Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The disturbances caused by electromagnetic field (EMF) noise of medical devices used near living tissues, as well as the corresponding functional control via the electromagnetic compatibility (EMC) of these devices are analyzed. These are minimally invasive and non-ionizing devices allowing various healthcare actions involving monitoring, assistance, diagnoses and image-guided medical interventions.
- EMF noise perturbation
- functional control
- EMC analysis
- devices working close to tissues
- monitoring
- assistance
- diagnosis
- image-guided interventions
- onboard devices
1. Introduction
Electromagnetic fields (EMFs) are used in many everyday pieces of equipment. They reflect wide ranges of strength and frequency. The environment close to the EMF source devices is subject to their exposure. Unwanted exposure to such fields can cause different types of disturbances and disorders in various areas. One of the greatest areas of concern is health safety. In this case, the disorder could directly involve body tissues or healthcare diagnostic, detection and intervention devices. Regarding EMF exposure, most of the affected source devices are characterized by significant stray fields, e.g., wireless devices. The EMF exposure of living tissues can cause different biological effects [1][2][3][4][5][6]. The other effect of EMF exposure on health safety is related to medical devices. Such exposure can disrupt several types of health devices. The devices most affected by health problems are those working near body tissues. Two important categories of devices are of concern. The first concerns imaging diagnostic procedures and image-assisted robotic interventions [7][8][9][10][11][12][13]. In such a case, disturbances due to EMF noise mainly concern universal non-ionizing procedures using MRI. The second category concerns body onboard embedded and wearable devices [14][15][16][17][18].
MRI as a diagnostic tool could be disrupted due to its sensitivity to EMF noise. This scanner is generally protected against exposure to external fields. However, EMF noise could be triggered due to the introduction of magnetic or conductive material inside or near the scaffold. This may occur due to objects inserted in the body under testing. The consequences would be image artifacts disrupting the diagnosis [19][20][21][22]. Considering the case of MRI-assisted robotic interventions, these can be surgical or implanted treatment. These procedures mainly concern situations requiring movement or location control. Thus, the assistance of concern offers remarkable means of localization and precise displacement control, improving the result of medical treatment and allowing precise minimally invasive actions. In this case, the EMF noise disturbance could be, in addition to the body tested, due to the presence of robotic accessories and medical tools within the scaffold [23][24][25][26][27][28][29].
The EMF noise perturbation of onboard devices is provoked by exposure to external fields (radiation). Embedded and portable devices are often used for ongoing medical assistance or for diagnostic and monitoring purposes. The case of wearable mechanisms correspond to a passive programmed role as sensing functions, e.g., [30][31][32]. The wearable biosensors involved behave as non-intrusive tools allowing real-time monitoring of patients, providing sufficient data to establish their health status and can constitute a first diagnosis. Devices integrated into the body can be static passive for permanent monitoring, e.g., [17], or active stimulating or assisting tools, e.g., [18]. All of these onboard tools enable diligent, personalized and tailored healthcare. Note that most of these devices may be of concern with the disturbances caused by the patient’s body inside the MRI mentioned above.
The different functional disorders of devices due to EMF noise mentioned above should be evaluated and controlled. Thus, a routine of functional control could be practiced on these devices. The functional control at large verifies the ability of a device to operate in a specific environment. Regarding EMFs, generally speaking, the increasing complexity and amplified practice of electronic tools have given rise to electromagnetic interference (EMI), which involves various signals emitted in an unsolicited manner. These can affect the operation of systems in a specific electromagnetic atmosphere, causing them to fail or reducing their performance. Indeed, EMI corresponds to the transmission of disturbing energy (noise) between two systems (source and receiver) via radiating and/or conductive tracks. Such noise can come from an artificial source (like radar or a cell phone) or a natural source (like lightning). Furthermore, the noise could be intrinsic to the system due to alterations of its physical characteristics (like the effect of the insertion of external materials). Concerning EMI, the creation of an electromagnetically compatible atmosphere in relation to the affected system (receiver) allows it to regain its intended operation. Thus, EMC can be achieved to respond to EMI threats. Therefore, due to the different types of noise mentioned, the functional EMC control can be termed as verifying the ability of a device to function properly in its electromagnetic environment without interference with itself or other systems in that environment.
Consequently, the various functional disturbances of medical devices conferred above can be evaluated and controlled with an EMC analysis, e.g., [25][27][29]. This can be achieved by experimental means (where possible) or by using numerical modeling tools. Such a numerical assessment in addition to functional control can assist in the redesign of disturbed devices, disturbing sources and introduced external materials. Additionally, a numerical EMC analysis enables shielding design with validity verification regarding sources and targets of disturbances [33].
2. Imaging Methodologies
Various imaging techniques are used in healthcare treatments. The chosen option depends on several conditions related to the concerned circumstances. Living tissue imaging involves soft tissues, bones, fluids and air gaps. The most widespread techniques use X-rays, magnetic fields, ultrasound or radioactive drugs (positron emission, gamma rays, etc.). The corresponding scanners are each relatively adapted to a specific case. Apart from magnetic and ultrasound imaging techniques, the others are subject to ionizing radiation. These tools can be used for diagnostic purposes or therapeutic assistance. In conventional diagnostic imaging, patient exposure is generally short-term, posing no risk from any scanner. On the other hand, persistent exposures such as, e.g., assisted treatments, infer and take into account the comfort and safety of the patient. In addition to a minimally invasive practice, non-ionizing conditions are required. In these circumstances, only MRI and ultrasound scanners are free from ionizing radiation [9][10][11][12][13]. However, each of these two scanners is subject to particular limitations. Ultrasound can only work in tissues devoid of bone and air [27][29]. MRI needs an atmosphere free from external EMF noise, which must be controlled [23][24][25][26][27][28][29]. From the above analysis, for image-assisted treatments, MRI seems the most adequate, conditioned on controlled external EMF noise (EMC control). Figure 1 illustrates the above analysis for imaging strategy options.
