Nano-sized plastics (NPLs, size < 100 nm) are characterized by a very small size and high reactivity, allowing them to interact with diverse phytoplankton species. The processes governing the interactions of NPLs with phytoplankton cells include absorption onto cell, penetration into cells via endocytosis or physical damage, and the obstruction of substance and energy exchange with the surrounding medium. Upon association with the cells, elevated concentrations of NPLs can reduce phytoplankton growth and photosynthesis, trigger overproduction of reactive oxygen species and damages, as well as alter cellular metabolic activity. NPLs can influence toxin production by cyanobacteria and release of extracellular polymeric substances by different phytoplankton species. Conversely, phytoplankton species can modulate NPL fate by secreting biomolecules that form an eco-corona around the NPLs, as well as taking part in the NPLs biotransformation.
- nanoplastics
- uptake
- toxicity
- trophic transfer
- algae
- cyanobacteria
1. Introduction
Plastic particles are considered as nanomaterials if at least half of them, in an unbound state or as an aggregate or an agglomerate state, have at least one dimension ranging from 1 to 100 nm [1]. As the usage of diverse plastic materials continues to expand, the accumulation of plastic waste is on the rise. Consequently, the presence of nanoplastics (NPLs) in the environment is increasing [2][3][4] together with the concerns regarding the possible environmental implications of NPLs [5][6]. Indeed, NPLs, originating from various primary or secondary sources, represent the least explored facet of plastic pollution [5][6]. In environmental settings, diverse plastic debris can release a large amount of NPLs through various physical and chemical degradation pathways, as previously reviewed [7]. The concentrations of NPLs in the environment have not yet been measured due to the constraints of current analytical methodologies. Multi-media model estimations provide average concentrations of NPLs in surface water of 280 µg L−1 [8]. More recently, NPLs’ abundance has been estimated to be in the range 0.3–488 μg L−1 in freshwaters, which are higher than those for marine environments (2.7–67 μg L−1) [9]. These concentrations are generally lower as compared to the predicted no-effect concentrations (PNECs) derived from probabilistic species sensitivity distributions, resulting in values of 99 μg L−1 and 72 μg L−1 for freshwater and marine datasets [10]. They are also below the estimated hazard concentration affecting 5% of the species (HC5) of 410 μg L−1 for marine plankton for two types of materials, polymethylmethacrylate (PMMA) and polystyrene (PS) [11], as well as HC5 for NPLs with a size of 50 nm in freshwater, 187.9 (8.0–2978.3) μg L−1 [12]. Nevertheless, some hotspots of NPLs pollution can be of risk for aquatic organisms, including phytoplankton. Consequently, considerable attention has been paid to the bioaccumulation of NPLs and their adverse effects within higher trophic levels [9]. Recent insigths into their accumulation within aquatic organisms have highlighted numerous challenges [13]. The accumulation of NPLs in aquatic organisms has been shown to lead to detrimental effects on various freshwater organisms [4][5][6][7][9][1][14][15][16][17][18][19][20]. These effects include oxidative stress and damage, inflammation, altered development, reduced growth, energy and movement, genotoxicity, etc., as recently reviewed in references [9][16][19][21][22][23][24]. Modifying factors, such as particle characteristics, concentration, size, exposure duration, and co-factors like presence of other organic or inorganic contaminants, food availability, species, development stage, and environmental conditions were extensively discussed in references [16][22][24][25]. However, there is a scarcity of scientific evidences regarding the interactions and possible impacts of NPLs on phytoplankton species.
