Vomeronasal Organ of the Korean Black Goat, Capra hircus coreanae. Acta Histochem. 2021, 123, 151684.
- Park, C.; Ahn, M.; Lee, J.-Y.; Lee, S.; Yun, Y.; Lim, Y.-K.; Taniguchi, K.; Shin, T. A Morphological Study of the Vomeronasal Organ and the Accessory Olfactory Bulb in the Korean Roe Deer, Capreolus pygargus. Acta Histochem. 2014, 116, 258–264.
- Vedin, V.; Eriksson, B.; Berghard, A. Organization of the Chemosensory Neuroepithelium of the Vomeronasal Organ of the Scandinavian Moose Alces alces. Brain Res. 2010, 1306, 53–61.
- Kondoh, D.; Nakamura, K.G.; Ono, Y.S.; Yuhara, K.; Bando, G.; Watanabe, K.; Horiuchi, N.; Kobayashi, Y.; Sasaki, M.; Kitamura, N. Histological Features of the Vomeronasal Organ in the Giraffe, Giraffa camelopardalis. Microsc. Res. Tech. 2017, 80, 652–656.
- Smith, T.D.; Bhatnagar, K.P.; Shimp, K.L.; Kinzinger, J.H.; Bonar, C.J.; Burrows, A.M.; Mooney, M.P.; Siegel, M.I. Histological Definition of the Vomeronasal Organ in Humans and Chimpanzees, with a Comparison to Other Primates. Anat. Rec. 2002, 267, 166–176.
- Meisami, E.; Bhatnagar, K.P. Structure and Diversity in Mammalian Accessory Olfactory Bulb. Microsc. Res. Tech. 1998, 43, 476–499.
- Takami, S.; Graziadei, P.P.C. Morphological Complexity of the Glomerulus in the Rat Accessory Olfactory Bulb—A Golgi Study. Brain Res. 1990, 510, 339–342.
- Nakajima, M.; Tsuruta, M.; Mori, H.; Nishikawa, C.; Okuyama, S.; Furukawa, Y. A Comparative Study of Axon-Surrounding Cells in the Two Nasal Nerve Tracts from Mouse Olfactory Epithelium and Vomeronasal Organ. Brain Res. 2013, 1503, 16–23.
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an Olfactory Sensory Map. Cell 1996, 87, 675–686.
- Vassar, R.; Chao, S.K.; Sitcheran, R.; Nuñez, J.M.; Vosshall, L.B.; Axel, R. Topographic Organization of Sensory Projections to the Olfactory Bulb. Cell 1994, 79, 981–991.
- Del Punta, K.; Puche, A.; Adams, N.C.; Rodriguez, I.; Mombaerts, P. A Divergent Pattern of Sensory Axonal Projections Is Rendered Convergent by Second-Order Neurons in the Accessory Olfactory Bulb. Neuron 2002, 35, 1057–1066.
- Crosby, E.C.; Humphrey, T. Studies of the Vertebrate Telencephalon. II. The Nuclear Pattern of the Anterior Olfactory Nucleus, Tuberculum Olfactorium and the Amygdaloid Complex in Adult Man. J. Comp. Neurol. 1941, 74, 309–352.
- Allison, A.C. The Structure of the Olfactory Bulb and Its Relationship to the Olfactory Pathways in the Rabbit and the Rat. J. Comp. Neurol. 1953, 98, 309–353.
- Salazar, I.; Sánchez-Quinteiro, P. A Detailed Morphological Study of the Vomeronasal Organ and the Accessory Olfactory Bulb of Cats. Microsc. Res. Tech. 2011, 74, 1109–1120.
- Larriva-Sahd, J. The Accessory Olfactory Bulb in the Adult Rat: A Cytological Study of Its Cell Types, Neuropil, Neuronal Modules, and Interactions with the Main Olfactory System. J. Comp. Neurol. 2008, 510, 309–350.
- Switzer, R.C., III; Johnson, J.I.; Kirsch, J.A.W. Phylogeny Through Brain Traits. Brain Behav. Evol. 1980, 17, 339–363.
- Baker, H.; Towle, A.C.; Margolis, F.L. Differential Afferent Regulation of Dopaminergic and GABAergic Neurons in the Mouse Main Olfactory Bulb. Brain Res. 1988, 450, 69–80.
- Gouda, M.; Matsutani, S.; Senba, E.; Tohyama, M. Peptidergic Granule Cell Populations in the Rat Main and Accessory Olfactory Bulb. Brain Res. 1990, 512, 339–342.
