1. Approaches for Drug Delivery through the Blood–Brain Barrier
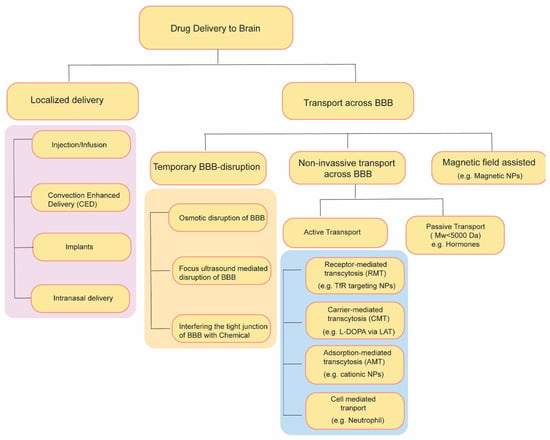
Over the past few decades, diverse strategies have emerged to enhance the transportation of drugs through the BBB (Figure 2). These strategies include temporary disruption of BBB via physical or chemical means as well as targeting some endogenous transporter systems over-expressed on BBB.
Figure 2. Summary of drug delivery strategies to brain.
2. Temporary Disruption of BBB
2.1. Osmotic Blood–Brain Barrier Disruption
In this process (
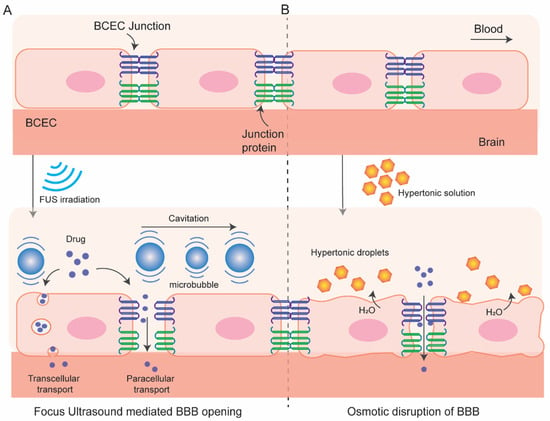
Figure 3), BBB permeation is achieved using a hyperosmotic agent which causes dehydration and shrinkage in BCEC resulting tight-junction dysfunction and transient disruption of BBB. This process of osmotic BBB disruption was first hypothesized by Rapoport et al. in 1972 [
24] following an improved BBB permeation of a dye Evan’s blue when co-administered with hypertonic arabinose and later supported by Brightman et al. who visualize the opening of tight junction with electron microscopy after intra-carotid infusion of mannitol [
25]. A variety of substances have been used as osmotic disruptors of the BBB including urea, lactamide, saline but mannitol has been most used for this purpose. Since 1980, intracarotid artery hyperosmotic mannitol (ICAHM) infusions has been used for drug delivery to brain in several pre-clinical and clinical studies [
26] many of which have produced encouraging results of enhanced survivability with clinical safety. For instance, a clinical study conducted in 17 patients with primary CNS lymphoma receiving cyclophosphamide and mannitol followed by radiotherapy significantly enhances the mean survivability (from 17.8 months to 44.5 months) compared with the control group receiving radiotherapy alone [
27]. Combination of carboplatin and etoposide delivered in this method exhibits an effective delivery in brain and dramatic responses in inhibiting CNS tumor in patients although unexpected high-frequency hearing loss limits the application of the combined chemotherapy [
28]. Some studies in animal models have demonstrated variable and inconsistent results in BBB permeability like nonselective opening of BBB, CNS toxicity and neuroinflammatory response become the major limitation of this approach [
29,
30,
31]. The success of this strategy depends on multiple factors, including injection speed, optimum mannitol dose, cerebral hemodynamics, and vascular anatomy. Strategies to overcome the limitation are currently under investigation, e.g., use of real time MRI guidance for optimum and targeted delivery of therapeutics [
32,
33]. Overall, mannitol mediated osmotic disruption of BBB for drug delivery to brain is safe and hold promise while further investigation is needed to improve its reproducibility and clinical effectiveness.
