Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
Conjugation of the proteins with carbohydrates, occurring in the early stages of the Maillard reactions, received increased attention because of the high potential to ensure the improvement of the biological activity and functional properties of the proteins of different origins.
- proteins
- Maillard reactions
- alternative processing techniques
1. Introduction
Considered one of biology’s most versatile polymers present in foods, proteins are playing roles as nutrients and structural building blocks for stabilizing texture and maintaining the food’s qualitative attributes [1,2,3]. Proteins have a strong contribution to the development of foods with functional properties influencing the bioavailability of nutrients, equilibrium with minerals, and other bioactive compounds [2]. The functionality of the proteins is associated with their ability to form and/or stabilize gels, films, foams, emulsions, etc. while maximizing their impact on nutrition and health [3]. On the other hand, the most important drawbacks of the food proteins are attributed to their sensibility to high temperatures, pH changes, high ionic strength, and specific proteolytic and organic agents that limit their use for industrial applications [4,5]. The Maillard reaction represents one of the most exploited strategies for improving the functionality of proteins. This approach involves a complex set of interrelated non-enzymatic reactions, where the reducing sugars react with the free amino groups of proteins or peptides to form Maillard reaction products. From a chemical point of view, the Maillard reaction occurs in three stages: early, intermediate, and advanced [5]. The early stage starts with the amino–sugar condensation (reversible Schiff base) accompanied by the Amadori rearrangement. The products that resulted in this stage are colorless, do not have the capacity to absorb ultraviolet light at 280 nm, and exhibit functional properties in terms of emulsification, foaming, heat stability, and gelation. The intermediate stage is characterized by sugar dehydration, fragmentation, and amino acid degradation, whereas the advanced stage includes aldol and aldehyde–amine condensation and the formation of heterocyclic nitrogen compounds [6]. In the intermediate/advanced stages, bioactive products are formed with antioxidant and/or antimicrobial activity, heterocyclic compounds, reductones, and melanoidins, accompanied by color shifting from colorless to yellow, which is more intense in the final stage of the Maillard reaction [6,7,8]. On the other hand, toxic compounds (with mutagenic, carcinogenic, and cytotoxic activity) can also be formed in the advanced stages, but the accumulation of these compounds can be prevented by controlling the reaction conditions [5]. Detailed chemical mechanisms of the Maillard reaction are provided by recent studies reported by Naik et al. [9], Zha et al. [8], and de Oliveira et al. [5]. The physicochemical characteristics in terms of molecular size of the reactants, the molar ratio between proteins/peptides, and carbohydrates, the number of available free amino and carbonyl groups, pH, water activity, temperature, and exposure time are playing an essential role in determining the rate and extent of Maillard conjugation, with positive or negative consequences on the functionality of the resulted conjugates.
The Maillard reaction compounds can be prepared by using conventional, alternative strategies, or a combination of both, as presented in Figure 1. To the best of our knowledge, there are no review papers focusing exclusively on the use of alternative treatments for obtaining Maillard conjugates. In a world where consumer requirements for food with health-related benefits that are at the same time fresh, tasty, and natural are increasing, food scientists and engineers have seen in the use of alternative technologies a good opportunity for satisfying the new consumer demands in a sustainable manner. Therefore, this review focuses on the recent works performed to improve the functionality and bioactivity of proteins through the Maillard reaction by using alternative technologies. The review critically assesses the contribution of the main alternative technologies used for the preparation of Maillard conjugates and their effects on changing the functional and biological properties of the proteins.
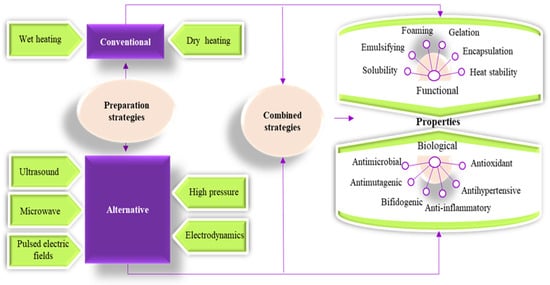
Figure 1. Preparation strategies for obtaining Maillard reaction compounds using alternative technologies and their impact on the functional and biological properties of the protein conjugates.