- McLean, J.H.; Shipley, M.T. Neuroanatomical Substrates of Olfaction. In Science of Olfaction; Serby, M.J., Chobor, K.L., Eds.; Springer: New York, NY, USA, 1992; pp. 126–171. ISBN 978-1-4612-7690-6.
- Frahm, H.D.; Stephan, H.; Baron, G. Comparison of Brain Structure Volumes in Insectivora and Primates. V. Area Striata (AS). J. Hirnforsch. 1984, 25, 537–557.
- Bonfanti, L.; Peretto, P.; Merighi, A.; Fasolo, A. Newly-Generated Cells from the Rostral Migratory Stream in the Accessory Olfactory Bulb of the Adult Rat. Neuroscience 1997, 81, 489–502.
- Defteralı, Ç.; Moreno-Estellés, M.; Crespo, C.; Díaz-Guerra, E.; Díaz-Moreno, M.; Vergaño-Vera, E.; Nieto-Estévez, V.; Hurtado-Chong, A.; Consiglio, A.; Mira, H.; et al. Neural Stem Cells in the Adult Olfactory Bulb Core Generate Mature Neurons In Vivo. Stem Cells 2021, 39, 1253–1269.
- Inaki, K.; Nishimura, S.; Nakashiba, T.; Itohara, S.; Yoshihara, Y. Laminar Organization of the Developing Lateral Olfactory Tract Revealed by Differential Expression of Cell Recognition Molecules. J. Comp. Neurol. 2004, 479, 243–256.
- Broadwell, R.D. Olfactory Relationships of the Telencephlaon and Diencephalon in the Rabbit. 1. An Autoradiographic Study of the Efferent Connections of the Main and Accessory Olfactory Bulbs. J. Comp. Neurol. 1975, 163, 329–345.
- Schwob, J.E.; Price, J.L. The Development of Axonal Connections in the Central Olfactory System of Rats. J. Comp. Neurol. 1984, 223, 177–202.
- Takami, S.; Fernandez, G.D.; Graziadei, P.P.C. The Morphology of GABA-Immunoreactive Neurons in the Accessory Olfactory Bulb of Rats. Brain Res. 1992, 588, 317–323.
- Takami, S.; Graziadei, P.P.C. Light Microscopic Golgi Study of Mitral/Tufted Cells in the Accessory Olfactory Bulb of the Adult Rat. J. Comp. Neurol. 1991, 311, 65–83.
- Hinds, J.W. Autoradiographic Study of Histogenesis in the Mouse Olfactory Bulb I. Time of Origin of Neurons and Neuroglia. J. Comp. Neurol. 1968, 134, 287–304.
- Rall, W.; Shepherd, G.M.; Reese, T.S.; Brightman, M.W. Dendrodendritic Synaptic Pathway for Inhibition in the Olfactory Bulb. Exp. Neurol. 1966, 14, 44–56.
- Martín-López, E.; Corona, R.; López-Mascaraque, L. Postnatal Characterization of Cells in the Accessory Olfactory Bulb of Wild Type and Reeler Mice. Front. Neuroanat. 2012, 6, 15.
- Jia, C.; Chen, W.R.; Shepherd, G.M. Synaptic Organization and Neurotransmitters in the Rat Accessory Olfactory Bulb. J. Neurophysiol. 1999, 81, 345–355.
- Del Punta, K. Sequence Diversity and Genomic Organization of Vomeronasal Receptor Genes in the Mouse. Genom. Res. 2000, 10, 1958–1967.
- Segovia, S.; Orensanz, L.M.; Valencia, A.; Guillamón, A. Effects of Sex Steroids on the Development of the Accessory Olfactory Bulb in the Rat: A Volumetric Study. Dev. Brain Res. 1984, 16, 312–314.
- Yokosuka, M. Histological Properties of the Glomerular Layer in the Mouse Accessory Olfactory Bulb. Exp. Anim. 2012, 61, 13–24.
- Frahm, H.D.; Bhatnagar, K.P. Comparative Morphology of the Accessory Olfactory Bulb in Bats. J. Anat. 1980, 130, 349–365.
- Jia, C.; Halpern, M. Calbindin D28k, Parvalbumin, and Calretinin Immunoreactivity in the Main and Accessory Olfactory Bulbs of the Gray Short-Tailed Opossum, Monodelphis domestica. J. Morphol. 2004, 259, 271–280.