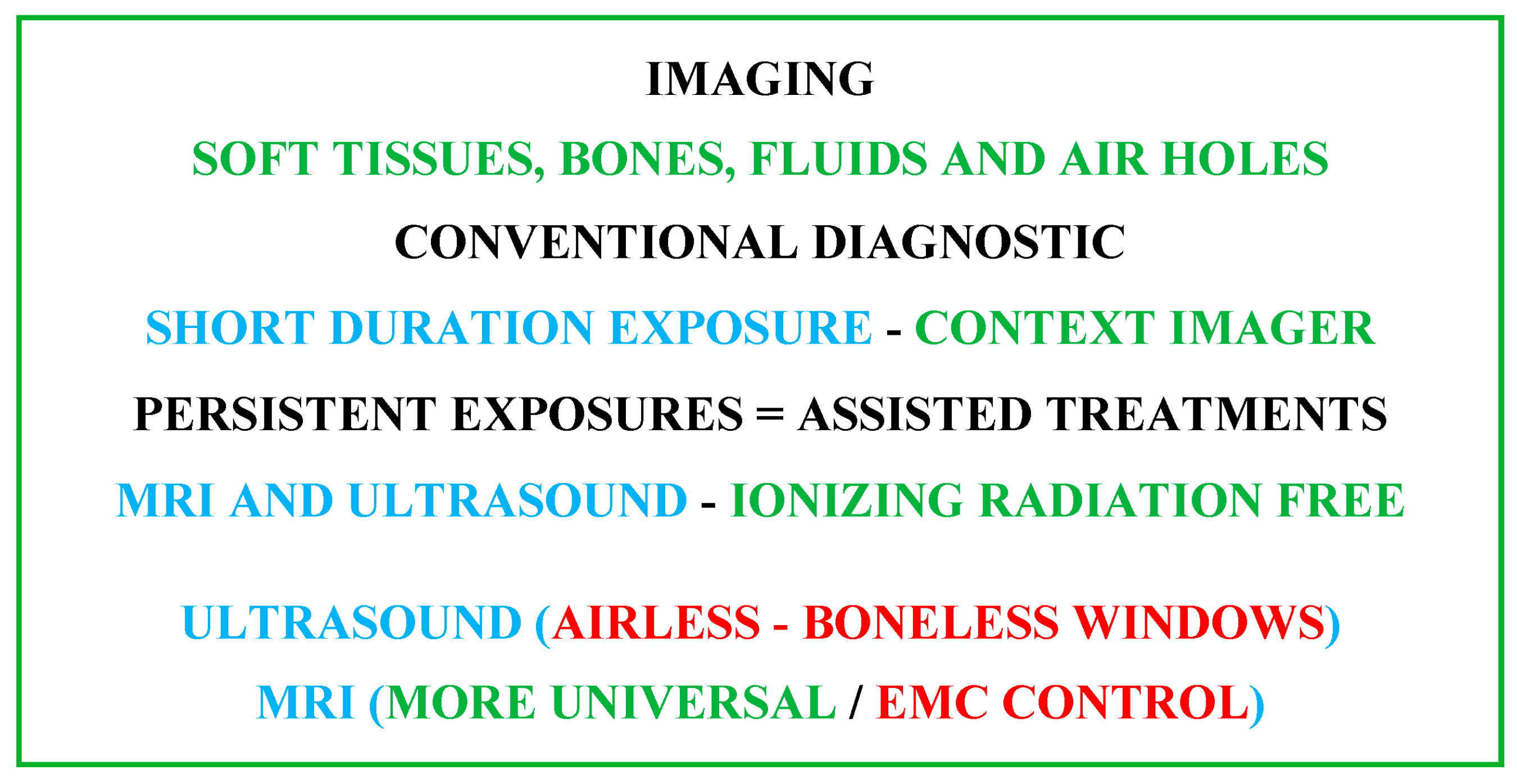
Figure 1. Summarizing diagram of imaging strategy options accounting for patient comfort and safety.
2.1. MRI Constituents
The shaped MRI image is produced using signals resulting from the interaction of biological tissues with magnetic fields. Three different feature fields are used to create 3D images. The first is a high-intensity static field. It generates a magnetizing vector in living tissue that aligns and measures the density of the protons involved. The second is linked to three low-frequency spatial gradient fields. These locate aligned tissue protons, establishing a 3D restoration of the different spatial divisions of tissue in the images. The third is a radio frequency field. This stimulates the magnetizing vector, allowing its identification with the scanner and the transformation of the effects on the tissues into images [27].
In fact, MRI theoretically aims to image the nuclei of hydrogen atoms, which are held inside living tissues. A hydrogen nucleus that is a proton is a mass of positive charge rotating on itself around an axis. In living tissue, protons are rotated randomly and do not rotate all together. As a result, they display zero subsequent magnetic field and operate out of phase. According to the principle of MRI, protons require three basic arrangements in the examined section of tissue, which align in a fixed direction all the protons, rotate them together and locate their distinct spatial origins. The aligning action could be fulfilled by the introduction of the concerned body tissue section in a high-intensity magnet to steer them simultaneously in the axial direction of its static field B0. To reach their joint spinning, a resonance action can be applied. Thus, one can use an excitation with a radiofrequency (RF) field, B1, having a frequency identical to that of proton rotation natural frequency fL (Larmor frequency of protons). Localizing protons’ distinct spatial positions can be achieved through the use of their related field distinctive values. Thus, a 3D space gradient, G(x, y, z), with pulsations of low-frequency repetitions can be joined to the field B0, permitting the detection of the distinct position values of B0d (x, y, z) = B0 + G(x, y, z). The last conferred fields B0, B1 and G(x, y, z) reflect different natures. It is worth noting that the value of protons’ Larmor frequency fL is a function of the B0 field value and equivalent to 42.5 MHz per tesla and hence the conforming position distinctive frequencies fLd (x, y, z) are functions of B0d (x, y, z).
These three fields allow establishing images as follows. The protons are subjected to excitation–relaxation sequences by an RF energy wave, leading to energy supply restoration actions. A suitable tuned RF antenna permits the detection of the restored energy corresponding signals. These are related to the B1 values with frequencies of fLd (x, y, z). Thus, coding of spatial imaging in the concerned tissues can be obtained. Note that B1 frequency is fL that is usually tuned to a value in the center of the explored tissue of fLd (x, y, z).
2.2. Features of MRI Fields B0, G(x, y, z) and B1
Respectively the strong magnet, gradient coils and RF coil produce these fields. In the standard procedure of MRI, its proper action necessitates the shield of the magnet and gradient coils and the compensation of their fields. Indeed, a virtuous MRI requests a uniform constant B0 (by using shimming coils) and linear uniform regulated field gradients. These fields necessitate corrections and compensations for reliable performing of the scanner.
The RF coil field seems the most exposed, and characterizes a fragility to noise fields and to near introduced external materials. The most widespread form of an RF coil is birdcage-like and is used as an exciting RF source as well as a tuned RF antenna. Figure 2 illustrates a representation of the three MRI components and their conforming fields.
