Exosomes are a subtype of extracellular vesicles (EVs) with a diameter of 30~150 nm (averaging ~100 nm) that are primarily produced through the endosomal pathway, and carry various components such as lipids, proteins, RNA, and other small molecular substances. Exosomes can mediate intercellular communication through the bioactive substances they carry, thus participating in different physiological activities. Metabolic syndrome (MS) is a disease caused by disturbances in the body’s metabolism, mainly including insulin resistance (IR), diabetes, obesity, non-alcoholic fatty liver disease (NAFLD), hyperlipidemia, and atherosclerosis (AS). Exosomes are closely related to the occurrence and development of MS. Exosomes can act as messengers to mediate signaling transductions between metabolic cells in the organism and play a bidirectional regulatory role in the MS process.
1. Introduction
EVs are a collective term for vesicles released by cells that have a membrane structure. According to their diameters, they can be simply classified into four types: exosomes (30~150 nm), microvesicles (100~1000 nm), apoptotic bodies (100~5000 nm), and oncosomes (1~10 μm). Exosomes are spherical bilayer vesicles formed by mammalian cells through a series of regulatory processes [
1]. They are usually observed in cell culture supernatants, plasma, saliva, urine, amniotic fluid, malignant ascites, and other biological fluids, and are rich in biologically active substances such as proteins, lipids, nucleic acids and metabolites [
2,
3]. As mediators of cell-to-cell communication via bio-active substances they carry, exosomes exert an important role in the progress of various diseases, such as MS, cardiovascular diseases and neuron diseases [
4]. In recent years, exosomes have been extensively studied due to their outstanding biochemical properties, and have emerged as an important means for disease diagnosis and drug delivery because of their biocompatibility, stability, and safety [
5,
6,
7,
8]. MS is a series of metabolic diseases caused by the impaired metabolism of various substances, such as proteins, fats, and carbohydrates in the human body. Due to lack of effective diagnostic methods and therapeutic strategies, the disease has progressed to become one of the most threatening public health problems affecting human health and quality of life. Recent documents have shown that the biological activity of exosomes is tightly related to the development of MS, and thus may have potential application for clinical diagnosis and treatment of MS.
2. Biogenesis of Exosomes
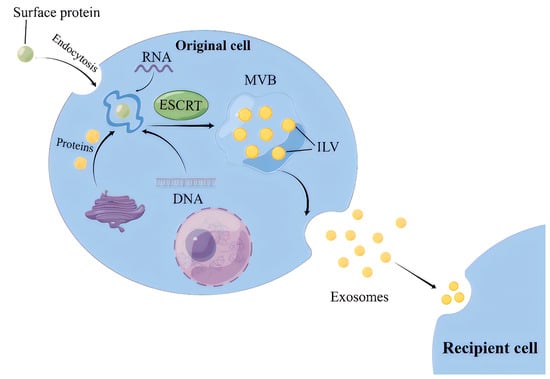
2.1. ILVs Production Involves Two Mechanisms according to Their Reliance on Endosomal Sorting Complex Required for Transport (ESCRT), Hence Referred to as ESCRT-Dependent and -Independent Pathways
For the ESCRT-dependent pathway, the initial stage involves the capture of ubiquitinated proteins by the ESCRT-0 complex on the cell membrane, thereby concentrating cargoes (such as DNA, RNA, and proteins) on the membrane [
10]. While the cargoes are aggregated, ESCRT-I and ESCRT-II complexes are recruited to the cell membrane, leading to membrane protrusion and the initial formation of intraluminal vesicles (ILVs) [
11]. Subsequently, the ubiquitinated cargoes interact with the ESCRT-I component TSG101 and the HRS protein in ESCRT-0 to be further concentrated and immobilized on the membrane [
12]. Finally, the ILVs maturation is promoted by the ESCRT-III complex, VPS4 protein, and auxiliary protein VTA1, which are able to provide energy. Thereafter, ILVs accumulate inside the cells and develop into multivesicular bodies (MVBs) (
Figure 1) [
13].
Figure 1. The process of exosomes intracellular biogenesis and secretion. MVB, Multivesicular body; ESCRT, Endosomal sorting complexes required for transport; ILV, intraluminal vesicles.