2. Strategies to Prepare Maillard Conjugates
As shown in Figure 1, the Maillard conjugates can be prepared by involving conventional and alternative strategies. Conventional strategies are based on using heat treatment applied to the wet samples (wet heating) or dry samples (dry heating). Thus, the main difference between dry and wet heating is related to the initial dissolution of reactants (both proteins and carbohydrates) in an aqueous solution [10]. Dry heating involves heating the dry sample under controlled conditions of relative humidity between 60 and 80% at a temperature ranging from 60 to 130 °C for a time interval ranging from minutes to days. Wet heating has the advantage of involving lower temperatures (frequently 60–95 °C) and shorter exposure times (minutes to hours) and does not require a drying step such as freeze drying or spray drying. On the other hand, in wet heating, random thermal agitation of the water molecules promotes a more intense contact between proteins and carbohydrates compared to dry heating, which promotes protein denaturation and subsequent polymerization at high temperatures, causing a lower grafting degree. However, macromolecular crowding is a promising method for reducing protein denaturation and polymerization as it has the potential to accelerate the Maillard reactions while stabilizing protein structure [10,11]. The preparation of Maillard conjugates by conventional strategies using different sources of proteins and carbohydrates is given elsewhere [5,9,10,12,13].
In recent years, alternative technologies have been considered to have remarkable potential for complementing, or in some cases, for replacing, the conventional methods for obtaining Maillard conjugates. These technologies have the advantage of improving the functional and biological properties of protein conjugates while limiting the formation of toxic compounds like acrylamide. Often, a combination of conventional and alternative treatments is considered more cost-effective for obtaining Maillard reaction products with a positive impact on the functionality and quality of the proteins. In principle, the effects of using combined treatments on the functionality of Maillard conjugates could be additive, synergistic, or antagonistic. The schematic representation of the different strategies involving the use of alternative technologies applied for the preparation of Maillard conjugates is presented in Figure 2. Four main strategies have been used so far for obtaining Maillard conjugates employing alternative technologies. The first strategy involves the pretreatment of the protein–carbohydrate mixture using an alternative technology, followed by the application of dry or wet heating. The second strategy uses an alternative treatment to pretreat; in the initial stage, only the protein. After the pretreatment, the protein is mixed with the carbohydrate and exposed to conventional heating. This treatment is preferred when working with protein hydrolysates. The third strategy prepares the Maillard conjugates by combining the alternative with conventional heating (e.g., ultrasound (US) treatment performed at an increased temperature), while the fourth strategy prepares the conjugates by using only the alternative treatments (e.g., US treatment performed at room temperature). The selection of one of these strategies is dependent on the desired protein functionality. The main parameters of alternative treatments and the associated mechanism under which the Maillard reaction products are formed are depicted in Figure 3, whereas the advantages and disadvantages of using the alternative technologies for producing Maillard conjugates are shown in Figure 4.
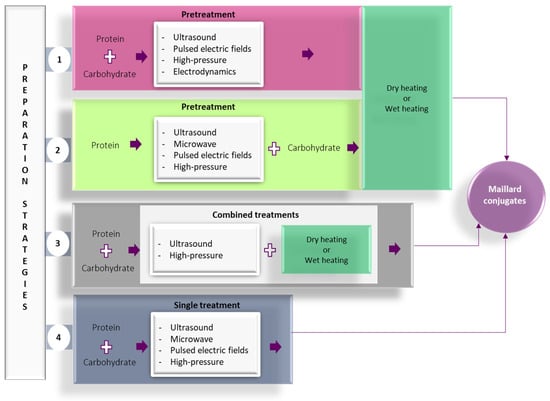
Figure 2. Strategies for preparation of Maillard conjugates based on the use of alternative technologies: (1) pretreatment of protein-carbohydrate mixture using alternative technology, followed by conventional heating; (2) pretreatment of the protein using alternative technologies, followed by the addition of carbohydrate and treatment with conventional heating; (3) the concomitant exposure of protein-carbohydrate system to combined treatment, including alternative technologies and conventional heating; (4) applying the alternative technology as a single treatment to the protein—carbohydrate mixtures.