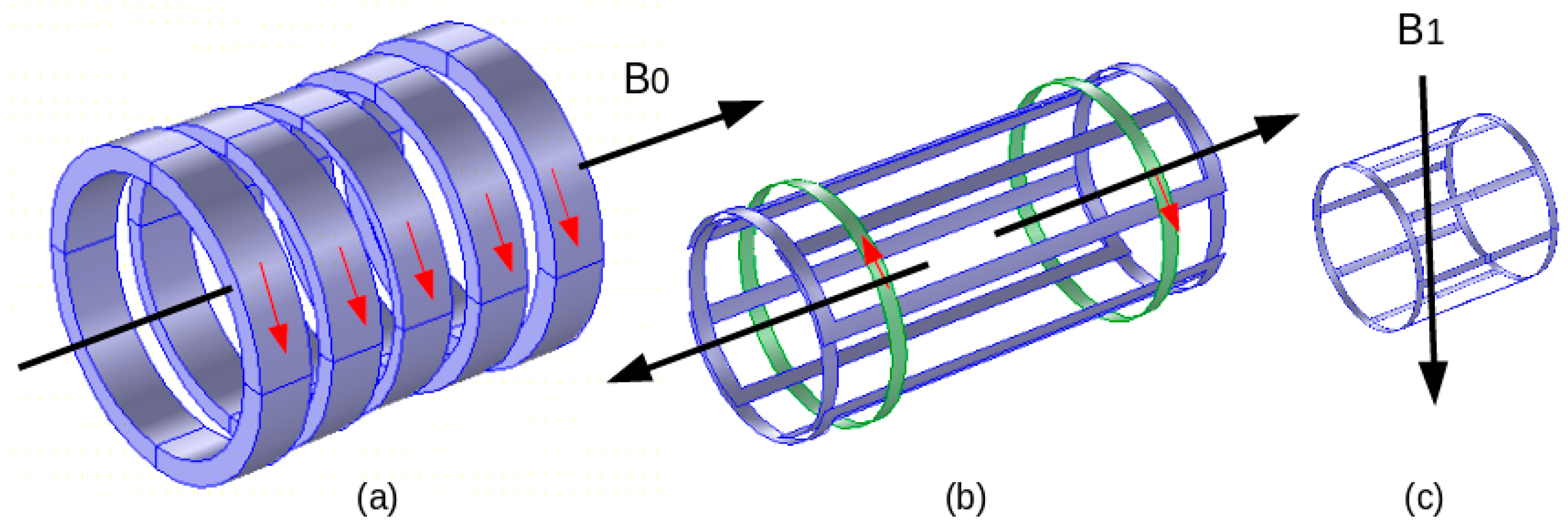
Figure 2. MRI components and corresponding fields: (a) electromagnet B0, (b) gradient coils (one axis couple), (c) RF coil B1.
The characteristics of these fields are different regarding their magnitude, pulsation and presence throughout the MRI functioning—B0: 0.2–10 T, 0 Hz, permanently present; G(x, y, z): 0–50 mT/m, 0–10 kHz, multiple pulses of few ms; and B1: 0–50 μT, 8–300 MHz, Amp. Mod. Pulses of few ms.
2.3. MRI Compatibility
As mentioned earlier, MRI is sensitive to EMF noise resulting from external field exposure or insertion of specific external matters (magnetic and conductor) within or close to the MRI scaffold. Conventionally, an MRI is shielded regarding external field exposure. We can largely typify an external object as MRI-compatible if it behaves in an MRI-safe manner, not affecting image quality, and working as expected. In addition, the static field magnet and the gradient coils are shielded and their fields are compensated for a reasonable size of inserted matters. As stated in the last section, only the RF coil and its field seem vulnerable for such insertion and it is necessary to control the compliance of the inserted matters with the correct functioning of the scanner. Thus, the inserted external materials should be MRI-compatible, i.e., not perturbing the RF field and image. Only non-magnetic and non-conductor matter can theoretically behave in an MRI-compatible manner. In general, magnetic materials are reduced to trivial-almost-zero sizes. Conducting materials have an indirect perturbing effect on the RF field. They exhibit eddy currents induced by the RF field that perturb the distribution of this field, which alters the image. Such induced currents are directly related to the conductor surface perpendicular to the RF field direction and hence can be reduced by minimizing such surface. Typically, a conducting sheet of negligible thickness positioned parallel to the field direction will almost not cause any field perturbation independent of its surface size. This phenomenon permits the use within the scaffold of conducting matters with specific shapes and orientations.
2.4. Image Artifacts
The image quality can be deteriorated for diverse reasons. The scanner itself can be the source of decreased image quality, as in the case of an inefficiently shimmed imager. Living tissues can also diminish the image quality due to susceptibility discrepancies, such as those between soft tissues and air holes in the brain. Moreover, embedded matters within the body such as prostheses and particularly metallic ones can also weaken image quality [34][35][36][37][38][39][40]. The image modification due to metallic materials, which present susceptibility variations, depends on the size, the shape and the direction with respect to B1 [27][29][33]. Additionally, an important cause of image artifacts could be the tools, particularly metallic ones, involved in under-imaging processing. Indeed, currents induced in metals not only mainly by the RF fields but also by low-frequency gradient fields can affect the image. These currents could interact mainly with the RF field, which is the most at risk among the fields of an MRI.
3. MRI-Assisted Robotic Treatments
As mentioned previously, in surgical or implanted treatments requiring movement or localization control with increased precision, we can use imaging-assisted robotic interventions allowing precise minimally invasive actions. Also, in such image-assisted treatments, non-ionizing MRI seems to be the most adequate, on the condition of controlled external EMF noise. Such noise can come from robotic accessories and medical tools [23][24][25][26][27][28][29].
3.1. Robotic External Matter Introductions
As mentioned in the last section, only non-magnetic and non-conducting materials can be used in MRI-guided robotic interventions. The robot’s mainframe and therapeutic tools are normally made of non-magnetic and non-conducting materials. However, a robot needs actionable movements. Most competent actuators are the electromagnetic ones using magnetic and conductive materials. Few types of non-electromagnetic actuators with performance suitable for MRI-assisted robotic operation can be used. This could be the case for piezoelectric actuators subject to their MRI compatibility; see, e.g., [41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56]. Such actuators are made of dielectric piezoelectric materials coated with trivial electrodes. The shape and orientation of these electrodes (conductors) relative to the RF field B1 allow possible MRI compatibility. Different keys are proposed for the mechatronic division of the robot containing electronics, sensors, actuators, etc., which address a difficult compatibility question [27][29][41][42][43][44][45][46][57][58][59][60][61].