Figure 3. Schematic of focus ultrasound-mediated (A) and osmotic (B) disruption of BBB.
2.2. BBB Disruption with Focused Ultrasound
In this method (Figure 3) local BBB permeation can be achieved by using focused ultrasound (FUS) in combination with intravenous microbubbles and can be monitored by using MR-imaging system. This method has several advantages over other methods. It is reproducible, non-invasive, and targeted opening of the BBB can be achieved. In addition, the BBB opening is transient which can be restored within 6 to 24 h allowing accumulation of therapeutics in the region of interest for a desired time window.
The FUS technology was first introduced in 1950s initially to treat psychiatric disorders and brain tumors although those early attempts were invasive involving craniectomy to introduce sonication into brain which has been evolved to non-invasive over time by decades of research [
34,
35,
36,
37]. Although the minimal invasiveness to reduce surgical trauma and recovery time are the driving force, the skull bone which varies in thickness and density among individuals greatly attenuates and distorts ultrasound. In addition, hair, which introduces air, significantly (up to 80%) distorts the delivery of ultrasound. The implementation of phased array transducers along with real-time MRI-thermal monitoring has been a breakthrough in this century to made non-invasive transcranial FUS feasible [
37].
The cellular and molecular mechanism of FUS-mediated enhanced BBB permeation is poorly understood. The sheer stress from the stable acoustic cavitation of the microbubbles induces structural and functional modulation in the BBB like higher caveolae formation, sonoporation, as well as opening of some tight junctions which enhance intracellular and paracellular transport [
38,
39,
40]. Although stable cavitation contributes to loosening of tight junction, inertial cavitation may contribute to hemorrhage and ischemia. Nonetheless, microbubble cavitation can be controlled by tuning ultrasound pressure amplitude and low-frequency FUS-mediated BBB opening rule out the thermal effect on the BBB. Notably, FUS activates PI3kinase/Akt pathway in neuronal cells which may play role in modulation of tight junction proteins ZO-1 and occludin [
41]. Cerebral vessels are resilient to such mechanical stress caused by stable microbubbles cavitation and quickly recover their integrity after the FUS.
As indicated before, FUS can induce local and targeted opening of the BBB with a desired time window. The extent of BBB opening can be controlled by tuning ultrasound pressure amplitude, transducer frequency, microbubble size and dosage, exposure duration and burst parameters [
42,
43,
44]. For instance, a study by Chen et al. has demonstrated that FUS can enable trans-BBB delivery of dextran molecule up to 2000 kDa (hydrodynamic diameter 2.3 to 54.4 nm) at a 0.84 MPa acoustic pressure [
45]. However, small opening (70 kDa) can be achieved by stable cavitation whereas larger BBB opening (>500 kDa) is associated with inertial cavitation. Thus, FUS has been demonstrated to markedly enhance the trans-BBB delivery of therapeutic antibodies (~150 kDa, e.g., Herceptin) [
7,
44,
46,
47,
48], chemotherapeutics [
49], and cells [
50,
51,
52] and shows clinical promise in treating brain tumor and other CNS diseases [
37,
53]. Furthermore, studies indicate that FUS can be utilized to target therapeutics in different regions of the brain such as the hippocampus [
54], striatum [
55], cortical targets [
46], and brainstem [
49]. The safety of FUS-mediated BBB opening is promising. A mild and short term (<2 weeks) immune response is reported after repeated administration [
56,
57,
58,
59]. Importantly, behavioral, morphological, and neuroimaging characteristics are retained even after long-term repeated administration of FUS in animal models (biweekly over 6 months in rats or 4 months in non-human primates) [
60,
61].
2.3. Radiation-Mediated BBB Disruption
Few studies have reported that radiation therapy, an important modality in treating brain tumor, may play a role in disrupting the BBB and enhance drug entry to brain [
62]. However, the role of radiation in increasing drug accumulation in brain and its underlying mechanisms are still uncertain. In addition, radiation induced BBB disruption is not temporary and the recovery time is much higher (in years) which often lead to radiation induced toxicity including headache, neurologic deficits or nausea [
63].