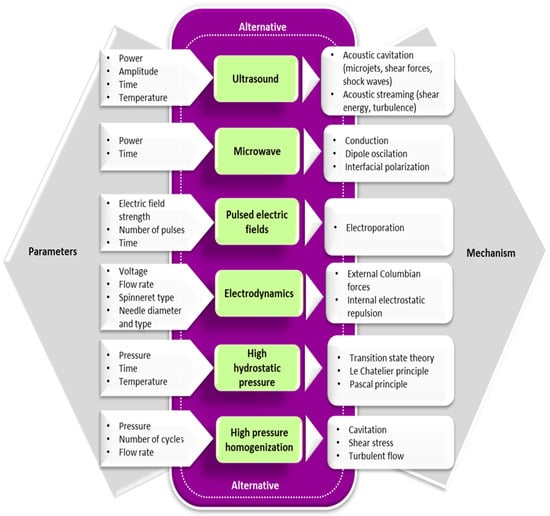
Figure 3. The main parameters of alternative treatments and associated mechanisms that influence the formation of the Maillard reaction products.
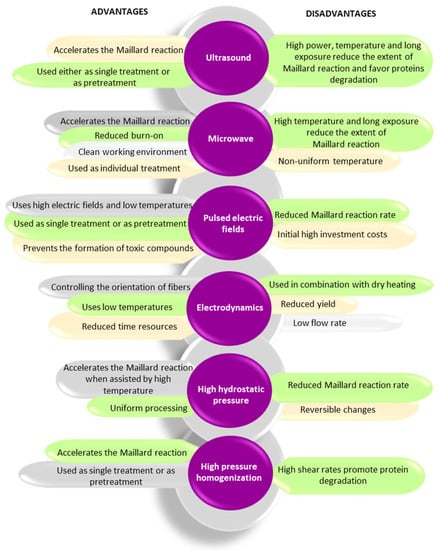
Figure 4. Advantages and disadvantages of using alternative technologies for producing Maillard reaction products.
2.1. Ultrasound (US)
US is a green, non-thermal technology that uses ultrasonic waves (above 16 kHz) to promote Maillard conjugation. During US exposure, acoustic cavitation induces local high temperatures and pressure, leading to mechanical and chemical effects that promote the formation of Maillard compounds. Acoustic cavitation is the result of the creation, expansion, and implosion of microbubbles in the medium that speed up the molecular motions of proteins and accelerate the rearrangement and expansion of protein molecules. These actions have an impact on the secondary and tertiary structure changes of proteins, which favor the exposure of reactive free amino groups of proteins, increasing the grafting speed [7]. In order to produce Maillard compounds with improved functionality, sonication has to be performed under controlled conditions. The formation of Maillard compounds during exposure to US is dependent on several factors, including power, temperature, time, and sample characteristics. Liu et al. [14] found that the US intensity between 0–150 W/cm2 increased the glycation rate for obtaining conjugates between α—lactalbumin and glucose, while Liu et al. [15] showed that exposure to US at 100–550 W for 25 min had no effect on the extent of glycation of β-lactoglobulin with ribose. On the other hand, too much power can decrease the glycation process due to the aggregation of proteins induced by acoustic cavitation [16]. Ma et al. [17] investigated the effects of US power (100–600 W), sonication time (15–35 min), and gum arabic concentration (0.25–1.5%) at a constant temperature of 80 °C, attempting to optimize the formation and properties of conjugates prepared with zein. The grafting degree of zein–gum arabic conjugates increased with increasing US power, exposure time, and polysaccharide concentration, reaching a maximum (32%) after 25 min of reaction at 400 W at a ratio of 2.66:1 (w/w). The increase was attributed to zein unfolding, which exposed more amino groups to participate in the Maillard reaction, and the generation of hydroxyl radicals, which enhanced the reaction between protein and polysaccharide. A further increase in power and time decreased the grafting degree due to the reduced exposure of the amino groups caused by protein aggregation (as a result of the mechanical, cavitation, and thermal effects induced by sonication on protein structure). Compared to conventional wet heating (80 °C, 20 min), the US treatment at 400 W produced conjugates with a higher degree of grafting. Similar results were reported by Jiang et al. [18] for pea protein isolate–inulin conjugates.