3.2. MRI-Compatible Materials
Materials governed by EMF behavior can be magnetic or non-magnetic and a conductor. The non-magnetic materials can behave between dielectric and electric conducting function of EMF wave frequency. Magnetic, dielectric or conductor materials are characterized, respectively, by the permeability μ (or the susceptibility χ), by the permittivity ε or by the conductivity σ. In highly magnetic material, μr >> 1 and μr ≈ χ; note that μ = μ0 · μr and χ = μr − 1. For non-magnetic material, μr = 1 and χ = 0. The relative values of σ and ω · ε (ω = 2 π f) characterize dielectric vs. electrically conducting behaviors of non-magnetic materials. For low f, σ >> ω · ε ≈ σ and for high f, ω · ε >> σ ≈ ω · ε and σ ≈ 0.
The materials’ compatibility in MRI is of two types, magnetic and electric, characterized, respectively, by μ (or χ) and σ. A fully MRI-compatible material has zero values for both χ and σ. The dielectric nature of matters does not affect the compatibility. Regarding the RF field distribution, the eventual introduced matters should have μr = 1 and χ = 0, with high ω · ε (will be naturally high in RF range), or conductors with a trivial cross section (e.g., very thin sheet) perpendicular to the RF field B1. With such features, the RF field distribution would not be altered. Note that the induced eddy currents (responsible for field perturbation) in the configured conductor by the current in the RF coil would be extremely negligible due to the trivial conductor section.
3.3. Conformity Control of MRI Compatibility
The MRI-compatibility control can be accomplished for existing image-guided MRI systems by using experimental means. This can be carried out by measuring the perturbations of the field resulting in the insertion of checked objects within or near the scaffold according to the case. This is generally accomplished via sensors positioned in specific points in the system. Such techniques in the case of MRI are relatively complex due to the necessity of special shielded expensive chambers and the self-perturbation effects of the measuring sensors. Additionally, the characteristics of a tested object could prove dangerous, leading to degradation of imager components. Furthermore, such a compatibility check is only possible for existing systems and cannot be used for the design of unbuilt systems. In these circumstances, a more advantageous solution could be a compatibility check with numerical modeling techniques via an EMC analysis for the different inserted objects [25][27][29][33]. In fact, disturbances in the distribution of EMFs in a given structure caused by the introduction of an external material are related to the EMFs produced in that material. In this case, if the EMF noise is reduced or removed, the field distribution of the target structure will be minorly or not affected.
4. Embedded, Wearable and Detachable Devices
Recent advances in the field of biocompatible and biodegradable materials [62][63][64][65][66][67][68][69][70][71][72][73] have enabled the development of implantable static passive tools enabling diagnoses and prediction via mini-sensors, thereby dramatically improving the value and efficiency of patient healthcare [74][75][76][77][78]. Other static but active implanted devices are proposed to stimulate or trigger an organ, as pumps, neuro-stimulators or pacemakers [79][80][81][82][83]. In addition, wearable devices, which behave as non-invasive tools in real-time, are available, allowing continuous monitoring of people under treatment and thus providing sufficient medical data to establish the general health status and, furthermore, a preliminary identification of the medical diagnosis [14][15][16][30][31][32]. Additionally, detachable and connectable smart sensing devices, which are also real-time health monitoring systems, exist for functional indications intimately associated with physical conditions. These indications concern the frequency of cardiac functioning, blood circulation pressure, respiratory rate, etc. Such individualized healthcare supervision provides appropriate medical information [84][85][86]. Figure 3 illustrates a summarized representation of the different onboard devices and their functions.
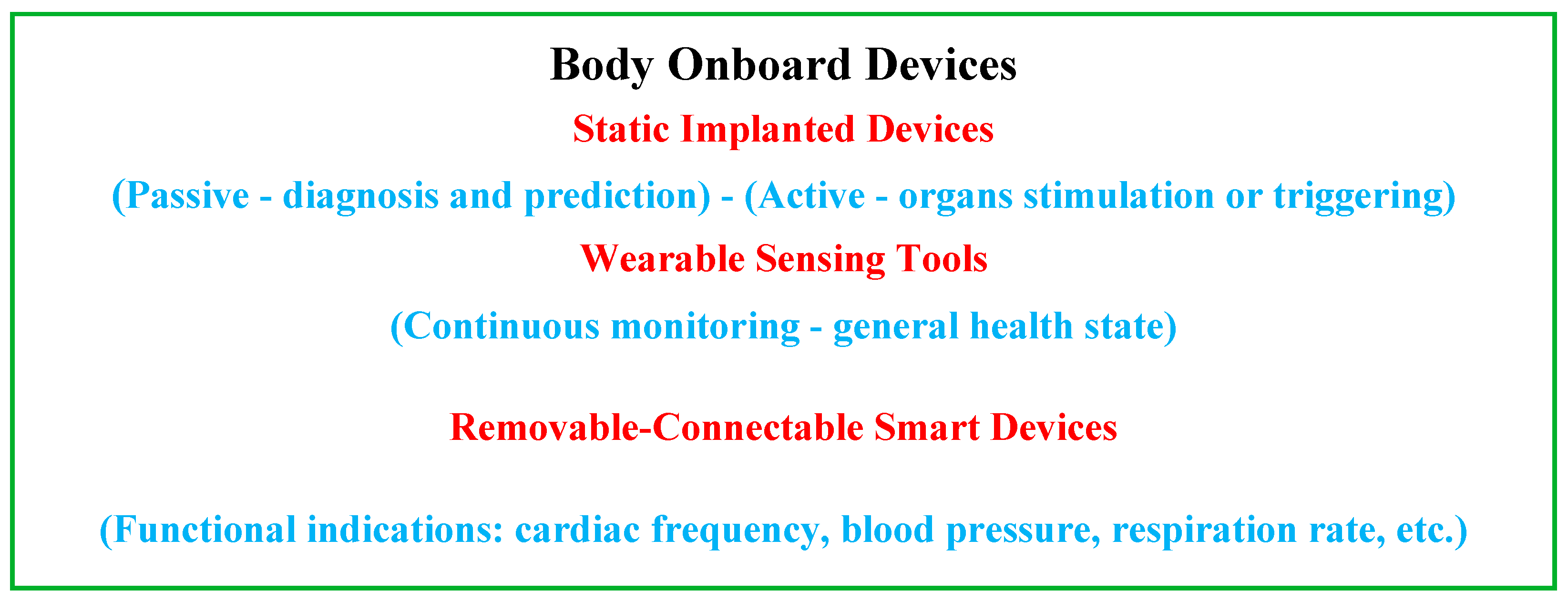
Figure 3. Summarizing diagram of the different onboard devices and their functions.