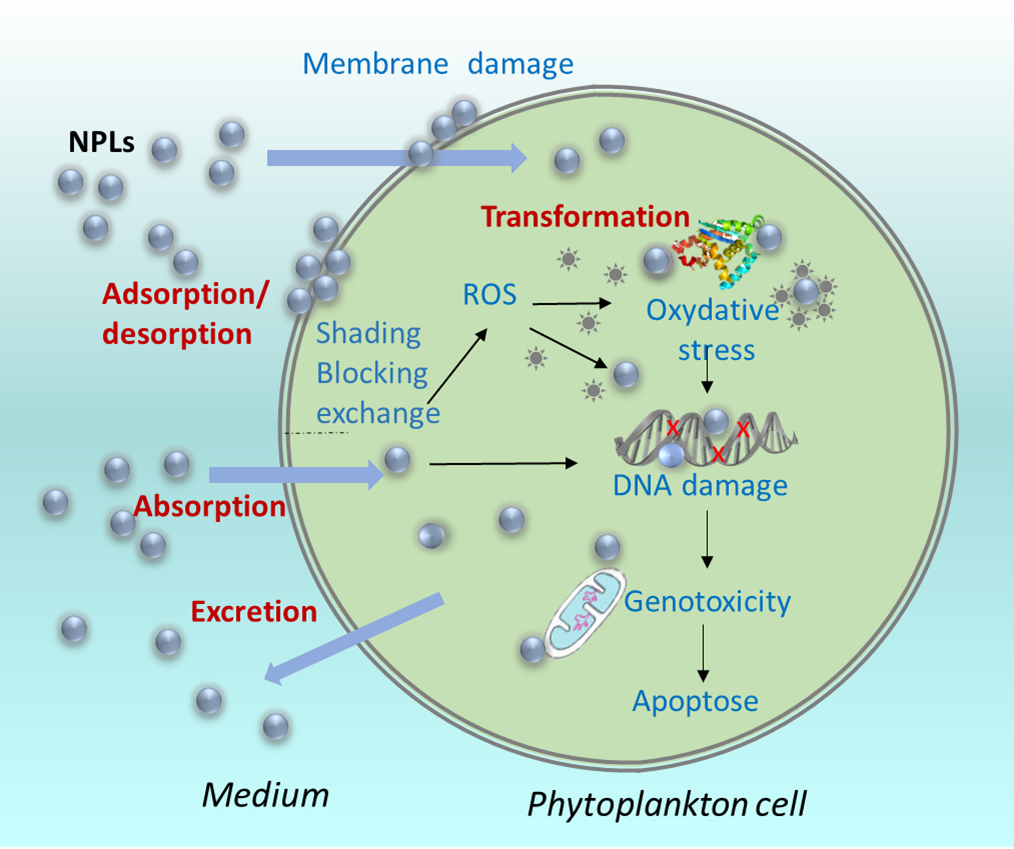
Figure 1. Key processes governing the interactions of the NPLs and phytoplankton cells.
Indeed, both amidine and carboxyl - functionalized polystyrene NPLs (PS-COOH) were found to adsorb onto the marine diatom Dunaliella tertiolecta. However, only amidine PS NPLs triggered the inhibition of algal growth, displaying an effective concentration for 50% of the algal population (EC50) of 12.97 μg mL−1 [28]. In another study, fluorescent-blue 50 nm amino - modified polystyrene NPLs (PS-NH2) adhered to the diatom Chaetoceros neogracile, leading to impairment of the photosynthetic machinery and an overproduction of reactive oxygen species (ROS) at both low (0.05 μg mL−1) and high (5 μg mL−1) exposure concentrations [29]. The adsorption onto the cells of green alga Pseudokirchneriella subcapitata was significantly higher for neutral and positively charged PS-NH2 NPLs at a concentration of 100 mg L−1. Conversely, negatively charged PS-COOH NPLs displayed minimal adsorption onto the algal cell wall [30]. These examples highlight the important role of the surface charge of NPL particles and the specificity of interactions with different algal species. The rapid adsorption and/or absorption of PS NPLs onto/in diatom Phaeodactylum tricornutum was evidenced through an observed increase in cell complexity, size and microalgae fluorescence induced by 100 nm fluoresbrite [31]. Fluorescent 51 nm PS NPLs attached to and penetrated the outer layer of green alga Chlamydomonas reinhardtii during cell division [32]. In a recent study involving metal - doped PS NPLs, it was demonstrated that more than 60% of Fe-PS or Eu-PS NPLs remained associated with algal cells of P. subcapitata after 72 hours [33]. A recent study uncovered that fluorescent aggregation - induced emission fluorogens-incorporated nanoparticles (AIE-NPs) of sizes 40, 70, and 85 nm were internally taken up via clathrin - dependent endocytosis in C. reinhardtii, while the 140 nm AIE-NPs remained surface-bound [34]. Notably, the authors highlighted the importance of endocytosis, algal cell membrane permeability, and the thickness of extracellular polymeric substances (EPS) and their cell cycle dependence in the uptake of AIE-NPs [34].