- Ortiz-Leal, I.; Torres, M.V.; Villamayor, P.R.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Can Domestication Shape Canidae Brain Morphology? The Accessory Olfactory Bulb of the Red Fox as a Case in Point. Ann. Anat. 2022, 240, 151881.
- Bird, D.J.; Jacquemetton, C.; Buelow, S.A.; Evans, A.W.; Van Valkenburgh, B. Domesticating Olfaction: Dog Breeds, Including Scent Hounds, Have Reduced Cribriform Plate Morphology Relative to Wolves. Anat. Rec. 2021, 304, 139–153.
- Bird, D.J.; Murphy, W.J.; Fox-Rosales, L.; Hamid, I.; Eagle, R.A.; Van Valkenburgh, B. Olfaction Written in Bone: Cribriform Plate Size Parallels Olfactory Receptor Gene Repertoires in Mammalia. Proc. R. Soc. B 2018, 285, 20180100.
- Wynn, E.H.; Sánchez-Andrade, G.; Carss, K.J.; Logan, D.W. Genomic Variation in the Vomeronasal Receptor Gene Repertoires of Inbred Mice. BMC Genom. 2012, 13, 415.
- Ortiz-Leal, I.; Torres, M.V.; López-Callejo, L.N.; Fidalgo, L.E.; López-Beceiro, A.; Sanchez-Quinteiro, P. Comparative Neuroanatomical Study of the Main Olfactory Bulb in Domestic and Wild Canids: Dog, Wolf and Red Fox. Animals 2022, 12, 1079.
- Dzięcioł, M.; Podgórski, P.; Stańczyk, E.; Szumny, A.; Woszczyło, M.; Pieczewska, B.; Niżański, W.; Nicpoń, J.; Wrzosek, M.A. MRI Features of the Vomeronasal Organ in Dogs (Canis familiaris). Front. Vet. Sci. 2020, 7, 159.
- Muñiz-de Miguel, S.; Barreiro-Vázquez, J.D.; Sánchez-Quinteiro, P.; Ortiz-Leal, I.; González-Martínez, Á. Behavioural Disorder in a Dog with Congenital Agenesis of the Vomeronasal Organ and the Septum Pellucidum. Vet. Rec. Case Rep. 2023, 11, e571.
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211.
- Puglisi, I.; Masucci, M.; Cozzi, A.; Teruel, E.; Navarra, M.; Cirmi, S.; Pennisi, M.G.; Siracusa, C. Effects of a Novel Gel Formulation of Dog Appeasing Pheromone (DAP) on Behavioral and Physiological Stress Responses in Dogs Undergoing Clinical Examination. Animals 2022, 12, 2472.
- Asproni, P.; Cozzi, A.; Verin, R.; Lafont-Lecuelle, C.; Bienboire-Frosini, C.; Poli, A.; Pageat, P. Pathology and Behaviour in Feline Medicine: Investigating the Link between Vomeronasalitis and Aggression. J. Feline Med. Surg. 2016, 18, 997–1002.
- Asproni, P.; Mainau, E.; Cozzi, A.; Carreras, R.; Bienboire-Frosini, C.; Teruel, E.; Pageat, P. Is There a Link between Vomeronasalitis and Aggression in Stable Social Groups of Female Pigs? Animals 2022, 12, 303.
- Mechin, V.; Asproni, P.; Bienboire-Frosini, C.; Cozzi, A.; Chabaud, C.; Arroub, S.; Mainau, E.; Nagnan-Le Meillour, P.; Pageat, P. Inflammation Interferes with Chemoreception in Pigs by Altering the Neuronal Layout of the Vomeronasal Sensory Epithelium. Front. Vet. Sci. 2022, 9, 936838.
- Mechin, V.; Pageat, P.; Boutry, M.; Teruel, E.; Portalier, C.; Asproni, P. Does the Environmental Air Impact the Condition of the Vomeronasal Organ? A Mouse Model for Intensive Farming. Animals 2023, 13, 1902.
- Salazar, I.; Cifuentes, J.M.; Quinteiro, P.S.; Caballero, G. The Vomeronasal System of the Mink, Mustela Vison. I. The Vomeronasal Organ. Funct. Dev. Morphol. 1994, 4, 113–117.
- Salazar, I.; Quinteiro, P.S.; Cifuentes, J.M.; Lombardero, M. The Accessory Olfactory Bulb of the Mink, Mustela Vison: A Morphological and Lectin Histochemical Study. Anat. Histol. Embryol. 1998, 27, 297–300.