In addition to the mentioned functions, implanted devices, wearable sensing tools and removable and connectable smart devices enable healthcare providers to monitor the biological characteristics of patients after treatment [87][88]. Additionally, it should be noted that an important aspect of health administration responsibilities is increased support for integration and connection strategies for the delivery of personalized care. Therefore, it is preferable to use the paradigms of connected services and home clinics rather than face-to-face care. All three mentioned implantable, wearable and detachable strategies are involved in such individualized healthcare [89][90][91][92].
An important safety issue is the protection of devices conferred above from exposure to EMF noise emitted by nearby everyday sources. This can be accomplished in different ways. The first is to bypass EMF-sensitive materials in their makeup where possible. The second option is to protect (by shielding) these devices as well as the tools that are sources of exposure. An additional precaution is to avoid exposing them to strong electromagnetic fields such as their insertion in the high static field of an MRI.
This entry is adapted from the peer-reviewed paper 10.3390/electronics12234780
References
- Petroulakis, N.; Mattsson, M.O.; Chatziadam, P.; Simko, M.; Gavrielides, A.; Yiorkas, A.M.; Zeni, O.; Scarfi, M.R.; Soudah, E.; Otin, R.; et al. NextGEM: Next-Generation Integrated Sensing and Analytical System for Monitoring and Assessing Radiofrequency Electromagnetic Field Exposure and Health. Int. J. Environ. Res. Public Health 2023, 20, 6085.
- Henschenmacher, B.; Bitsch, A.; de Las Heras Gala, T.; Forman, H.J.; Fragoulis, A.; Ghezzi, P.; Kellner, R.; Koch, W.; Kuhne, J.; Sachno, D.; et al. The effect of radiofrequency electromagnetic fields (RF-EMF) on biomarkers of oxidative stress in vivo and in vitro: A protocol for a systematic review. Environ. Int. 2022, 158, 106932.
- Hamed, T.; Maqsood, M. SAR Calculation & Temperature Response of Human Body Exposure to Electromagnetic Radiations at 28, 40 and 60 GHz mm Wave Frequencies. Prog. Electromagn. Res. M 2018, 73, 47–59.
- Lagorio, S.; Blettner, M.; Baaken, D.; Feychting, M.; Karipidis, K.; Loney, T.; Orsini, N.; Röösli, M.; Paulo, M.S.; Elwood, M. The effect of exposure to radiofrequency fields on cancer risk in the general and working population: A protocol for a systematic review of human observational studies. Environ. Int. 2021, 157, 106828.
- Romeo, S.; Zeni, O.; Sannino, A.; Lagorio, S.; Biffoni, M.; Scarfì, M.R. Genotoxicity of radiofrequency electromagnetic fields: Protocol for a systematic review of in vitro studies. Environ. Int. 2021, 148, 106386.
- Pophof, B.; Burns, J.; Danker-Hopfe, H.; Dorn, H.; Egblomasse-Roidl, C.; Eggert, T.; Fuks, K.; Henschenmacher, B.; Kuhne, J.; Sauter, C.; et al. The effect of exposure to radiofrequency electromagnetic fields on cognitive performance in human experimental studies: A protocol for a systematic review. Environ. Int. 2021, 157, 106783.
- Kovács, A.; Bischoff, P.; Haddad, H.; Kovács, G.; Schaefer, A.; Zhou, W.; Pinkawa, M. Personalized Image-Guided Therapies for Local Malignencies: Interdisciplinary Options for Interventional Radiology and Interventional Radiotherapy. Front. Oncol. 2021, 11, 616058.
- Zhao, J.; Zhi, Z.; Zhang, H.; Zhao, J.; Di, Y.; Xu, K.; Ma, C.; Liu, Z.; Sui, A.; Wang, J. Efficacy and safety of CT guided 125I brachytherapy in elderly patients with non small cell lung cancer. Oncol. Lett. 2020, 20, 183–192.
- Park, B.K. Ultrasound-guided genitourinary interventions: Principles and techniques (Review Article). Ultrasonography 2017, 36, 336–348.
- Pinto, P.A.; Chung, P.H.; Rastinehad, A.R.; Baccala, A.A., Jr.; Kruecker, J.; Benjamin, C.J.; Xu, S.; Yan, P.; Kadoury, S.; Chua, C.; et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J. Urol. 2011, 186, 1281–1285.
- Fiard, G.; Hohn, N.; Descotes, J.L.; Rambeaud, J.J.; Troccaz, J.; Long, J.A. Targeted MRI-guided prostate biopsies for the detection of prostate cancer: Initial clinical experience with real-time 3-dimensional transrectal ultrasound guidance and magnetic resonance/transrectal ultrasound image fusion. Urology 2013, 81, 1372–1378.
- Veltri, A.; Garetto, I.; Pagano, E.; Tosetti, I.; Sacchetto, P.; Fava, C. Percutaneous RF thermal ablation of renal tumors: Is US guidance really less favorable than other imaging guidance techniques? Cardiovasc. Intervent. Radiol. 2009, 32, 76–85.
- Bassignani, M.; Moore, Y.; Watson, L.; Theodorescu, D. Pilot experience with real-time ultrasound guided percutaneous renal mass cryoablation. J. Urol. 2004, 171, 1620–1623.
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813.
- Xin, Y.; Liu, T.; Sun, H.; Xu, Y.; Zhu, J.; Qian, C.; Lin, T. Recent progress on the wearable devices based on piezoelectric sensors. Ferroelectrics 2018, 531, 102–113.
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, 1706910.
- Sener, T.; Haenen, W.; Smits, P.; Hans, G.H. Large-scale real-life implementation of technology-enabled care to maximize hospitals’ medical surge preparedness during future infectious disease outbreaks and winter seasons: A viewpoint. Front. Public Health 2023, 11, 1149247.
- Navarro-Valverde, C.; Ramos-Maqueda, J.; Romero-Reyes, M.J.; Esteve-Ruiz, I.; García-Medina, D.; Pavón-Jiménez, R.; Rodríguez-Gómez, C.; Leal-del-Ojo, J.; Cayuela, A.; Molano-Casimiro, F.J. Magnetic resonance imaging in patients with cardiac implantable electronic devices: A prospective study. Magn. Reson. Imaging 2022, 91, 9–15.
- Hirano, M.; Muto, Y.; Kuroda, M.; Fujiwara, Y.; Sasaki, T.; Kuroda, K.; Kamizaki, R.; Imajoh, S.; Tanabe, Y.; Al-Hammad, W.E.; et al. Quantitative evaluation of the reduction of distortion and metallic artifacts in magnetic resonance images using the multiacquisition variable resonance image combination selective sequence. Exp. Ther. Med. 2023, 25, 109.