2.2. MVBs Transportation and Exosomes Release
MVBs transportation from the site of generation to the cell membrane occurs mainly through a network of microtubules, where small GTPases (e.g., Rab proteins) play a key role [
20]. The fusion of MVBs with the cell membrane is mediated by the soluble N-ethylmaleimide sensitive factor attachment receptor (SNARE) protein complex. The exosome transport and release are influenced by a variety of organelles, mainly lysosomes, which can significantly reduce the release of exosomes once it fuses with MVBs [
21].
3. Biological Characteristics of Exosomes
The outer membrane of exosomes is rich in cholesterol, sphingolipids, ceramides, glycolipids, and glycerophospholipid chains, which together play crucial roles in the cellular microenvironment. The main roles of the lipid components in the exosome membrane are to provide some stiffness, ensure bioavailability, and prevent the bioactive materials carried from degradation. Moreover, evidence have shown that exosomes lipids are also involved in the transport of lipids in vivo [
26,
27]. Different cells produce different exosomes that carry similar conserved proteins on their surface alone, such as the major histocompatibility complex MHC I and MHC II molecules, heat-shock proteins, four transmembrane proteins (CD9, CD63, CD81), integrins, cytoskeletal proteins and some biological enzymes [
4,
28,
29,
30]. The inclusions of exosomes mainly include proteins, including cytokines, miRNA, mRNA, DNA, and lipids.
The composition of the exosome inclusions of different cellular sources and states is in a state of dynamic change. The highest proportion of exosomes RNAs are miRNAs [
34], which is a unique feature that distinguishes exosomes from other extracellular vesicles. The types and amount of RNAs present in the exosome reflect the secretory-cell types and its physiological/pathological state [
35]; however, the types and expression levels of these RNAs differ significantly from these secretory cells [
36], suggesting that there is a sorting mechanism for RNA assembly in exosomes, although the exact mechanism remains elusive.
4. Exosome Separation and Technical Challenges
Several prominent methods have been established and utilized for exosome extraction and separation, encompassing ultracentrifugation, size-exclusion chromatography, ultrafiltration, immunomagnetic bead separation, and microfluidic chip technology. Ultracentrifugation is the most prevalent technique and is highly regarded for its simplicity and affordability. However, ultracentrifugation tends to require longer processing times, with higher losses and lower purity due to centrifugal forces, thus having an impact on subsequent identification [
37,
38]. Size-exclusion chromatography is the second most used technique after ultracentrifugation and offers a simple operation for exosome extraction while preserving biological activity, making it suitable for experiments of various scales and effective in isolation of large exosomes. However, it also presents some limitations, such as sample loss, limited separation efficiency, time cost, and unsuitable for the extraction of small exosomes [
39]. Extraction of exosomes using ultrafiltration is high yielding, simple and fast. Nevertheless, it is prone to clogging the membrane pores and contaminating large protein particles, resulting in lower exosome recovery as well as purity [
40]. The immunomagnetic bead separation, grounded in the principle of antigen–antibody binding, can procure remarkably pure exosomes. However, it comes at an elevated cost and demands advanced technical proficiency [
41].
5. Biological Functions of Exosomes
Exosomes are natural transport carriers, and their inner lumen can be loaded with various biomolecules, such as proteins and nucleic acids. Exosomes also embed and anchor various protein ligands on their surface, which upon recognition and binding to the recipient cells can lead to reactive changes, thus affecting intracellular signaling pathways and the physiological state of the recipient cells. Therefore, exosomes can serve as carriers for biological molecules and signals for communication between cells.
Different cells achieve intercellular communication by secreting exosomes carrying different components, and these exosomes are taken up by the recipient cells to exchange molecules or signals through substance exchange and release of endosomes, thus leading to the subsequent influence of recipient cells behavior and phenotype features. The intercellular communications mainly undergo via the following mechanisms. First, exosomes surface proteins can modulate the signaling pathways of target cells via directly binding to its cell receptors; Jodo et al. showed that the membrane of exosomes secreted by T cells contains a signaling molecule, Fas L, which specifically binds to transmembrane protein, Fas, on the receptor cell membrane and induces its trimerization, thus forming an apoptosis-inducing complex to initiate cell death [
47]. Second, exosomes can fuse with cell membranes and deliver functional proteins, miRNAs, mRNAs, and other biomolecules to target cells.