- Salazar, I.; Lombardero, M.; Alemañ, N.; Sánchez Quinteiro, P. Development of the Vomeronasal Receptor Epithelium and the Accessory Olfactory Bulb in Sheep. Microsc. Res. Tech. 2003, 61, 438–447.
- Tirindelli, R.; Mucignat-Caretta, C.; Ryba, N.J.P. Molecular Aspects of Pheromonal Communication via the Vomeronasal Organ of Mammals. Trends Neurosci. 1998, 21, 482–486.
- Alonso, J.R.; Briñón, J.G.; Crespo, C.; Bravo, I.G.; Arévalo, R.; Aijón, J. Chemical Organization of the Macaque Monkey Olfactory Bulb: II. Calretinin, Calbindin D-28k, Parvalbumin, and Neurocalcin Immunoreactivity: Calcium-Binding Proteins in the Monkey OB. J. Comp. Neurol. 2001, 432, 389–407.
- Briñón, J.G.; Weruaga, E.; Crespo, C.; Porteros, A.; Arévalo, R.; Aijón, J.; Alonso, J.R. Calretinin-, Neurocalcin-, and Parvalbumin-Immunoreactive Elements in the Olfactory Bulb of the Hedgehog (Erinaceus europaeus). J. Comp. Neurol. 2001, 429, 554–570.
- Weruaga, E. A Sexually Dimorphic Group of Atypical Glomeruli in the Mouse Olfactory Bulb. Chem. Senses 2001, 26, 7–15.
- Smithson, L.J.; Kawaja, M.D. A Comparative Examination of Biomarkers for Olfactory Ensheathing Cells in Cats and Guinea Pigs. Brain Res. 2009, 1284, 41–53.
- Dehmelt, L.; Halpain, S. The MAP2/Tau Family of Microtubule-Associated Proteins. Genome Biol. 2005, 6, 204.
- Benowitz, L.I.; Perrone-Bizzozero, N.I.; Neve, R.L.; Rodriguez, W. Chapter 26 GAP-43 as a Marker for Structural Plasticity in the Mature CNS. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1990; Volume 86, pp. 309–320. ISBN 978-0-444-81121-9.
- Shapiro, L.S.; Roland, R.M.; Halpern, M. Development of Olfactory Marker Protein and N-CAM Expression in Chemosensory Systems of the Opossum, Monodelphis domestica. J. Morphol. 1997, 234, 109–129.
- Mugnaini, E.; Oertel, W.H.; Wouterlood, F.F. Immunocytochemical Localization of GABA Neurons and Dopamine Neurons in the Rat Main and Accessory Olfactory Bulbs. Neurosci. Lett. 1984, 47, 221–226.
- Le Jeune, H.; Aubert, I.; Jourdan, F.; Quirion, R. Comparative Laminar Distribution of Various Autoradiographic Cholinergic Markers in Adult Rat Main Olfactory Bulb. J. Chem. Neuroanat. 1995, 9, 99–112.
- Woo, C.C.; Leon, M. Distribution and Development of β-Adrenergic Receptors in the Rat Olfactory Bulb. J. Comp. Neurol. 1995, 352, 1–10.
- Huang, Z.; Thiebaud, N.; Fadool, D.A. Differential Serotonergic Modulation across the Main and Accessory Olfactory Bulbs: Differential Serotonin Modulation in Olfactory System. J. Physiol. 2017, 595, 3515–3533.
- Matsutani, S.; Senba, E.; Tohyama, M. Neuropeptide- and Neurotransmitter-Related Immunoreactivities in the Developing Rat Olfactory Bulb. J. Comp. Neurol. 1988, 272, 331–342.
- Lundberg, L.-M.; Alm, P.; Wharton, J.; Polak, J.M. Protein Gene Product 9.5 (PGP 9.5): A New Neuronal Marker Visualizing the Whole Uterine Innervation and Pregnancy-Induced and Developmental Changes in the Guinea pig. Histochemistry 1988, 90, 9–17.
- Nacher, J.; Crespo, C.; McEwen, B.S. Doublecortin Expression in the Adult Rat Telencephalon: Doublecortin Expression in the Adult Rat. Eur. J. Neurosci. 2001, 14, 629–644.