Zhao et al. [19] highlighted that the effect of sonication was dependent on the type of carbohydrate used in conjugation. The authors used glucose or maltose for the conjugation of soy proteins, and the effect of sonication on the glycation rate was more pronounced when using glucose. The results were attributed, on one hand, to increased reactivity of monosaccharides than disaccharides and to acoustic cavitation induced during US exposure that led to the unfolding of protein chains that exposed more reactive groups for reactions with carbonyl groups of the carbohydrate.
The ratio between reactants has to be properly chosen, as it can reach a level of saturation above which any further increase of reactants may have a negative impact on glycation, as reported by Dev et al. [20]. The authors investigated the influence of US on the conjugation of the whey protein isolate-gellan gum mixture by varying the ratio between whey protein isolate:gellan gum from 1:1 to 3:1 (w/w) and from 1:1 to 3:1 (w/w) for the gellan gum:whey protein isolate ratio, respectively. The grafting degree increased with the increase in gellan gum concentration up to a 1:2 (w/w) whey proteins:gellan gum ratio, while beyond this ratio, the degree of glycation drastically decreased. US treatment (300 W, duty cycle 50%, 60 min) was able to produce whey protein isolate–gellan gum conjugates with an increased grafting degree (17.22%) in a shorter time than the conventional method (8.41%) performed at 90 °C for 4 h. The results were associated with the US ability to depolymerize the gellan gum into smaller chains, promoting and increasing interactions that favor the extent of glycation.
The initial pH of the biopolymer system has a strong contribution to the glycation reaction; alkaline conditions promote higher intramolecular electrostatic repulsions leading to the unfolding of the proteins; and there is an increase in the number of amino groups available for the Maillard reaction. Similar to other parameters, excessive alkaline conditions have a negative impact on the grafting reaction. The influence of pH (7.0–11.0) on the formation of casein–dextran conjugates obtained using sonication as pretreatment (duty cycle 40%, 15 min) was recently tested [21]. During US exposure, the grafting degree increased up to pH 9.0 due to the formation of Schiff bases in the initial stage of the Maillard reaction, which led to the exposure of more active amino groups. Under more alkaline conditions, the condensation step of the Maillard reaction was promoted, causing a decrease in the grafting degree.
The glycation extent varies with temperature; the optimum temperature depends on the protein structure and conformation. Usually, the applied temperature has to favor protein unfolding, which increases the number of amino groups at the protein surface, making them available to react with the carbonyl groups of the carbohydrate counterpart. On the other hand, the beneficial impact of the US in the reaction system is reduced at higher temperatures, with several papers reporting a negative effect on the glycation rate. For example, in the whey proteins–gellan gum conjugates exposed to US, the grafting degree increased with the temperature up to 70 °C when the maximum exposure of the amino groups at the surface was obtained, which allowed them to react with the carbonyl ends of the gellan gum counterpart. Above 70 °C, it was reported that a smaller US power introduced in the reaction system limited the interaction between reactants, which, at the end, led to a decrease in glycation degree [20]. The optimum temperature of glycation for whey proteins–gum acacia conjugates ranged between 60 and 90 °C, for whey proteins-gum acacia conjugates between 50 and 70 °C, and from 60 °C to 80 °C for peanut protein-glucomannan conjugates [16,22,23]. On the other hand, Stanic–Vucinic et al. [24] applied US to obtain β-lactoglobulin–ribose conjugates with a high grafting degree (about 38%) under non-denaturing thermal conditions (10–15 °C).
