Sugarcane (Saccharum spp. hybrid) is the chief source of sugar and biofuel globally and is prominent among cash crops. Sucrose is the main required product in sugarcane, and many studies have been performed to understand the phenomena of sucrose synthesis, metabolism, and accumulation in sugarcane. However, none of the studies concluded that a single gene is responsible for the sucrose content. Instead, a complex mechanism consisting of several genes, such as sucrose phosphate synthase genes (SPS1, SPS2, SPS4, SPS5), sucrose synthase genes (SuSy1, SuSy2, SuSy4), invertase genes (INV, CWIN, NIN1, CINV2), and phytohormone, trehalose, transcription factor (TF), protein kinase, and sugar transporter genes are working spatiotemporally in sugarcane. Plant hormones are the main regulatory tools involved in growth and development, which play a dominant role in integrating interior and exterior signals that temper development.
- sugarcane
- sucrose
- phytohormone
- IAA
- ETH
- GA
- ABA
1. Introduction
2. IAA
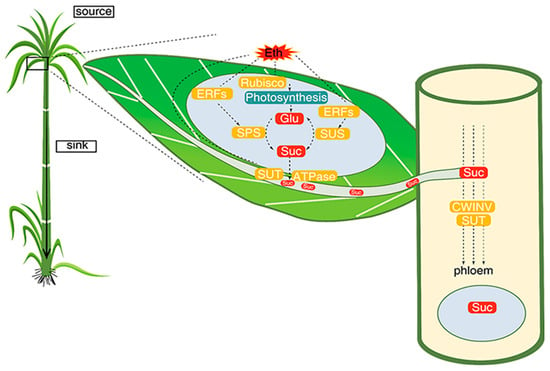
3. ETH
4. GA
5. ABA
| Sucrose phosphate synthase (SPS); SPS1, SPS2, SPS4, SPS5 |
| Sucrose synthase (SuSy); SuSy1, SuSy2, SuSy4 |
| Soluble acid invertase (SAI) |
| Cell wall invertase (CWI) |
| Neutral invertase (NI) |
| Sucrose transporter; SWEET1b, SWEET13c, SWEET4a/4b, SUT1, SUT4, SUT5, SUT6, ShPST2a, ShPST2b, ShSUT4. |
| SNF1-like kinases |
| Trehalose-phosphate synthase |
| Cellulose synthase (CeS); CesA1, CesA7, CesA9, bk2l3,CesA10, CesA11, CesA12 |
| Trehalose 6-phosphate (T6P) |
| Trehalose-6-phosphate phosphatase (TPP) |
| Transcription factors (TF); WRKY, MYB, NAC, AP2/ERF |
| Basic helix-loop-helix (bHLH) |
| ScFBHs and ScACS2 |
| Mitogen-activated PTK (MAPK) |
| Sucrose-nonfermentation1-related protein kinase1-2 (ScSnRK1-2) |
| BCL2 antagonist/killer 1 (ScBAK1) |
| Phytohormones; Auxin (IAA), AUX/IAA, Gibberellin (GA), Cytokinin (CTK), Abscisic acid (ABA), Ethylene (ETH). |
| Brassinosteroid (BR), jasmonates, salicylic acid, a peptide hormone, and strigolactone |
| Gretchen Hagen3 (GH3), small auxin-up RNAs (SAUR |
| Ethylene receptor (ETR), Ethylene response sensor (ERS), Ethylene insensitive (EIN) ETP, EIN2, Targeting protein genes EIL, EIN3 like, EBF (EIN3-F) |
| GA1, GA3, GA4 and GA7 A19, GA20 and GA29 |
| ABA receptor pyrabactin resistance 1 (PYR1), PYR1-like (PYL) |
This entry is adapted from the peer-reviewed paper 10.3390/agronomy13122957
References
- Watson, L.; Clifford, H.T.; Dallwitz, M.J. The classification of Poaceae: Subfamilies and supertribes. Aust. J. Bot. 1985, 33, 433–484.
- Bonnett, G.D.; Henry, R.J. Saccharum. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin/Heidelberg, Germany, 2011; pp. 165–177.
- Cheavegatti-Gianotto, A.; de Abreu, H.M.C.; Arruda, P.; Bespalhok Filho, J.C.; Burnquist, W.L.; Creste, S.; di Ciero, L.; Ferro, J.A.; de Oliveira Figueira, A.V.; de Sousa Filgueiras, T. Sugarcane (Saccharum X officinarum): A reference study for the regulation of genetically modified cultivars in Brazil. Trop. Plant Biol. 2011, 4, 62–89.
- Moore, P.H. Temporal and spatial regulation of sucrose accumulation in the sugarcane stem. Funct. Plant Biol. 1995, 22, 661–679.
- Bihmidine, S.; Hunter, C.T., III; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 177.
- Braun, D.M.; Wang, L.; Ruan, Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735.
- Yadav, U.P.; Ayre, B.G.; Bush, D.R. Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: Assessing the potential to improve crop yields and nutritional quality. Front. Plant Sci. 2015, 6, 275.
- McCormick, A.; Watt, D.; Cramer, M. Supply and demand: Sink regulation of sugar accumulation in sugarcane. J. Exp. Bot. 2009, 60, 357–364.
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8.
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, R365–R373.
- Botha, F.C.; Lakshmanan, P.; O’Connell, A.; Moore, P.H. Hormones and growth regulators. In Sugarcane: Physiology, Biochemistry, and Functional Biology; Wiley: Hoboken, NJ, USA, 2013; pp. 331–377.
- Gray, W.M. Hormonal regulation of plant growth and development. PLoS Biol. 2004, 2, e311.
- Tabashnik, B.E. Communal benefits of transgenic corn. Science 2010, 330, 189–190.