2. Effect of Nanoplastics on Phytoplankton Species
3. Effect of Phytoplankton Species on Nanoplastics
Phytoplankton species could influence the NPLs fate in the aquatic environment and thus their impact via release of biomolecules such as extracellular polymeric substance and via cellular transformations. Recent reviews have shed light on how phytoplankton can influence the fate and biological availability of NPLs by excreting diverse EPS, leading to the formation of the eco-corona [58][59]. Studies indicate that EPS produced by the marine phytoplankton play a role in forming an eco-corona arround various NPLs, thereby influencing their reactivity [58]. For example, the EPS derived from the diatom P. tricornutum, containing proteins with molecular weight ranging from 30 to 100 kDa along with high molecular weight carbohydrates, formed an eco-corona on 60 nm-sized PS-COOH NPLs, effectively reducing NPLs’ aggregation [60]. However, when EPS from P. tricornutum, Ankistrodesmus angustus, and Amphora sp. interacted with 23 nm PS NPLs, it led to the formation of gel-like micrometer aggregates, which was presumably driven by hydrophobic interactions [61]. The formation of the eco-corona has been found to depend on NPLs size, charges, and incubation duration [58]. Alginate, used as a model polysaccharide, formed an eco-corona on amidine functionalized PS NPLs, altering the surface charge, although aggregation was minimal [62][63]. Furthermore, aminated, carboxylated and plain NPLs aged in EPS reduced the oxidative stress and mitigated toxic effects in the marine alga Chlorella sp. [64].
Overall, the accumulating evidences obtained from model NPLs demonstrated that these particles interact with phytoplanktonic organisms, potentially causing harm when present in concentrations significantly higher than those typically found in aquatic environments. However, further investigations are indispensible to understand the intricate interplays between phytoplankton species and more realistic nanoplastic materials, such as secondary NPLs and aged NPLs, at concentrations closer to those anticipated in aquatic settings. Enhanced and quantitative understanding of the fundamental processes governing the interactions between NPLs and phytoplankton species is pivotal for a comprehensive grasp of their potential impacts on aquatic ecosystems. Notably, phytoplankton play a critical role in global elemental cycling, contributing to nearly half of the global primary production, and occupies a foundational position at the base of aquatic food chains.
This entry is adapted from the peer-reviewed paper 10.3390/microplastics2040029
References
- European Commission. Chemicals Strategy for Sustainability. Available online: https://environment.ec.europa.eu/strategy/chemicals-strategy_en (accessed on 17 November 2023).
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166.
- Zhang, B.; Chao, J.Y.; Chen, L.; Liu, L.C.; Yang, X.; Wang, Q. Research progress of nanoplastics in freshwater. Sci. Total Environ. 2021, 757, 143791.
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724.
- Kukkola, A.; Krause, S.; Lynch, I.; Sambrook Smith, G.H.; Nel, H. Nano and microplastic interactions with freshwater biota—Current knowledge, challenges and future solutions. Environ. Int. 2021, 152, 106504.
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E.M. Nanoplastics in aquatic systems—Are they more hazardous than microplastics? Environ. Pollut. 2021, 272, 115950.
- Chae, Y.; An, Y.J. Effects of micro- and nanoplastics on aquatic ecosystems: Current research trends and perspectives. Mar. Pollut. Bull. 2017, 124, 624–632.
- Besseling, E.; Redondo-Hasselerharm, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80.
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of nanoplastics in the aquatic environment and possible impacts on aquatic organisms. Sci. Total Environ. 2023, 906, 167404.
- Yang, T.; Nowack, B. A meta-analysis of ecotoxicological hazard data for nanoplastics in marine and freshwater systems. Environ. Toxicol. Chem. 2020, 39, 2588–2598.
- Venâncio, C.; Ferreira, I.; Martins, M.A.; Soares, A.M.V.M.; Lopes, I.; Oliveira, M. The effects of nanoplastics on marine plankton: A case study with polymethylmethacrylate. Ecotoxicol. Environ. Saf. 2019, 184, 109632.
- Takeshita, K.M.; Iwasaki, Y.; Sinclair, T.M.; Hayashi, T.I.; Naito, W. Illustrating a species sensitivity distribution for nano- and microplastic particles using bayesian hierarchical modeling. Environ. Toxicol. Chem. 2022, 41, 954–960.
- Atugoda, T.; Piyumali, H.; Wijesekara, H.; Sonne, C.; Lam, S.S.; Mahatantila, K.; Vithanage, M. Nanoplastic occurrence, transformation and toxicity: A review. Environ. Chem. Lett. 2023, 21, 363–381.
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82.
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326.
- Kogel, T.; Bjoroy, O.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050.
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.-J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699.
- Huang, D.; Tao, J.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.; Li, R. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 2021, 407, 124399.