When used as a pretreatment, US showed a positive effect on conjugation. For example, Zhao et al. [25] prepared Maillard conjugates by heating aqueous dispersions of soy protein isolate and maltodextrin after sonication at 138 W/cm2 at 20 kHz for up to 25 min. The sonication promoted the glycation reaction as a result of the increase in the β-type ordered secondary structure, leading to a less compact tertiary conformation of conjugates.
The exposure to US of proteins/peptide hydrolysates followed by conventional conjugation was indicated as having good potential for obtaining Maillard conjugates with improved functional and biological properties. Abdelhedi et al. [26] tested the effect of US (160 W, 30 min, 40 °C) on the evolution of glycation induced between peptides with molecular weights lower than 1 kDa obtained from enzymatic hydrolysis (with Esperase) of hound viscera proteins and sucrose. The grafting degree of the conjugates increased significantly during sonication compared to the conjugates obtained by conventional wet heating (90 °C, 2 h), due to the US’s ability to generate free radical-mediated oxidation reactions of free amino acids. Other studies reported an opposite effect of US on the glycation of whey peptides with galactose, as reported by Liu et al. [27]. The sonication of the whey peptide–galactose mixture had a negative impact on the glycation extent that decreased with increasing US exposure from 15 to 60 min, with the highest grafting degree of 21.80% being reported for the conjugates obtained by wet heating (90 °C, 2 h). The US exposure combined with wet heating promoted the exposure of free amino groups in the mixture that were previously hidden.
The US pretreatment of protein from grass carp prior to hydrolysis and conjugation with glucose proved to be very effective in promoting Maillard glycation [28]. US pretreatment at 100 W, 20 min, and 30 °C provided enough energy to change the protein secondary structure and favored the production of peptides/amino acids that accelerated the Maillard reaction. In another study, it was indicated that the formation of Maillard conjugates between the brewer’s yeast-peanut meal hydrolysate and glucose was affected by the US power, and conjugates with improved functional and sensorial properties were obtained when the US pretreatment was performed at 200 W [29]. Above this limit, the formation of Maillard products decreased and flavor characteristics were inferior because sonication inhibited the polymerization of intermediates and consequently the conversion to melanoidins. A recent study investigated the potential of using US at higher temperatures to obtain Maillard conjugates from protein hydrolysates [30]. The authors investigated the changes in the Maillard reaction between mussel meat hydrolysate and glucose/xylose under ultrasonic treatment (300 W, 2 h, pulsation mode, 60 °C), wet heating (115 °C, 2 h), and ultrasonic-assisted wet heating. The grafting degree of the conjugates prepared with ultrasonic treatment was similar to that of wet heating. The ultrasonic (300 W, 30 min, pulsation mode, 60 °C)-assisted wet heating (115 °C, 2 h) was found to have the highest Maillard reaction rate, as the acoustic cavitation and acoustic streaming induced by US exposure lead to the exposure of amino groups to the surface and the disintegration of the polypeptides into low molecular weight peptides.
Water content was shown to have a strong contribution to the glycation process, with too high or too low water content having a negative influence on the grafting degree of the Maillard reaction. Recently, the potential of US-assisted wet heating in a natural deep eutectic solvent (NADES) system for obtaining protein hydrolysate–xylose conjugates with superior functionality was investigated [31]. These eutectic solvents have the potential to regulate the glycation process by controlling the water content of biological systems exposed to Maillard glycation. The authors optimized the glycation reaction by varying the water content (0–50%), temperature (60–85 °C), US power (240–480 W), and reaction time (20–40 min). Optimized conjugates with superior functional properties were obtained when performing the Maillard reaction at 300 W, 80 °C, for 35 min, using a water content of 10%.