2.4. Interfering the Tight Junction of BBB with Chemicals
Disengaging the tight junctions of BBB is another strategy to improve permeability across BBB. Bradykinin (BK), a peptide containing 9 aminoacids upon administration causes dilation of arterioles and enhances paracellular transport by down-regulating expression of the tight junction proteins (occluding, ZO-1, and claudin-5) and improves transcellular transport by upregulating caveolin mediated pinocytotic vesicles [
64]. The BBB opening potential of bradykinin, and its synthetic analogs, have been explored [
65,
66,
67] especially in brain tumors due to the high expression of BK receptor at BTB [
68]. However, it did not go through Phase-II mainly because the extremely transient opening of BBB and the adverse side effects due to the wide distribution of BK receptors at numerous additional sites beyond the BBB [
69]. BBB disruption via targeting claudin-5, a major component of BBB tight junctions, via siRNA mediated knockdown or using anti-claudin5-antibody also demonstrated to enhance BBB permeation transiently and reversibly [
70,
71]. It also suffers similar limitations of transient effects and adverse side effects due to wider distribution of receptor expression. To this end, targeting Angulin-1, another functional constituent of BBB tricellular tight junctions which is majorly expressed in BBB and selectively blocks entry of macromolecules into the brain, can address the adverse effects [
72]. Angubindin-1, a ligand of angulin-1, is demonstrated to enhance the entry of macromolecules across BBB by removing angulin-1 and disrupting the tricellular tight junctions [
73].
3. Drug Transport without Disrupting BBB: Active and Passive Transport Pathways
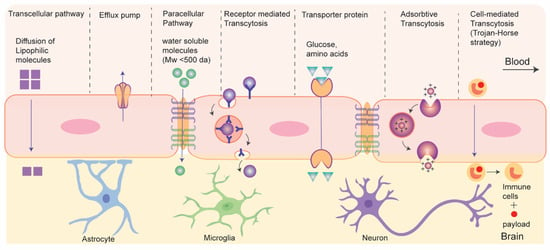
Recent strategies of drug delivery to brain without disrupting BBB can be classified into two types based on their energy (adenosine triphosphate (ATP)) requirements during the process: passive and active transport (
Figure 4) [
74]. Passive transport is an energy-independent process that lacks specificity. It includes the diffusion of small molecules through paracellular and transcellular pathways. Paracellular diffusion involves solute molecules moving between adjacent endothelial cells due to a negative concentration gradient. Only water-soluble molecules can pass through the paracellular space. In transcellular diffusion, non-ionic solute (molecular weight < 400 Da) with a desirable lipophilicity (e.g., hormones and steroids) can diffuse through the endothelial cells to brain [
75]. However, in addition to the tight junction, some efflux pumps present at the luminal side of BCEC also limit the drug transport across BBB. Efflux pumps function in two phases, it initially inhibit cellular uptake of drug molecules in BCEC and later expel the drugs molecules (like doxorubicin, daunorubicin etc.) into blood against a negative concentration gradient in ATP dependent pathway [
76]. P-glycoprotein (P-gp) is an example of efflux pump that plays a role in drug resistance in tumors. Regulating efflux pumps at the BBB represents another strategy for drug delivery to brain tumors. It is important to note that efflux pumps, although beneficial for protecting the healthy brain from harmful neurotoxins, can also pose challenges in drug delivery to brain tumors.
Figure 4. Schematics for drug transport pathways across BBB.
The active transport pathway often exploits endogenous receptor or transporter proteins that are expressed on the luminal side of BBB. Active transport routes include receptor mediated transcytosis (RMT), carrier mediated transcytosis, adsorption-mediated transcytosis, and cell-mediated transcytosis, all of which require ATP. In RMT, particles cross BBB via interaction with specific receptors expressed on apical surface of BCEC. It is an important pathway and is widely being explored for delivery of macromolecular biopharmaceuticals (e.g., protein or recombinant peptide-based therapeutics) or nanocarrier-mediated drug transport to the brain. The mechanism of RMT centers on endocytosis, where a ligand selectively binds to a receptor. This binding leads to the creation of an intracellular vesicle through membrane invagination. These vesicles are then transported and fused with the basolateral membrane, subsequently releasing the payloads as they detach from the membrane. It is worth mentioning that, like general endocytosis, in addition to the transcytosis from blood to brain some vesicles undergo lysosomal degradation while some others are recycled to the apical side in RMT. This process often targets specific receptors, including transferrin receptors, low-density lipoprotein (LDL) receptors, and insulin receptors for drug delivery to the brain.