- Amann, N.; Johnson, S.; Chagarlamudi, K.; Gupta, A.; Faraji, N. Scheduling Musculoskeletal MRI for Patients with Metallic Hardware: Initial Observations on Decreasing Nondiagnostic and Repeat Examinations at a Multisite Academic Medical Center. Curr. Probl. Diagn. Radiol. 2023, 52, 327–329.
- Fujiwara, Y.; Sasaki, T.; Muto, Y.; Hirano, M.; Kamizaki, R.; Murakami, K.; Miura, N.; Fujibuchi, Y.; Ohmukai, N.; Ueda, N.; et al. Multiacquisition Variable-Resonance Image Combination Selective Can Improve Image Quality and Reproducibility for Metallic Implants in the Lumbar Spine. Acta Med. Okayama 2021, 75, 187–197.
- Choo, H.J.; Lee, S.J.; Lee, Y.H. Metallic Artifacts on MR Imaging and Methods for Their Reduction. Taehan Yongsang Uihakhoe chi 2020, 81, 41–57.
- Chinzei, K.; Kikinis, R.; Jolesz, F.A. MR compatibility of mechatronic devices: Design criteria. In Medical Image Computing and Computer-Assisted Intervention—MICCAI’99; Springer: Berlin/Heidelberg, Germany, 1999; Volume 1679, pp. 1020–1030.
- Tsekos, N.V.; Khanicheh, A.; Christoforou, E.; Mavroidis, C. Magnetic resonance-compatible robotic and mechatronics systems for image guided interventions and rehabilitation: A Review Study. Annu. Rev. Biomed. Eng. 2007, 9, 351–387.
- Khairi, R.; Razek, A.; Bernard, L.; Corcolle, R.; Bernard, Y.; Pichon, L.; Poirier-Quinot, M.; Ginefri, J.C. EMC analysis of MRI environment in view of Optimized performance and cost of image guided interventions. Int. J. Appl. Electromagn. Mech. 2016, 51, S67–S74.
- Su, H.; Kwok, K.W.; Cleary, K.; Iordachita, I.; Cavusoglu, M.C.; Desai, J.P.; Fischer, G.S. State of the art and future opportunities in MRI-guided robot-assisted surgery and interventions. Proc. IEEE 2022, 110, 968–992.
- Razek, A. Towards an image-guided restricted drug release in friendly implanted therapeutics. Eur. Phys. J. Appl. Phys. 2018, 82, 31401.
- Velazco Garcia, J.D.; Navkar, N.V.; Gui, D.; Morales, C.M.; Christoforou, E.G.; Ozcan, A.; Abinahed, J.; Al-Ansari, A.; Webb, A.; Seimenis, I.; et al. A Platform Integrating Acquisition, Reconstruction, Visualization, and Manipulator Control Modules for MRI-Guided Interventions. J. Digit. Imaging 2019, 32, 420–432.
- Razek, A. Assessment of Supervised Drug Release in Cordial Embedded Therapeutics. Athens J. Technol. Eng. 2019, 6, 77–91.
- Chakrabarti, S.; Biswas, N.; Jones, L.D.; Kesari, S.; Ashili, S. Smart Consumer Wearables as Digital Diagnostic Tools: A Review. Diagnostics 2022, 12, 2110.
- Escobar-Linero, E.; Muñoz-Saavedra, L.; Luna-Perejón, F.; Sevillano, J.L.; Domínguez-Morales, M. Wearable Health Devices for Diagnosis Support: Evolution and Future Tendencies. Sensors 2023, 23, 1678.
- Devi, D.H.; Duraisamy, K.; Armghan, A.; Alsharari, M.; Aliqab, K.; Sorathiya, V.; Das, S.; Rashid, N. 5G Technology in Healthcare and Wearable Devices: A Review. Sensors 2023, 23, 2519.
- Razek, A. Biological and Medical Disturbances Due to Exposure to Fields Emitted by Electromagnetic Energy Devices—A Review. Energies 2022, 15, 4455.
- Sato, Y.; Takeuchi, T.; Fuju, A.; Takahashi, M.; Hashimoto, M.; Okawa, R.; Hayashi, N. MRI safety for leave-on powdered hair thickeners under 1.5-T and 3.0-T MRI: Measurement of deflection force, MRI artifact, and evaluation of preexamination screening. Phys. Eng. Sci. Med. 2023, 46, 915–924.
- Akdogan, G.; Istanbullu, O. Analysing the effects of metallic biomaterial design and imaging sequences on MRI interpretation challenges due to image artefacts. Phys. Eng. Sci. Med. 2022, 45, 1163–1174.
- Germann, C.; Nanz, D.; Sutter, R. Magnetic Resonance Imaging Around Metal at 1.5 Tesla: Techniques from Basic to Advanced and Clinical Impact. Investig. Radiol. 2021, 56, 734–748.
- Germann, C.; Falkowski, A.L.; von Deuster, C.; Nanz, D.; Sutter, R. Basic and Advanced Metal-Artifact Reduction Techniques at Ultra-High Field 7-T Magnetic Resonance Imaging-Phantom Study Investigating Feasibility and Efficacy. Investig. Radiol. 2022, 57, 387–398.
- Inaoka, T.; Kitamura, N.; Sugeta, M.; Nakatsuka, T.; Ishikawa, R.; Kasuya, S.; Sugiura, Y.; Nakajima, A.; Nakagawa, K.; Terada, H. Diagnostic Value of Advanced Metal Artifact Reduction Magnetic Resonance Imaging for Periprosthetic Joint Infection. J. Comput. Assist. Tomogr. 2022, 46, 455–463.
- Haskell, M.W.; Nielsen, J.F.; Noll, D.C. Off-resonance artifact correction for MRI: A review. NMR Biomed. 2023, 36, e4867.
- Spronk, T.; Kraff, O.; Kreutner, J.; Schaefers, G.; Quick, H.H. Development and evaluation of a numerical simulation approach to predict metal artifacts from passive implants in MRI. Magma 2022, 35, 485–497.
- Farooq, M.U.; Ko, S.Y. A Decade of MRI Compatible Robots: Systematic Review. Trans. Robot. 2023, 39, 862–884.
- Farooq, M.U.; Ko, S.Y.; Seung, S.; Kim, C.; Cha, K.; Oh, S.S.; You, H. An MRI-compatible endonasal surgical robotic system: Kinematic analysis and performance evaluation. Mechatronics 2023, 94, 103029.
- Manjila, S.; Rosa, B.; Price, K.; Manjila, R.; Mencattelli, M.; Dupont, P.E. Robotic Instruments Inside the MRI Bore: Key Concepts and Evolving Paradigms in Imaging-enhanced Cranial Neurosurgery. World Neurosurg. 2023, 176, 127–139.