2.2. Microwave (MW)
Microwaves use electromagnetic waves in the frequency range of 300–300 GHz that induce molecular vibrations at the molecular level and changes in water polarity orientation [32]. Thus, during MW irradiation, the electromagnetic energy is transferred and converted to heat as a result of interaction with material molecules in a very short period of time [33]. The MW treatment was employed as a single treatment for obtaining Maillard conjugates from different protein sources. Hu et al. [33] tested the effect of MW (480 W and 640 W) between 5 and 15 min on the glycation extent of a solid-state ovoalbumin–glucose mixture. The degree of glycation increased with MW power and exposure time and accelerated the interactions between the free amino groups of ovalbumin and the carbonyl groups of glucose. After exposure for 15 min at 480 W and 640 W, the temperature in the system was 93.4 °C and 109 °C, respectively. When compared to dry-heating glycation, the authors reported that MW is more efficient at improving the functionality of the proteins.
The effect of the ratio between reactants and the exposure time (15–120 min) at a MW power of 450 W on the formation of bovine serum albumin-maltodextrin conjugates was investigated by Nasrollahzadeh et al. [34]. The authors varied the weight ratio between bovine serum albumin:maltodextrin from 1:1 to 1:5 and showed that the proportion of maltodextrin had a strong effect on the Maillard reaction progression. The grafting degree reached a maximum of 40% at a 1:5 bovine serum albumin:maltodextrin weight ratio. Also, the degree of glycation increased significantly with increasing time and reached a maximum after 120 min of MW treatment. The authors concluded that MW was able to speed up the formation of Maillard reaction products compared to conventional wet heating. The MW heating was found to promote the glycation of casein with glucose and β-cyclodextrin and improve the gel properties [35]. Guan et al. [36] indicated that MW heating promoted the Maillard reaction of soy proteins–glucose conjugates and reduced the occurrence of caramelization. Moreover, the authors indicated that, compared to wet heating, exposure to MW had a lower effect on protein agglomeration, suggesting better availability of free amino groups to react with carbonyl groups. It seems that during MW exposure, the protein presents a triple state where molecules have electrons with different mutual spin orientations, with consequences on the biochemical reaction rate of the proteins [34].
2.3. Pulsed Electric Fields (PEF)
PEF is a nonthermal technology based on the use of short pulses of high electric fields for a very short period of time, causing an increase in internal energy in the carbon backbone of biopolymers. This energy is strong enough to weaken the hydrogen bonds between hydroxyl groups, leading to the depolymerization and decomposition of biopolymer chains and changes in the protein surface hydrophobicity due to the polarization of amino acids. Under controlled conditions, the covalent or electrostatic interactions between the proteins and carbohydrates are promoted, allowing the formation of Maillard reaction products [9,10].
Previous studies showed that PEF is able to unfold some proteins, like ovalbumin and bovine serum albumin, promoting the formation of Maillard products upon glycation [37]. PEF pretreatment (25 kV/cm, 60 μs) combined with dry glycation (55 °C, 79% relative humidity, 4 h) increased the glycation degree and promoted the reduction of IgG and IgE binding ability of the β-lactoglobulin–mannose conjugate through covalent binding with mannose [37].
Other authors have demonstrated the potential of using PEF as a single treatment for obtaining Maillard conjugates. For example, the influence of PEF (15 kV/cm, 30 kV/cm, 7.35 ms) on the glycation of the whey protein–dextran system was investigated by Sun et al. [38]. The formation of Maillard reaction products increased with increasing electric field intensity, with positive effects on the functionality of whey protein–dextran conjugates; however, the reaction rate was lower compared with conventional heating. Similar results were reported by Guan et al. [39] for the bovine serum albumin–dextran system, where the Maillard reaction was highly promoted at field intensities above 10 kV/cm. The authors explained that exposure to PEF increases proteolytic activity by forming a dynamic balance between the proteolytic and non-proteolytic forms of bovine serum albumin. Moreover, with the addition of dextran to the system, the balance was broken, increasing the number of exposed amino groups that could participate in the formation of Maillard compounds. Similar results have been reported for bovine serum albumin glycated with glucose or mannose [40]. PEF treatment performed at 10 kV/cm and 20 kV/cm had a strong effect on the conjugation rate, producing an increase from 25.92% to 30.11% in the grafting degree of the conjugates obtained with mannose. When compared to glucose, the grafting degree of conjugates prepared with mannose was always higher; however, the action mechanism was not yet elucidated.