- Salazar, I.; Sánchez Quinteiro, P. Differential Development of Binding Sites for Four Lectins in the Vomeronasal System of Juvenile Mouse: From the Sensory Transduction Site to the First Relay Stage. Brain Res. 2003, 979, 15–26.
- Takami, S.; Graziadei, P.P.C.; Ichikawa, M. The Differential Staining Patterns of Two Lectins in the Accessory Olfactory Bulb of the Rat. Brain Res. 1992, 598, 337–342.
- Taniguchi, K.; Nii, Y.; Ogawa, K. Subdivisions of the Accessory Olfactory Bulb, as Demonstrated by Lectin-Histochemistry in the Golden Hamster. Neurosci. Lett. 1993, 158, 185–188.
- Shapiro, L.S.; Halpern, M.; Ee, P.-L. Lectin Histochemical Identification of Carbohydrate Moieties in Opossum Chemosensory Systems during Development, with Special Emphasis on VVA-Identified Subdivisions in the Accessory Olfactory Bulb. J. Morphol. 1995, 224, 331–349.
- Hovis, K.R.; Ramnath, R.; Dahlen, J.E.; Romanova, A.L.; LaRocca, G.; Bier, M.E.; Urban, N.N. Activity Regulates Functional Connectivity from the Vomeronasal Organ to the Accessory Olfactory Bulb. J. Neurosci. 2012, 32, 7907–7916.
- Raisman, G. An Experimental Study of the Projection of the Amygdala to the Accessory Olfactory Bulb and Its Relationship to the Concept of a Dual Olfactory System. Exp. Brain Res. 1972, 14, 395–408.
- Scalia, F.; Winans, S.S. The Differential Projections of the Olfactory Bulb and Accessory Olfactory Bulb in Mammals. J. Comp. Neurol. 1975, 161, 31–55.
- Winans, S.S.; Scalia, F. Amygdaloid Nucleus: New Afferent Input from the Vomeronasal Organ. Science 1970, 170, 330–332.
- Shipley, M.T.; Adamek, G.D. The Connections of the Mouse Olfactory Bulb: A Study Using Orthograde and Retrograde Transport of Wheat Germ Agglutinin Conjugated to Horseradish Peroxidase. Brain Res. Bull. 1984, 12, 669–688.
- Dulac, C.; Wagner, S. Genetic Analysis of Brain Circuits Underlying Pheromone Signaling. Annu. Rev. Genet. 2006, 40, 449–467.
- Price, J.L. Beyond the Primary Olfactory Cortex: Olfactory-Related Areas in the Neocortex, Thalamus and Hypothalamus. Chem. Senses 1985, 10, 239–258.
- Courtiol, E.; Wilson, D.A. The Olfactory Thalamus: Unanswered Questions about the Role of the Mediodorsal Thalamic Nucleus in Olfaction. Front. Neural Circuits 2015, 9, 49.
- Cádiz-Moretti, B.; Otero-García, M.; Martínez-García, F.; Lanuza, E. Afferent Projections to the Different Medial Amygdala Subdivisions: A Retrograde Tracing Study in the Mouse. Brain Struct. Funct. 2016, 221, 1033–1065.
- Devor, M. Fiber Trajectories of Olfactory Bulb Efferents in the Hamster. J. Comp. Neurol. 1976, 166, 31–47.
- Martinez-Marcos, A. On the Organization of Olfactory and Vomeronasal Cortices. Prog. Neurobiol. 2009, 87, 21–30.
- Gutiérrez-Castellanos, N.; Pardo-Bellver, C.; Martínez-García, F.; Lanuza, E. The Vomeronasal Cortex—Afferent and Efferent Projections of the Posteromedial Cortical Nucleus of the Amygdala in Mice. Eur. J. Neurosci. 2014, 39, 141–158.
- Stowers, L.; Liberles, S.D. State-Dependent Responses to Sex Pheromones in Mouse. Curr. Opin. Neurobiol. 2016, 38, 74–79.
- Pardo-Bellver, C.; Cádiz-Moretti, B.; Novejarque, A.; Martínez-García, F.; Lanuza, E. Differential Efferent Projections of the Anterior, Posteroventral, and Posterodorsal Subdivisions of the Medial Amygdala in Mice. Front. Neuroanat. 2012, 6, 33.
- Lo, L.; Anderson, D.J. A Cre-Dependent, Anterograde Transsynaptic Viral Tracer for Mapping Output Pathways of Genetically Marked Neurons. Neuron 2011, 72, 938–950.