- Zhao, Z.; Carvalho, P.A.; Tang, H.; Pooladvand, K.; Gandomi, K.Y.; Nycz, C.J.; Furlong, C.; Fischer, G.S. Preliminary Characterization of a Plastic Piezoelectric Motor Stator Using High-Speed Digital Holographic Interferometry. In Advancement of Optical Methods & Digital Image Correlation in Experimental Mechanics; Lin, M.T., Furlong, C., Hwang, C.H., Eds.; Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2021.
- Carvalho, P.A.; Tang, H.; Razavi, P.; Pooladvand, K.; Castro, W.C.; Gandomi, K.Y.; Zhao, Z.; Nycz, C.J.; Furlong, C.; Fischer, G.S. Study of MRI Compatible Piezoelectric Motors by Finite Element Modeling and High-Speed Digital Holography. In Advancements in Optical Methods & Digital Image Correlation in Experimental Mechanics; Lin, M.T., Furlong, C., Hwang, C.H., Eds.; Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2020; Volume 3.
- Zhang, S.J.; Liu, Y.; Deng, J.; Gao, X.; Li, J.; Wang, W.Y.; Xun, M.X.; Ma, X.F.; Chang, Q.B.; Liu, J.K.; et al. Piezo robotic hand for motion manipulation from micro to macro. Nat. Commun. 2023, 14, 500.
- Mohith, S.; Upadhya, A.R.; Navin, K.P.; Kulkarni, S.M.; Rao, M. Recent trends in piezoelectric actuators for precision motion and their applications: A review. Smart Mater. Struct. 2020, 30, 013002.
- Gao, X.; Yang, J.; Wu, J.; Xin, X.; Li, Z.; Yuan, X.; Shen, X.; Dong, S. Piezoelectric Actuators and Motors: Materials, Designs, and Applications. Adv. Mater. Technol. 2020, 5, 1900716.
- Qiao, G.; Li, H.; Lu, X.; Wen, J.; Cheng, T. Piezoelectric stick-slip actuators with flexure hinge mechanisms: A review. J. Intell. Mater. Syst. Struct. 2022, 33, 1879–1901.
- Liu, J.; Gao, X.; Jin, H.; Ren, K.; Guo, J.; Qiao, L.; Qiu, C.; Chen, W.; He, Y.; Dong, S.; et al. Miniaturized electromechanical devices with multi-vibration modes achieved by orderly stacked structure with piezoelectric strain units. Nat. Commun. 2022, 13, 6567.
- Fu, D.K.; Fan, P.Q.; Yuan, T.; Wang, Y.S. A novel hybrid mode linear ultrasonic motor with double driving feet. Rev. Sci. Instrum. 2022, 93, 025003.
- Li, Z.; Guo, Z.; Han, H.; Su, Z.; Sun, H. Design and characteristic analysis of multi-degree-of-freedom ultrasonic motor based on spherical stator. Rev. Sci. Instrum. 2022, 93, 025004.
- Wang, S.; Zhou, S.; Zhang, X.; Xu, P.; Zhang, Z.; Ren, L. Bionic Stepping Motors Driven by Piezoelectric Materials. J. Bionic Eng. 2023, 20, 858–872.
- Hernandez, C.; Bernard, Y.; Razek, A. Design and manufacturing of a piezoelectric traveling-wave pumping device. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1949–1956.
- Yang, Z.; Li, X.; Tang, J.; Huang, H.; Zhao, H.; Cheng, Y.; Liu, S.; Li, C.; Xiong, M. A Bionic Stick–Slip Piezo-Driven Positioning Platform Designed by Imitating the Structure and Movement of the Crab. J. Bionic Eng. 2023, 20, 2590–2600.
- Virtanen, J. Enhancing the Compatibility of Surgical Robots with Magnetic Resonance Imaging. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2006. Available online: http://urn.fi/urn:isbn:9514280660 (accessed on 1 November 2023).
- Tada, M.; Sasaki, S.; Ogasawara, T. Development of an optical 2-axis force sensor usable in MRI environments. In Proceedings of the SENSORS, 2002 IEEE, Orlando, FL, USA, 12–14 June 2002; Volume 2, pp. 984–989.
- Tada, M.; Kanade, T. An MR-Compatible Optical Force Sensor for Human Function Modeling. In Medical Image Computing and Computer-Assisted Intervention—MICCAI 2004. Lecture Notes in Computer Science; Barillot, C., Haynor, D.R., Hellier, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 3217.
- Jolesz, F.A.; Morrison, P.R.; Koran, S.J.; Kelley, R.J.; Hushek, S.G.; Newman, R.W.; Fried, M.P.; Melzer, A.; Seibel, R.M.; Jalahej, H. Compatible instrumentation for intraoperative MRI: Expanding resources. J. Magn. Reson. Imaging 1998, 8, 8–11.
- Shellock, F.G. Pocket Guide to MR Procedures and Metallic Objects: Update 1998; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1998; Available online: https://archive.org/details/pocketguidetomrp0000shel_y5n3 (accessed on 1 November 2023).
- Schenck, J.F. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med. Phys. 1996, 23, 815–850.
- Traverson, M.; Heiden, M.; Stanciu, L.A.; Nauman, E.A.; Jones-Hall, Y.; Breur, G.J. In Vivo Evaluation of Biodegradability and Biocompatibility of Fe30Mn Alloy. Vet. Comp. Orthop. Traumatol. 2018, 31, 10–16.
- Wang, Y.; Venezuela, J.; Dargusch, M. Biodegradable shape memory alloys: Progress and prospects. Biomaterials 2021, 279, 121215.
- Li, H.; Lin, G.; Wang, P.; Huang, J.; Wen, C. Nutrient alloying elements in biodegradable metals: A review. J. Mater. Chem. B 2021, 9, 9806–9825.
- Rabeeh, V.P.M.; Hanas, T. Progress in manufacturing and processing of degradable Fe-based implants: A review. Prog. Biomater. 2022, 11, 163–191.
- Babacan, N.; Kochta, F.; Hoffmann, V.; Gemming, T.; Kühn, U.; Giebeler, L.; Gebert, A.; Hufenbach, J. Effect of silver additions on the microstructure, mechanical properties and corrosion behavior of biodegradable Fe-30Mn-6Si. Mater. Today Commun. 2021, 28, 102689.
- Tai, C.-C.; Lo, H.-L.; Liaw, C.-K.; Huang, Y.-M.; Huang, Y.-H.; Yang, K.-Y.; Huang, C.-C.; Huang, S.-I.; Shen, H.-H.; Lin, T.-H.; et al. Biocompatibility and Biological Performance Evaluation of Additive-Manufactured Bioabsorbable Iron-Based Porous Suture Anchor in a Rabbit Model. Int. J. Mol. Sci. 2021, 22, 7368.