Other reports indicated that above a threshold limit of electric field strength, the glycation degree decreases, as investigated recently by Taha et al. [41] for bovine serum albumin–starch conjugates. PEF treatment performed between 3.5 and 5.7 kV/cm promoted the Maillard reaction with a maximum degree of glycation of 8.92 ± 1.59%, while at a higher electric field strength of 8.1 kV/cm, the glycation degree decreased to 4.33% due to the lower availability of free amino groups caused by protein aggregation. Some studies mentioned the potential of PEF for obtaining Maillard conjugates with desired functionality while reducing the content of advanced glycation end products. This effect is correlated with the reduced temperature induced during PEF treatment, which lowers the extent of the glycation reaction [42].
2.4. Electrodynamics
Electrohydrodynamic processes like electrospinning or electrospraying are performed by applying an electrical field to a polymer solution that can be spun or sprayed to obtain fibers or particles, respectively [60]. Applying high-voltage electric fields (15–25 kV/cm) extrusion is produced at the end of the needle tip, doubled by the external action of Coulombic forces and internal electrostatic repulsions of the charges accumulated on the surface of polymer solution that induce distortions of the hemispherical droplet into a Taylor cone. When the critical value of the electrical field counteracts the surface tension, fibers or particles are formed [61].
These techniques have found many applications in the food industry where electrospun fibers/particles can be successfully used as protective films or delivery systems [55]. Compared to conventional methods, electrospinning is an excellent alternative for accelerating the formation of Maillard conjugates. The glycation process during electrospinning is affected by the preparation of the polysaccharide–protein fibers/particles and the dry conjugation process known as the annealing process. In the annealing process, the electropun fibers/particles resulted from electrohydrodynamics are maintained under controlled conditions of temperature and relative humidity for a specified time interval [10].
The electrospinning method was found to be very promising for obtaining protein–polysaccharide conjugates with shorter incubation times and higher conjugate yields, as reported by Baier et al. [62]. The authors patented the application of electrospinning as a pretreatment to produce whey proteins–dextran conjugates and reported that the most important parameters that affected fiber formation and conjugation were: dextran concentration, mixture viscosity, protein-to-polysaccharide ratio in the mixture, and time allowed for annealing. The glycation extent increased with increasing annealing time and decreasing the molecular weight of dextran. Good fiber formation and conjugation were reported at 100 kDa dextran, whey proteins:dextran weight ratio above 1:2, annealing time between 4 and 24 h at 60 °C, and a relative humidity of 74%. Kutzli et al. [63] used a combination of electrospinning and dry heating to promote the glycation of the pea proteins–maltodextrins system. Maltodextrins with different dextrose equivalents (2, 10, 21) were tested to investigate their effect on electrospinnability (63.8 kV) and dry glycation (60 °C, 75% relative humidity, 6–24 h). The glycation extent was affected by the maltodextrin composition and incubation time. The addition of two maltodextrins (2 and 10 dextrose equivalent) increased the grafting degree, which reached a maximum after 6 h of heating at 60 °C. The authors did not observe any influence of fiber diameter on the glycation process. More recently, the positive influence of glycation with xylose on the physical properties of gluten/zein nanofibrous films produced by electrospinning was reported by Zhang et al. [64]. The composite gluten/zein electrospun films, further glycated via the Maillard reaction, presented physical properties required for active food packaging applications.