Carrier- or transporter- mediated transcytosis (CMT) represents another dynamic active transport mechanism across the BBB, facilitating the transportation of essential nutrients such as amino acids and glucose into the brain. Nutrient molecules bind to the specific transporter proteins on the luminal side, causing conformational changes that enable the transfer of these nutrients into the brain. Glucose transporter isoform (GLUT-1) and large amino-acid transporter (LAT) are examples of such transporter. Small molecule drugs like L-DOPA and gabapentin utilize CMT to reach the CNS. However, the high specificity required for the interaction between transporters and ligands in this process limits its applicability in transporting macromolecular therapeutics [
77]. Charged particles such as nanocarriers, predominantly traverse the BBB through adsorptive mediated transcytosis (AMT), relying on the electrostatic interactions between the negatively charged cell surface of BCEC and the particles [
78]. Such interactions are non-specific, and many nanocarriers can be delivered; however, this is not devoid of the non-specific accumulation in other organs under systemic circulation. Cell-mediated transcytosis, which utilizes blood cells capable of BBB crossing for delivering drug to brain, has recently emerged as biomimetic strategy. In this method, immune cells or platelets are incorporated with drug-loaded nanocarriers which then cross BBB and navigate towards the inflammation sites within the brain by responding to chemotaxis signals and undergoing diapedesis [
79]. More recently, extracellular vesicles, e.g., exosomes, have attracted significant attention as biomimetic drug carrier for CNS drug delivery. In addition, viral vectors have shown promise for gene delivery to brain. Further nanocarrier-mediated approaches have gained significant interest for efficient delivery to brain.
3.1. Nanocarriers Mediated Drug Transport across BBB
Nanoparticles (NPs) such as liposomes, polymeric NPs, inorganic NPs, etc., are being used as drug carriers for decades [
80,
81,
82,
83,
84,
85]. Drug loading in NPs enhances circulation life of hydrophobic drugs in blood, protect nucleic-acid-based therapeutics from serum nucleases, or reduce the adverse off-target effects of drugs [
86,
87,
88,
89,
90]. Surface of NPs can be engineered with PEG to achieve longer circulation life or with cell-penetrating peptide to enhance cellular uptake [
91], or with targeting ligand to selectively deliver the payloads at targeted tissue [
92]. Furthermore, drug release at the targeted tissue can be externally controlled by using stimuli-responsive nanocarriers [
82]. Over the past few decades, many nanocarriers with size range ~10–300 nm have been explored for delivering small molecules, nucleotides, peptides, or proteins-based therapeutics to brain for combating various CNS diseases including brain tumor, neurodegenerative disorders, neuroHIV, stroke, etc. [
82,
88,
89,
90,
93,
94,
95,
96,
97,
98,
99,
100,
101,
102,
103]. Such nanocarriers can cross the BBB by passive diffusion or can be engineered with some ligand at their exo-surfaces actively targeting some endogenous receptor/transporter protein on the BBB. For instance, liposomal encapsulation of cytotoxic anti-neoplastic agent doxorubicin has significantly mitigated the adverse effect of systemic chemotherapy as indicated by the enhanced safety index in a phase I trial involving 13 children with recurrent/refractory high-grade glioma (NCT02861222) [
104]. Similarly, liposomal encapsulation of irinotecan has improved the safety profile of systemic chemotherapy in another phase I study with 34 high-grade glioma patients (NCT02022644) permitting its progression for Phase II trial [
105]. However, although such encapsulation of cytotoxic chemotherapeutics improved the safety index of systemic chemotherapy in patients, the efficacy of nanoformulations might be facilitated by their passive accumulation via compromised integrity of the BBB around high-grade tumors. Clearly, there is a need for an active transport mechanism across the BBB for delivering drugs to combat low-grade tumor or other CNS diseases with intact or less compromised integrity of BBB.