6. Application of Exosomes
Due to their excellent properties, exosomes play an extremely important role in disease diagnosis and treatment. First, given their presence in fluids like blood, urine, and saliva, exosomes can be isolated using liquid biopsy, providing a non-invasive strategy alternative to traditional biopsies [
54], Second, the compositions of exosomes can be analyzed by mass spectrometry and other analytical methods to obtain a large amount of information about molecular carriers inside and outside the cells, on the basis of which a comprehensive diagnosis can be achieved at different states of disease progress. It was reported that 120 plasma exosome samples collected from the patients were used to screen the specific biomarkers of 16 different types of tumors by proteomic analysis. The results show that they achieved 95% sensitivity and 90% specificity for the classification of various tumors [
55]. EPI-CE kit (Exosome dx company) and ExoDx Prostate-IntelliScore Diagnostics Product (Bio-Techne company) have been developed and marketed for the diagnosis of prostate cancer by screening RNA biomarkers originating from prostate-specific or urine exosomes [
56,
57]. Third, exosomes are natural carriers with the advantages of long half-life and natural non-toxicity. They also have the unique ability to target homing and deliver the substances to the target cells. The biological nature of exosomes allows them to easily cross the blood–brain barrier, making them an ideal cargo for drug delivery into the brain. For instance, exosomes can bind to an anti-CD22 monoclonal antibody fragment (CD22-F (ab’) 2) and wrap doxorubicin (DOX) to form CD22-F (ab’) 2-Exo-DOX, which can penetrate the BBB and accurately deliver DOX to lymphoma cells of primary central nervous system, thus enhancing anti-tumor activity in tumor-bearing mice [
58].
7. Exosomes Involved in MS Progress
7.1. IR
IR is a central factor contributing to MS and a driving force for the development of cardiovascular complications associated with type 2 diabetes mellitus (T2DM) and hypertension [
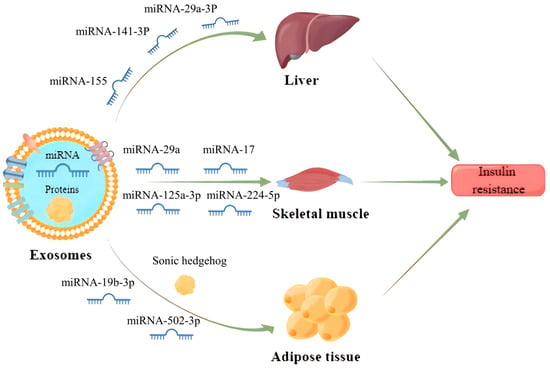
61]. It has been found that exosomes can enter the receptor cells by wrapping different inclusions to regulate the transduction of the insulin signaling pathway, which contributes to IR development (
Figure 3). Exosomes carrying miR-155 secreted by adipose tissue macrophages into hepatocytes can downregulate glucose transporter-4 (Glut-4) by targeting peroxisome proliferator-activated receptor γ (PPARγ), which ultimately leads to a decrease in insulin sensitivity in the liver and exacerbates the process of diabetes in obese mice [
62].
Figure 3. Exosomes released from parent cells promote IR in the liver, skeletal muscle and adipose tissue by delivering the miRNAs and proteins they carry to receipt cells.
Gestational diabetes mellitus is also one of the more studied types of diabetes mellitus and its relationship with exosomes has recently received more attention. It has been reported that exosomes miRNA-125a-3p and miRNA-224-5p released from the placenta of patients with gestational diabetes mellitus can effectively regulate the expression of glypican and CD40, and inhibit the PI3K/AKT/Glut-4 signaling axis in skeletal muscle, thus resulting in diabetic IR [
65].