2.5. Pressure-based Processing (HP) Techniques
HP technologies are among the most successful nonthermal technologies, well recognized for their advantages of preserving many food products while keeping their freshness. HP treatments represent a good alternative to conventional thermal treatments for modifying the functionality of proteins. Thus, under the action of high pressure, the quaternary, tertiary, and secondary structures of proteins are modified, making them structurally more available to participate in the Maillard reaction and affecting their functionality. Also, pressure favors the formation of the reactive forms of the reducing carbohydrate; however, the addition of carbohydrates with low ionic strength can increase the pressure stability of some proteins, which enables them to rearrange their secondary structure after the pressure treatment [65,66].
Based on the processing conditions applied, Maillard conjugates can be produced with high hydrostatic pressure (HHP) followed by thermal treatment, by high pressure-high temperature (HPHT), also known as pressure-assisted thermal sterilization, by dynamic high-pressure microfluidization (DHPM), or low-pressure homogenization (LPH).
Applied as a single treatment, HHP was shown to be less effective in promoting the formation of Maillard products that enhance the functionality of the proteins. Under the action of pressure, the formation of furfurals or reductones from Amadori rearrangement products is retarded, whereas the breakdown of Amadori rearrangement products is delayed due to nitrogen loss [65]. When HHP is performed at moderate pressure and temperature, the formation of Maillard conjugates is reported. For example, Liu et al. [59] showed that the formation of Maillard conjugates between soybean protein isolate and flaxseed gum was promoted after 3 days of exposure at moderate pressure (100 MPa) and mild temperature (60 °C). The same authors showed that above 200 MPa, pressure had a negative effect on glycation extent. A similar effect was reported in another study, where HHP treatment at 400 MPa was combined for 1 h with mild thermal treatment at 60 °C [67]. It was obtained with a lower effect on the conjugation of β-lactoglobulin with lactose than conventional dry heating (50 °C, 44% relative humidity, 120 h). The authors attributed these results to conformational changes induced by HHP treatment that hindered the accessibility of carbonyl groups of carbohydrate to the free amino groups of the protein.
In comparison with conventional heat treatment, HPHT at 123 °C and 700 MPa for up to 15 min was able to retard the formation of Maillard reaction products in the initial and advanced stages of whey protein–sugar solutions, regardless of the pH investigated [68]. During LPH and DHPM, the protein structure and conformation are affected as a result of cavitation, shearing, turbulence, and heating for short periods, leading to protein unfolding and aggregation [57]. The difference between LPH and DHPM is based on the applied pressure. For high-pressure homogenization, a pump has the ability to deliver at least 100 MPa, regardless of the flow rate [69].
Zhao et al. [57] modified the functionality of pea proteins by using a combination of LPH (80 MPa, 3 passes), US (400 W, 20 min, 70 °C), and wet glycation (20 min, 70 °C) with xylo-oligosaccharides. The authors tested the influence of xylo-oligosaccharides on grafting degree and found that glycation extent increased with increasing xylo-oligosaccharide concentration. However, above a pea proteins:xylo-oligosaccharides weight ratio of 1:3, the grafting degree decreased. Moreover, it was found that the combination of LPH and US was more effective in increasing the glycation degree than the single treatments, due to the LPH’s ability to break down the aggregates and the sonication that induced the partial protein unfolding. On the other hand, glycation was not able to suppress protein aggregation (near isoelectric point) due to the low molecular weight of xylo-oligosaccharides that generated weak steric repulsive forces.
Recently, DHPM was employed as a single treatment for obtaining Maillard conjugates. The exposure of the lotus seed protein–dextran mixture between 40 and 160 MPa accelerated the conjugation, with the highest degree of glycation (17.8%) being reported at 120 MPa [58]. A further increase in pressure had a negative effect on the grafting degree because the molecular forces responsible for maintaining the protein’s spatial structure were broken, leading to protein aggregation. Regardless of the pressure applied in DHPM (40–160 MPa), the degree of grafting was always higher when compared to conventional heating (70 °C, 4 h).
This entry is adapted from the peer-reviewed paper 10.3390/foods12193588
This entry is offline, you can click here to edit this entry!