Active targeting of receptor or transporter proteins expressed in brain capillary endothelial cells (BCEC) is the most widely explored nanocarrier-based drug delivery strategy across BBB. In this method, nanocarriers are surface engineered with targeting ligands of such receptors/transporters to deliver payload in brain via RMT or CMT which has been reviewed elsewhere in detail [
106,
107]. Although Transferrin, LDL family receptors (LDLR), insulin, and integrin receptors are widely explored receptors due to their high receptor-ligand affinity, GLUT and LAT-1 are some transporter proteins that are explored for drug delivery to brain.
Transferrin Receptor: Transferrin receptors (TfRs) control iron homeostasis via their natural ligand transferrin. TfRs are highly expressed in the luminal side of BBB and in brain tumors which makes them an attractive target for drug delivery to the brain [
108]. Different TfRs ligands such as transferrin (Tf) itself (~80 kDa) [
109], antibodies or antibody fragments [
110], and peptides [
111,
112] are explored to examine their brain targeting efficacy by grafting such ligands with the biopharmaceuticals or at the exo-surface of nanocarriers which is reviewed in detail elsewhere [
113]. For instance, Lam et al. have developed a transferrin-functionalized PEGylated liposomes for simultaneous delivery of temozolomide (TMZ) and bromodomain inhibitor in brain tumor. The combined chemotherapy regimen overcome the drug resistance of TMZ, reduced the tumor size, and improved the survival of mice with glioma compared to control groups, all while showing minimal systemic drug toxicity [
109]. To overcome the plausible inhibition of RMT by competitive binding of endogenous Tf, nanocarriers are also surface engineered with monoclonal antibody (mAb), or peptide fragments targeting TfR. For instance, Yue et al. has conjugated OX26 antibody, a monoclonal antibody against TfR1, with micelles to develop an immunomicelle which shows high BBB-crossing ability [
110]. A TfR specific heptapeptide T7 (HAIYPRH) with high binding affinity (K
d = 10 nM) has also been explored to target nucleotides and neoplastic drugs in glioma tissue in preclinical model [
111,
112]. Although such studies are at the preclinical stages, some have shown initial clinical promise [
95]. For instance, a fusion of lysosomal enzyme iduronate 2- sulfatase (IDS) with anti- TfR antibody (JR-141) enabled successful delivery of the fusion protein into the CNS of patients with Hunter Syndrome under systemic settings (i.v.) in a phase I/II trial (NCT03128593) which shows promising therapeutic efficacy with no significant safety issue [
114]. Notably, the use of TfR-targeting Tf-toxin conjugates has demonstrated clinical potential in anti-glioma therapy. Human Tf is linked to a diphtheria toxin featuring a CRM107 point mutation, resulting in the creation of Tf-CRM107. This conjugate displayed tumor growth inhibition when administered directly into the tumor in a U251 mouse model [
115]. Subsequently, a phase I study following intra-tumoral injection revealed no adverse effects, leading to a phase II study involving patients with recurrent high-grade brain tumors. Although 35% of the patients displayed positive tumor responses and improved survival, the phase III was discontinued due to probable CNS toxicity with an indication for more targeted delivery of the toxin [
116].
The sub-optimal clinical efficiency of TfRs targeting may be related to the high recycling rate (~90%) of endocytosed TfRs by BCEC to the luminal side as indicated by studies in mouse brain [
117] where only 10% of TfR-NPs are able to reach brain parenchyma. Efforts to improve rate of transcytosis via varying ligand density on nanocarrier [
118] or increasing receptor-ligand affinity are being examined [
119]. Bivalent TfR antibodies with high receptor-affinity diverts the trafficking into lysosomes and subsequent degradation of the therapeutics indicating requirement of optimum receptor-ligand affinity for effective transcytosis [
120,
121]. Furthermore, interspecies variation of receptors, such as 2.5 times higher expression level of TfRs in mouse brain microvessels compared with that in human also contributes to the reduced efficacy during clinical translation of such active targeting strategies. Finally, ubiquitous expression of TfRs in other organs (liver, spleen, and bone-marrow) and uptakes of drugs in non-peripheral tissues also contribute to the compromised therapeutic efficacy of such targeting strategy [
118].