- Bakhsheshi-Rad, H.R.; Najafinezhad, A.; Hadisi, Z.; Iqbal, N.; Daroonparvar, M.; Sharif, S.; Ismail, A.F.; Akbari, M.; RamaKrishna, S.; Berto, F. Characterization and biological properties of nanostructured clinoenstatite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2021, 259, 123969.
- Sun, Y.; Chen, L.; Liu, N.; Wang, H.; Liang, C. Laser-modified fe–30mn surfaces with promoted biodegradability and biocompatibility toward biological applications. J. Mater. Sci. 2021, 56, 13772–13784.
- Saliba, L.; Sammut, K.; Tonna, C.; Pavli, F. FeMn and FeMnAg Biodegradable Alloys: An In Vitro And In Vivo Investigation. Available online: https://ssrn.com/abstract=4325636 (accessed on 1 November 2023).
- Hao, S.; Yang, T.; Zhang, A.; Wang, P.; Jiang, H.; Shen, D.; Guo, L.; Ye, M. Evaluation of Biodegradable Alloy Fe30Mn0.6N in Rabbit Femur and Cartilage through Detecting Osteogenesis and Autophagy. BioMed Res Int. 2023, 2023, 3626776.
- Biffi, C.A.; Fiocchi, J.; Bregoli, C.; Gambaro, S.; Copes, F.; Mantovani, D.; Tuissi, A. Ultrashort Laser Texturing for Tuning Surface Morphology and Degradation Behavior of the Biodegradable Fe–20Mn Alloy for Temporary Implants. Adv. Eng. Mater. 2022, 24, 2101496.
- Putra, N.E.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zhou, J.; Zadpoor, A.A. Extrusion-based 3D printing of ex situ-alloyed highly biodegradable MRI-friendly porous iron-manganese scaffolds. Acta Biomater. 2021, 134, 774–790.
- Soliman, M.M.; Chowdhury, M.E.H.; Khandakar, A.; Islam, M.T.; Qiblawey, Y.; Musharavati, F.; Zal Nezhad, E. Review on Medical Implantable Antenna Technology and Imminent Research Challenges. Sensors 2021, 21, 3163.
- Gupta, A.; Kumar, V.; Bansal, S.; Alsharif, M.H.; Jahid, A.; Cho, H.-S. A Miniaturized Tri-Band Implantable Antenna for ISM/WMTS/Lower UWB/Wi-Fi Frequencies. Sensors 2023, 23, 6989.
- Chowdhury, M.E.H.; Khandakar, A.; Alzoubi, K.; Mansoor, S.; Tahir, A.M.; Reaz, M.B.I.; Al-Emadi, N. Real-Time Smart-Digital Stethoscope System for Heart Diseases Monitoring. Sensors 2019, 19, 2781.
- Moon, K.S.; Lee, S.Q. A Wearable Multimodal Wireless Sensing System for Respiratory Monitoring and Analysis. Sensors 2023, 23, 6790.
- Khan Mamun, M.M.R.; Sherif, A. Advancement in the Cuffless and Noninvasive Measurement of Blood Pressure: A Review of the Literature and Open Challenges. Bioengineering 2022, 10, 27.
- Bhuva, A.N.; Moralee, R.; Brunker, T.; Lascelles, K.; Cash, L.; Patel, K.P.; Lowe, M.; Sekhri, N.; Alpendurada, F.; Pennell, D.J.; et al. Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices. Eur. Heart J. 2022, 43, 2469–2478.
- Joo, H.; Lee, Y.; Kim, J.; Yoo, J.S.; Yoo, S.; Kim, S.; Arya, A.K.; Kim, S.; Choi, S.H.; Lu, N.; et al. Soft Implantable Drug Delivery Device Integrated Wirelessly with Wearable Devices to Treat Fatal Seizures. Sci. Adv. 2021, 7, eabd4639.
- Cheng, Y.; Xie, D.; Han, Y.; Guo, S.; Sun, Z.; Jing, L.; Man, W.; Liu, D.; Yang, K.; Lei, D.; et al. Precise management system for chronic intractable pain patients implanted with spinal cord stimulation based on a remote programming platform: Study protocol for a randomized controlled trial (PreMaSy study). Trials 2023, 24, 580.
- Thotahewa, K.M.S.; Redouté, J.; Yuce, M.R. Electromagnetic and thermal effects of IR-UWB wireless implant systems on the human head. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 5179–5182.
- Corbett, G.D.; Buttery, P.C.; Pugh, P.J.; Cameron, E.A.B. Endoscopy and implantable electronic devices. Frontline Gastroenterol. 2012, 3, 72–75.
- Pantelopoulos, A.; Bourbakis, N.G. A survey on wearable sensor-based systems for health monitoring and prognosis. IEEE Trans. Syst. Man Cybern. Part C 2010, 40, 1–12.
- Chan, M.; Esteve, D.; Fourniols, J.Y.; Escriba, C.; Campo, E. Smart wearable systems: Current status and future challenges. Artif. Intell. Med. 2012, 56, 137–156.
- Kim, J.; Campbell, A.S.; de Ávila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406.
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 2016, 28, 4373–4395.
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 1–17.
- Hurley, N.C.; Spatz, E.S.; Krumholz, H.M.; Jafari, R.; Mortazavi, B.J. A Survey of Challenges and Opportunities in Sensing and Analytics for Risk Factors of Cardiovascular Disorders. ACM Trans. Comput. Healthc. 2021, 2, 9–21.
- Talal, M.; Zaidan, A.A.; Zaidan, B.B.; Albahri, A.S.; Alamoodi, A.H.; Albahri, O.S.; Alsalem, M.A.; Lim, C.K.; Tan, K.L.; Shir, W.L.; et al. Smart Home-based IoT for Real-time and Secure Remote Health Monitoring of Triage and Priority System using Body Sensors: Multi-driven Systematic Review. J. Med. Syst. 2019, 43, 42.
- Patel, V.; Orchanian-Cheff, A.; Wu, R. Evaluating the Validity and Utility of Wearable Technology for Continuously Monitoring Patients in a Hospital Setting: Systematic Review. JMIR Mhealth Uhealth 2021, 9, e17411.
- Osama, M.; Ateya, A.A.; Sayed, M.S.; Hammad, M.; Pławiak, P.; Abd El-Latif, A.A.; Elsayed, R.A. Internet of Medical Things and Healthcare 4.0: Trends, Requirements, Challenges, and Research Directions. Sensors 2023, 23, 7435.
This entry is offline, you can click here to edit this entry!