- Han, Y.R.; Lian, F.; Xiao, Z.G.; Gu, S.G.; Cao, X.S.; Wang, Z.Y.; Xing, B.S. Potential toxicity of nanoplastics to fish and aquatic invertebrates: Current understanding, mechanistic interpretation, and meta-analysis. J. Hazard. Mater. 2022, 427, 127870.
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. 2019, 252, 511–521.
- Zhu, H.L.; Fu, S.F.; Zou, H.; Su, Y.Y.; Zhang, Y.F. Effects of nanoplastics on microalgae and their trophic transfer along the food chain: Recent advances and perspectives. Environ. Sci. Process. Impacts 2021, 23, 1873–1883.
- Larue, C.; Sarret, G.; Castillo-Michel, H.; del Real, A.E.P. A critical review on the impacts of nanoplastics and microplastics on aquatic and terrestrial photosynthetic organisms. Small 2021, 17, 2005834.
- Nguyen, M.-K.; Lin, C.; Nguyen, H.-L.; Le, V.-G.; Haddout, S.; Um, M.-J.; Chang, S.W.; Nguyen, D.D. Ecotoxicity of micro- and nanoplastics on aquatic algae: Facts, challenges, and future opportunities. J. Environ. Manag. 2023, 346, 118982.
- Gong, H.; Li, R.X.; Li, F.; Guo, X.W.; Xu, L.J.; Gan, L.; Yan, M.T.; Wang, J. Toxicity of nanoplastics to aquatic organisms: Genotoxicity, cytotoxicity, individual level and beyond individual level. J. Hazard. Mater. 2023, 443, 130266.
- Agathokleous, E.; Iavicoli, I.; Barcelo, D.; Calabrese, E.J. Micro/nanoplastics effects on organisms: A review focusing on ‘dose’. J. Hazard. Mater. 2021, 417, 126084.
- Zhu, H.; Fan, X.; Zou, H.; Guo, R.-B.; Fu, S.-F. Effects of size and surface charge on the sedimentation of nanoplastics in freshwater. Chemosphere 2023, 336, 139194.
- Gigault, J.; Halle, A.t.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034.
- Bergami, E.; Pugnalini, S.; Vannuccini, M.L.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.A.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2017, 189, 159–169.
- González-Fernández, C.; Toullec, J.; Lambert, C.; Le Goïc, N.; Seoane, M.; Moriceau, B.; Huvet, A.; Berchel, M.; Vincent, D.; Courcot, L.; et al. Do transparent exopolymeric particles (tep) affect the toxicity of nanoplastics on Chaetoceros neogracile? Environ. Pollut. 2019, 250, 873–882.
- Nolte, T.M.; Hartmann, N.B.; Kleijn, J.M.; Garnæs, J.; van de Meent, D.; Jan Hendriks, A.; Baun, A. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat. Toxicol. 2017, 183, 11–20.
- Sendra, M.; Staffieri, E.; Yeste, M.P.; Moreno-Garrido, I.; Gatica, J.M.; Corsi, I.; Blasco, J. Are the primary characteristics of polystyrene nanoplastics responsible for toxicity and ad/absorption in the marine diatom Phaeodactylum tricornutum? Environ. Pollut. 2019, 249, 610–619.
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284.
- Monikh, F.A.; Chupani, L.; Vijver, M.G.; Peijnenburg, W. Parental and trophic transfer of nanoscale plastic debris in an assembled aquatic food chain as a function of particle size. Environ. Pollut. 2021, 269, 116066.
- Yan, N.; Tang, B.Z.; Wang, W.X. Cell cycle control of nanoplastics internalization in phytoplankton. ACS Nano 2021, 15, 12237–12248.
- Zhao, Y.; Tao, S.; Liu, S.; Hu, T.; Zheng, K.; Shen, M.; Meng, G. Research advances on impacts micro/nanoplastics and their carried pollutants on algae in aquatic ecosystems: A review. Aquat. Toxicol. 2023, 264, 106725.
- Corsi, I.; Bellingeri, A.; Eliso, M.C.; Grassi, G.; Liberatori, G.; Murano, C.; Sturba, L.; Vannuccini, M.L.; Bergami, E. Eco-interactions of engineered nanomaterials in the marine environment: Towards an eco-design framework. Nanomaterials 2021, 11, 1903.
- Wang, S.; Liu, M.; Wang, J.; Huang, J.; Wang, J. Polystyrene nanoplastics cause growth inhibition, morphological damage and physiological disturbance in the marine microalga Platymonas helgolandica. Mar. Pollut. Bull. 2020, 158, 111403.