3.2. Magnetic Field Assisted Crossing of BBB
Application of an external magnetic field is another physical method for drug delivery to the brain which not only spatially guides the magnetic nanoparticle to the targeted region but also significantly improves the speed and time for drug delivery. In this method, paramagnetic nanoparticles (PMNP), especially superparamagnetic iron oxide nanoparticles (SPIONs) with sizes ~10–100 nm, are used. Although magnetic nanoparticles (MNPs) and liposomes in diameters of 70 nm do not cross the BBB, the application of a static magnetic field facilitates its delivery across BBB. Particle size controls the magnetic susceptibility under a fixed static magnetic field. Small SPIONs exhibit higher magnetic susceptibility (highest with crystalline domain with 10–30 nm) than larger paramagnetic nanoparticles containing many crystalline domains mutually diminish the net magnetic moment. In addition, nanoparticles of 10–100 nm are considered optimum due to their longer systemic circulation times. The size of the nanoparticles determines their effect on BBB. For instance, SPION with ~117 nm under 0.39 Tesla did not disrupt BBB integrity whereas magnetic nanoparticles with a size of 800 nm cause leakage in BBB under the same magnetic field strength. In addition, the lower particle size with higher magnetic susceptibility requires less field strength, although no adverse effect in cells is reported with the static magnetic field as strong as up to 10 Tesla. Although the transcellular migration through BCEC via uptake or nanoporation is presumed to be the major pathway, some recent studies indicate interaction of SPIONs with junction proteins such as VE-cadherin may contribute to additional involvement of the paracellular pathway for BBB crossing [
148,
149].
SPIONs are used in clinics for MRI as a contrast agent and hold potential for other biomedical purposes including targeted drug delivery, image-guided drug delivery, hyperthermia, etc., for the management of CNS diseases [
96,
150,
151]. SPIONs can be surface-functionalized with different polymers, lipids etc. to achieve desired drug loading or pharmacokinetic property. For instance, polystyrene-coated SPIONs (~100 nm) under 0.1 T external magnetic field cross the BBB, accumulate in the brain parenchyma, and exhibit 25 times greater retention with minimal neurotoxicity. Similarly, transferrin-coated PEGylated magneto liposomes (~130 nm) exhibit complete transmigration across an in vitro BBB under 0.08 T magnetic field without affecting the BBB [
152]. Beyond small molecule anti-cancer drugs [
150], magneto liposomes also have been used to facilitate delivery of therapeutic peptide [
153], brain-derived neurotrophic factor (BDNF) [
154] or antiretroviral agents across the BBB [
155,
156]. For instance, to enhance the BBB permeation of antiretroviral agent 3′Azido-3′deoxythymidine-5′-triphosphate (AZT), it is complexed with SPIONs (~25 nm) followed by coating with liposome. This magneto liposome containing encapsulated AZT (~150 nm) crosses the BBB (in vitro) under 0.3 T field and results in three times higher accumulation of AZT across BBB compared to the only AZT [
155]. Importantly, to further gain control for on-demand drug release, MNP are modified to electromagnetic nanoparticles (MENP) [
157] which exhibit brain accumulation under low ac magnetic field with no adverse effect in rodents [
158] and non-human primates [
159] and can facilitate delivery of hydrophilic therapeutics including siRNA [
160], CRISPR [
161] across in vitro BBB (
Figure 5). It is worth mentioning that such MENP can also be used for non-invasive deep brain stimulation to control neuroactivity in Parkinson’s disease [
162]. Many studies have claimed lysosomal degradation of SPIONs as histopathological evaluation of major organs involved in systemic circulation revealed no iron-positive pigment or related macrophage accumulation [
158,
163]. However, some recent studies have reported toxicity of SPIONs as it causes an imbalance in iron homeostasis which may induce oxidative stress and inflammation leading to genotoxicity due to its differential interaction with mitochondria [
164,
165]. Clearly, in-depth evaluation of in vivo toxicity in long-term exposure to SPION is needed.