- Trotier, D. Vomeronasal Organ and Human Pheromones. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 184–190.
- Pro-Sistiaga, P.; Mohedano-Moriano, A.; Ubeda-Bañon, I.; Del Mar Arroyo-Jimenez, M.; Marcos, P.; Artacho-Pérula, E.; Crespo, C.; Insausti, R.; Martinez-Marcos, A. Convergence of Olfactory and Vomeronasal Projections in the Rat Basal Telencephalon. J. Comp. Neurol. 2007, 504, 346–362.
- Cadiz, B.; Martinez-Garcia, F.; Lanuza, E. Neural Substrate to Associate Odorants and Pheromones: Convergence of Projections from the Main and Accessory Olfactory Bulbs in Mice. In Chemical Signals in Vertebrates 12; Springer: New York, NY, USA, 2013; pp. 3–16. ISBN 978-1-4614-5926-2.
- Mohrhardt, J.; Nagel, M.; Fleck, D.; Ben-Shaul, Y.; Spehr, M. Signal Detection and Coding in the Accessory Olfactory System. Chem. Senses 2018, 43, 667–695.
- Záborszky, L.; Carlsen, J.; Brashear, H.R.; Heimer, L. Cholinergic and GABAergic Afferents to the Olfactory Bulb in the Rat with Special Emphasis on the Projection Neurons in the Nucleus of the Horizontal Limb of the Diagonal Band. J. Comp. Neurol. 1986, 243, 488–509.
- De Saint Jan, D. Target-Specific Control of Olfactory Bulb Periglomerular Cells by GABAergic and Cholinergic Basal Forebrain Inputs. eLife 2022, 11, e71965.
- Liberia, T.; Blasco Ibáñez, J.M.; Nácher, J.; Varea, E.; Lanciego, J.L.; Crespo, C. Synaptic Connectivity of the Cholinergic Axons in the Olfactory Bulb of the Cynomolgus Monkey. Front. Neuroanat. 2015, 9, 28.
- Böhm, E.; Brunert, D.; Rothermel, M. Input Dependent Modulation of Olfactory Bulb Activity by HDB GABAergic Projections. Sci. Rep. 2020, 10, 10696.
- Gomez, D.M.; Newman, S.W. Differential Projections of the Anterior and Posterior Regions of the Medial Amygdaloid Nucleus in the Syrian Hamster. J. Comp. Neurol. 1992, 317, 195–218.
- Fan, S.; Luo, M. The Organization of Feedback Projections in a Pathway Important for Processing Pheromonal Signals. Neuroscience 2009, 161, 489–500.
- Inbar, T.; Davis, R.; Bergan, J.F. A Sex-specific Feedback Projection from Aromatase-expressing Neurons in the Medial Amygdala to the Accessory Olfactory Bulb. J. Comp. Neurol. 2022, 530, 648–655.
- Pardo-Bellver, C.; Vila-Martin, M.E.; Martínez-Bellver, S.; Villafranca-Faus, M.; Teruel-Sanchis, A.; Savarelli-Balsamo, C.A.; Drabik, S.M.; Martínez-Ricós, J.; Cervera-Ferri, A.; Martínez-García, F.; et al. Neural Activity Patterns in the Chemosensory Network Encoding Vomeronasal and Olfactory Information in Mice. Front. Neuroanat. 2022, 16, 988015.
- Keverne, E.B.; Brennan, P.A. Olfactory Recognition Memory. J. Physiol. 1996, 90, 399–401.
- Brennan, P.A.; Kendrick, K.M.; Keverne, E.B. Neurotransmitter Release in the Accessory Olfactory Bulb during and after the Formation of an Olfactory Memory in Mice. Neuroscience 1995, 69, 1075–1086.
- Brennan, P.A. The Nose Knows Who’s Who: Chemosensory Individuality and Mate Recognition in Mice. Horm. Behav. 2004, 46, 231–240.
- Takahashi, Y.; Kaba, H. Muscarinic Receptor Type 1 (M1) Stimulation, Probably through KCNQ/Kv7 Channel Closure, Increases Spontaneous GABA Release at the Dendrodendritic Synapse in the Mouse Accessory Olfactory Bulb. Brain Res. 2010, 1339, 26–40.
- Wilson, R.I.; Mainen, Z.F. Early Events in Olfactory Processing. Annu. Rev. Neurosci. 2006, 29, 163–201.
This entry is offline, you can click here to edit this entry!