- Li, R.; Wang, B.; Nan, F.; Lv, J.; Liu, X.; Liu, Q.; Feng, J.; Xie, S. Effects of polystyrene nanoplastics on the physiological and biochemical characteristics of microalga Scenedesmus quadricauda. Environ. Pollut. 2023, 319, 120987.
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, W.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68.
- Yang, W.; Gao, P.; Li, H.; Huang, J.; Zhang, Y.; Ding, H.; Zhang, W. Mechanism of the inhibition and detoxification effects of the interaction between nanoplastics and microalgae Chlorella pyrenoidosa. Sci. Total Environ. 2021, 783, 146919.
- Hazeem, L.J.; Yesilay, G.; Bououdina, M.; Perna, S.; Cetin, D.; Suludere, Z.; Barras, A.; Boukherroub, R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar. Pollut. Bull. 2020, 156, 111278.
- Xiao, Y.; Jiang, X.; Liao, Y.; Zhao, W.; Zhao, P.; Li, M. Adverse physiological and molecular level effects of polystyrene microplastics on freshwater microalgae. Chemosphere 2020, 255, 126914.
- Liao, Y.; Jiang, X.; Xiao, Y.; Li, M. Exposure of microalgae Euglena gracilis to polystyrene microbeads and cadmium: Perspective from the physiological and transcriptional responses. Aquat. Toxicol. 2020, 228, 105650.
- Feng, L.-J.; Li, J.-W.; Xu, E.G.; Sun, X.-D.; Zhu, F.-P.; Ding, Z.; Tian, H.; Dong, S.-S.; Xia, P.-F.; Yuan, X.-Z. Short-term exposure to positively charged polystyrene nanoparticles causes oxidative stress and membrane destruction in cyanobacteria. Environ. Sci. Nano 2019, 6, 3072–3079.
- Seoane, M.; González-Fernández, C.; Soudant, P.; Huvet, A.; Esperanza, M.; Cid, Á.; Paul-Pont, I. Polystyrene microbeads modulate the energy metabolism of the marine diatom Chaetoceros neogracile. Environ. Pollut. 2019, 251, 363–371.
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 714, 136767.
- Tamayo-Belda, M.; Pérez-Olivares, A.V.; Pulido-Reyes, G.; Martin-Betancor, K.; González-Pleiter, M.; Leganés, F.; Mitrano, D.M.; Rosal, R.; Fernández-Piñas, F. Tracking nanoplastics in freshwater microcosms and their impacts to aquatic organisms. J. Hazard. Mater. 2023, 445, 130625.
- Gomes, T.; Almeida, A.C.; Georgantzopoulou, A. Characterization of cell responses in Rhodomonas baltica exposed to pmma nanoplastics. Sci. Total Environ. 2020, 726, 138547.
- Tamayo-Belda, M.; Vargas-Guerrero, J.J.; Martín-Betancor, K.; Pulido-Reyes, G.; González-Pleiter, M.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Understanding nanoplastic toxicity and their interaction with engineered cationic nanopolymers in microalgae by physiological and proteomic approaches. Environ. Sci. Nano 2021, 8, 2277–2296.
- Cesarini, G.; Secco, S.; Taurozzi, D.; Venditti, I.; Battocchio, C.; Marcheggiani, S.; Mancini, L.; Fratoddi, I.; Scalici, M.; Puccinelli, C. Teratogenic effects of environmental concentration of plastic particles on freshwater organisms. Sci. Total Environ. 2023, 898, 165564.
- Baudrimont, M.; Arini, A.; Guégan, C.; Venel, Z.; Gigault, J.; Pedrono, B.; Prunier, J.; Maurice, L.; Ter Halle, A.; Feurtet-Mazel, A. Ecotoxicity of polyethylene nanoplastics from the north atlantic oceanic gyre on freshwater and marine organisms (microalgae and filter-feeding bivalves). Environ. Sci. Pollut. Res. 2020, 27, 3746–3755.
- Tamayo-Belda, M.; Pulido-Reyes, G.; González-Pleiter, M.; Martín-Betancor, K.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Identification and toxicity towards aquatic primary producers of the smallest fractions released from hydrolytic degradation of polycaprolactone microplastics. Chemosphere 2022, 303, 134966.