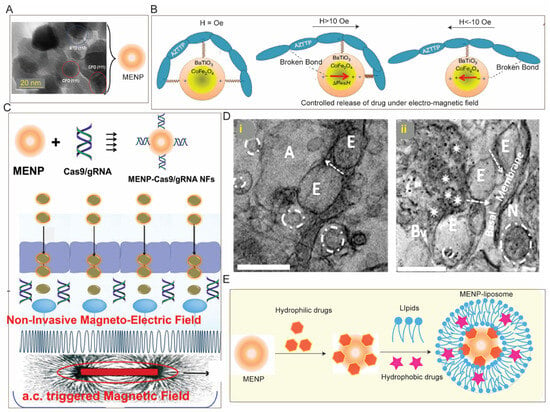
Figure 5. Magnetic field assisted drug delivery to the brain: (
A) TEM of a magneto electric nanoparticle (MENP) containing CoFe
2O
4 at the core with a shell of BaTiO
3 (
B) which enables controlled drug release under alternating electric stimuli, (
C) MENP (~30 nm) can be loaded with CRISPR via hydrophilic interaction which allows their non-invasive delivery across BBB (in vitro) under electromagnetic field, (
D) TEM of brain tissue from (
i) untreated mice and (
ii) mice intravenously administered with 10 mg/kg BW of MENP indicating brain accumulation of MENP (black dot in
D(
ii)). (
E) Schematic for potential simultaneous delivery of hydrophilic and hydrophobic payload to the brain using MENP-liposome composed of a lipid coating embedded with hydrophobic drug onto the hydrophilic drug-loaded MENP. Adopted with permission from Refs. [
148,
157,
158,
161].
3.3. Cell-Based Biomimetic Strategy of BBB Crossing
Bioinspired carriers such as blood cells, cell-membrane-coated nanocarriers, exosomes, etc., are being explored recently for drug delivery across BBB due to their longer circulation life and biocompatibility [
79]. Leukocytes such as macrophages, monocytes, and neutrophils are most explored for brain delivery due to their inherent chemotactic recruitment property, especially brain diseases with inflammation. In such methods, drugs are first loaded in nanocarriers which are then incorporated into cells to facilitate delivery across the BBB. For instance, Xue et al. have used neutrophils to deliver paclitaxel loaded liposomes in residual tissue post-surgery which have suppressed the recurrence of glioma growth [
166]. To treat ischemic stroke, Xu et al. have developed a ‘nanoplatelet’ by coating a neuroprotective agent loaded dextran-based nanocarrier with platelet membrane surface-engineered with thrombin-responsive anti-ischemic drug and TAT peptide. This ‘nanoplatelet’ crosses the BBB, clears the thrombus clog at the ischemic site in the brain and delivers neuroprotective agent to combat ischemic stroke [
167]. In another study, to combat encephalitis Yuan et al. have utilized a macrophage-derived exosome for delivering brain-derived neurotrophic factor (BDNF) to inflamed brain [
168]. Such BDNF-loaded exosomes crosses BBB via intercellular adhesion molecule 1 (ICAM-1) which is upregulated under encephalitis-related inflammation. However, cell-based carriers suffer from some common limitations such as viability of cell-based carriers arising due to leaching of drug from nanocarriers. In addition, there are some cell-specific limitations like risk of immune activation while using leukocytes or activation of platelets while using it as drug carrier that may cause undesired thrombosis or bleeding. Exosomes are extracellular vesicles which show ~0.5% passive brain accumulation and have attracted attention for drug delivery to brain and treating neurological conditions. For instance, i.v. administration of dopamine-loaded exosomes enhanced the dopamine levels (15 times) in mouse brain [
169]. Further understanding of the interaction between the BBB and bio-mimetic carriers are necessary for proper engineering of such carrier to maximize therapeutic benefit. Extracellular vesicles (EVs) derived from the cells have been explored for neuroprotective applications including traumatic brain injury. In one example, the EVs derived from mesenchymal stromal cells (MSCs) were examined from neuronal cell protection using in vitro models [
170].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15122658