7.2. DM and Its Related Complications
DM is a long-term systemic chronic metabolic disease, mainly classified into type 1 diabetes mellitus (T1DM) and T2DM. Recent studies suggest that miRNAs in exosomes may serve as new diagnostic markers and therapeutic interventions for DM. The levels of circulating exosomes miR-15a, miR-375, miR-126, miR-1, and miR-133 are altered during the early stages of diabetes in rodent and human models and could be potential diagnostic markers for the disease [
68]. From a therapeutic point of view, exosomes miR-106b-5p and miR-222-3p released from BM cells can effectively repair damaged pancreatic β-cells in T1DM mice [
69]. Exosomes miR-29 secreted by β-cells during the pre-DM phase contributes to the induction of an inflammatory response in macrophages and monocytes, leading to IR and progression of T2DM [
70].
Exosomes are also involved in the course of many diabetic complications, including diabetic peripheral neuropathy (DPN), diabetic kidney disease (DKD), diabetic foot ulcers (DFU) and diabetic cardiomyopathy (DCM). It has been shown that Schwann cell-derived exosomes miR-21 can regulate neurite growth in DPN rats by affecting the AKT signaling pathway, opening up new perspectives for the prevention and treatment of DPN [
73]. Exosomes derived from renal tubular epithelial cells can promote macrophage glycolysis, renal inflammation, and fibrosis by upregulating HIF-1α expression, which accelerates the progression of DKD [
74].
7.3. Obesity
Obesity is one of the most common metabolic diseases, and the prevalence of obesity has greatly increased in past decades in the world [
83]. Evidence have demonstrated that circulating exosomes miRNAs primarily secreted by adipose tissue exhibit different profiles between obese patients and healthy individuals [
84], and exosomes play a vital role in the exchange of information between adipose tissue and other tissues, thereby contributing to the pathogenesis of obesity and its related diseases [
85]. Adipocyte-derived exosomes miR-27a can regulate the hepatic lipid synthesis pathway by inhibiting PPARγ [
86]. Exosomes also play an important role in lipid transport by regulating the expression of classical lipid transporters (e.g., ABCA1, ABCG1, LDLR, CD36), where plasma exosomes miR-30e and miR-92a can disrupt lipid metabolism and cause inadequate cholesterol efflux by inhibiting ABCA1 and ABCG1 [
87].
7.4. NAFLD
NAFLD, like obesity and diabetes, is a highly prevalent global metabolic disease that affects about a quarter of the global adult population and poses a serious health burden [
89]. There is growing evidence that exosomes are involved in the development and occurrence of NAFLD [
90]. The severity of liver inflammation in NAFLD patients positively correlates with the level of toxic hepatocyte-derived exosomes miR-192-5p [
91], indicating that exosomes miR-192-5p can be used as a molecular marker for NAFLD. The release of miR-223-rich exosomes from macrophages can inhibit the expression of transcriptional activator with PDZ-binding motif (TAZ) in hepatocytes, thereby alleviating the progression of NAFLD to NASH and even liver fibrosis [
92].
7.5. Hyperlipidemia and AS
Hyperlipidemia is caused by higher percentages of fatty droplets and oxidized low-density lipoprotein (Ox-LDL) in blood composition. Such substances can increase the viscosity of the blood, which leads to a decrease in the oxygen-carrying capacity of the blood and promotes damage to the mucous membranes on the walls of blood vessels, resulting in the formation of atheromatous plaques and AS. It has been shown that macrophage-derived exosomes miR-223 promotes macrophage differentiation, inflammatory response, and disturbs lipid metabolism in adipose tissue, thus exacerbating the progression of AS [
95,
96].
7.6. Hypertension
Hypertension is a complex multifactorial disease, primarily attributable to the interaction between genetic and environmental factors [
105]. There is emerging evidence showing that exosomes play a key role in the progression of hypertension. A study has revealed that miRNAs and proteins contained in exosomes derived from endothelial cells and immune cells drastically elicit VSMC proliferation and migration, leading to vascular remodeling, a core mechanism in the etiology of hypertension [
106]. Specific miRNAs, such as miR-155, miR-21, miR-34a, miR-145, and miR-22, present in circulating exosomes have been demonstrated to play a crucial role in the pathogenesis of hypertension. They can modulate vascular dilation and constriction by carrying or altering the expression of endothelial nitric oxide synthase (eNOS), thereby participating in blood pressure regulation [
107].
This entry is adapted from the peer-reviewed paper 10.3390/biology12121480