- González-Pleiter, M.; Tamayo-Belda, M.; Pulido-Reyes, G.; Amariei, G.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environ. Sci. Nano 2019, 6, 1382–1392.
- Liu, Q.; Tang, X.X.; Zhang, B.H.; Li, L.Y.; Zhao, Y.R.; Lv, M.C.; Li, J.; Kan, C.X.; Zhao, Y. The effects of two sized polystyrene nanoplastics on the growth, physiological functions, and toxin production of Alexandrium tamarense. Chemosphere 2022, 291, 132943.
- Zheng, X.; Yuan, Y.; Li, Y.; Liu, X.; Wang, X.; Fan, Z. Polystyrene nanoplastics affect growth and microcystin production of Microcystis aeruginosa. Environ. Sci. Pollut. Res. 2021, 28, 13394–13403.
- Shiu, R.-F.; Vazquez, C.I.; Chiang, C.-Y.; Chiu, M.-H.; Chen, C.-S.; Ni, C.-W.; Gong, G.-C.; Quigg, A.; Santschi, P.H.; Chin, W.-C. Nano- and microplastics trigger secretion of protein-rich extracellular polymeric substances from phytoplankton. Sci. Total Environ. 2020, 748, 141469.
- Mitrano, D.M.; Wick, P.; Nowack, B. Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 2021, 16, 491–500.
- Junaid, M.; Wang, J. Interaction of nanoplastics with extracellular polymeric substances (eps) in the aquatic environment: A special reference to eco-corona formation and associated impacts. Water Res. 2021, 201, 117319.
- Liu, W.; Worms, I.A.M.; Jakšić, Ž.; Slaveykova, V.I. Aquatic organisms modulate the bioreactivity of engineered nanoparticles: Focus on biomolecular corona. Front. Toxicol. 2022, 4, 933186.
- Grassi, G.; Gabellieri, E.; Cioni, P.; Paccagnini, E.; Faleri, C.; Lupetti, P.; Corsi, I.; Morelli, E. Interplay between extracellular polymeric substances (eps) from a marine diatom and model nanoplastic through eco-corona formation. Sci. Total Environ. 2020, 725, 138457.
- Chen, C.-S.; Anaya, J.M.; Zhang, S.; Spurgin, J.; Chuang, C.-Y.; Xu, C.; Miao, A.-J.; Chen, E.Y.; Schwehr, K.A.; Jiang, Y. Effects of engineered nanoparticles on the assembly of exopolymeric substances from phytoplankton. PLoS ONE 2011, 6, e21865.
- Saavedra, J.; Stoll, S.; Slaveykova, V.I. Influence of nanoplastic surface charge on eco-corona formation, aggregation, and toxicity to freshwater zooplankton. Environ. Pollut. 2019, 252, 715–722.
- Pochelon, A.; Stoll, S.; Slaveykova, V.I. Polystyrene nanoplastic behavior and toxicity on crustacean Daphnia magna: Media composition, size, and surface charge effects. Environments 2021, 8, 101.
- Natarajan, L.; Omer, S.; Jetly, N.; Jenifer, M.A.; Chandrasekaran, N.; Suraishkumar, G.K.; Mukherjee, A. Eco-corona formation lessens the toxic effects of polystyrene nanoplastics towards marine microalgae Chlorella sp. Environ. Res. 2020, 188, 109842.
- Chia, W.Y.; Tang, D.Y.Y.; Khoo, K.S.; Kay Lup, A.N.; Chew, K.W. Nature’s fight against plastic pollution: Algae for plastic biodegradation and bioplastics production. Environ. Sci. Ecotechnol. 2020, 4, 100065.
- He, Y.; Deng, X.; Jiang, L.; Hao, L.; Shi, Y.; Lyu, M.; Zhang, L.; Wang, S. Current advances, challenges and strategies for enhancing the biodegradation of plastic waste. Sci. Total Environ. 2023, 906, 167850.
- Kim, J.W.; Park, S.B.; Tran, Q.G.; Cho, D.H.; Choi, D.Y.; Lee, Y.J.; Kim, H.S. Functional expression of polyethylene terephthalate-degrading enzyme (petase) in green microalgae. Microb. Cell. Fact. 2020, 19, 97.
- Kim, J.W.; Park, S.B.; Tran, Q.G.; Cho, D.H.; Choi, D.Y.; Lee, Y.J.; Kim, H.S. Functional expression of polyethylene terephthalate-degrading enzyme (petase) in green microalgae. Microb. Cell. Fact. 2020, 19, 97.