- Adamowski, M.; Friml, J. PIN-dependent auxin transport: Action, regulation, and evolution. Plant Cell 2015, 27, 20–32.
- Vert, G.; Chory, J. Crosstalk in cellular signaling: Background noise or the real thing? Dev. Cell 2011, 21, 985–991.
- Boivin, S.; Fonouni-Farde, C.; Frugier, F. How auxin and cytokinin phytohormones modulate root microbe interactions. Front. Plant Sci. 2016, 7, 1240.
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177.
- Li, Z.; Hua, X.; Zhong, W.; Yuan, Y.; Wang, Y.; Wang, Z.; Ming, R.; Zhang, J. Genome-wide identification and expression profile analysis of WRKY family genes in the autopolyploid Saccharum spontaneum. Plant Cell Physiol. 2020, 61, 616–630.
- Zhu, F.; Wai, C.M.; Zhang, J.; Jones, T.C.; Nagai, C.; Ming, R. Differential expression of hormone related genes between extreme segregants of a Saccharum interspecific F2 population. Euphytica 2018, 214, 55.
- Liscum, E.; Reed, J. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400.
- Ntambo, M.S.; Meng, J.Y.; Rott, P.; Royer, M.; Lin, L.H.; Zhang, H.L.; Gao, S.J. Identification and characterization of Xanthomonas albilineans causing sugarcane leaf scald in China using multilocus sequence analysis. Plant Pathol. 2019, 68, 269–277.
- Masood, A.; Iqbal, N.; Khan, N.A. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012, 35, 524–533.
- Nazar, R.; Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Involvement of ethylene in reversal of salt-inhibited photosynthesis by sulfur in mustard. Physiol. Plant. 2014, 152, 331–344.
- Acevedo, A.; Tejedor, M.T.; Erazzu, L.E.; Cabada, S.; Sopena, R. Pedigree comparison highlights genetic similarities and potential industrial values of sugarcane cultivars. Euphytica 2017, 213, 121.
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence tolerant rice: SUB1′s journey from landrace to modern cultivar. Rice 2010, 3, 138–147.
- Chen, Y.-F.; Etheridge, N.; Schaller, G.E. Ethylene signal transduction. Ann. Bot. 2005, 95, 901–915.
- Chang, C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016, 14, 7.
- Chen, Z.; Qin, C.; Wang, M.; Liao, F.; Liao, Q.; Liu, X.; Li, Y.; Lakshmanan, P.; Long, M.; Huang, D. Ethylene-mediated improvement in sucrose accumulation in ripening sugarcane involves increased sink strength. BMC Plant Biol. 2019, 19, 285.
- Cunha, C.P.; Roberto, G.G.; Vicentini, R.; Lembke, C.G.; Souza, G.M.; Ribeiro, R.V.; Machado, E.C.; Lagôa, A.M.; Menossi, M. Ethylene-induced transcriptional and hormonal responses at the onset of sugarcane ripening. Sci. Rep. 2017, 7, 43364.
- de Almeida Silva, M.; Caputo, M.M. Ripening and the use of ripeners for better sugarcane management. In Crop Management—Cases and Tools for Higher Yield and Sustainability; InTech: London, UK, 2012; Chapter 1.
- Gao, X.X.; Fan, X.; Dao, J.M.; Deng, J.; Li, R.D.; Zhang, Y.B.; Guo, J.W.; Liu, S.C. Relationship between endogenous ethylene production and natural defoliation traits during the maturation of sugarcane. Bragantia 2015, 74, 189–195.
- Yang, C.; Lu, X.; Ma, B.; Chen, S.-Y.; Zhang, J.-S. Ethylene signaling in rice and Arabidopsis: Conserved and diverged aspects. Mol. Plant 2015, 8, 495–505.
- Jan, A.; Komatsu, S. Functional characterization of gibberellin-regulated genes in rice using microarray system. Genom. Proteom. Bioinform. 2006, 4, 137–144.
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421.
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9.
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760.
- MacMillan, J. Occurrence of gibberellins in vascular plants, fungi, and bacteria. J. Plant Growth Regul. 2001, 20, 387–442.
- Moore, P.; Osgood, R.; Carr, J.; Ginoza, H. Sugarcane studies with gibberellin. V. Plot harvests vs. stalk harvests to assess the effect of applied GA3 on sucrose yield. J. Plant Growth Regul. 1982, 1, 205–210.
- Gupta, R.; Chakrabarty, S. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504.
- Resende, P.; Soares, J.; Hudetz, M. Moddus, a plant growth regulator and management tool for sugarcane production in Brazil. Sugar Cane Int. 2000, 103, 5–9.
- Claeys, H.; De Bodt, S.; Inzé, D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239.
- Zhang, X.; Ling, H.; Liu, F.; Huang, N.; Wang, L.; Mao, H.; Li, C.; Tang, H.; Su, W.; Su, Y. Cloning and expression analysis of a II d sub-group WRKY transcription factor gene from sugarcane. Sci. Agric. Sin. 2018, 51, 4409–4423.
- Wang, Z.; Zhao, F.; Zhao, X.; Ge, H.; Chai, L.; Chen, S.; Perl, A.; Ma, H. Proteomic analysis of berry-sizing effect of GA3 on seedless Vitis vinifera L. Proteomics 2012, 12, 86–94.
- Pribil, M.; Hermann, S.; Dun, G.; Karno, X.; Ngo, C.; O’neill, S.; Wang, L.; Bonnett, G.; Chandler, P.; Beveridge, C. Altering sugarcane shoot architecture through genetic engineering: Prospects for increasing cane and sugar yield. In Proceedings of the Australian Society of Sugar Cane Technologists, Cairns, Australia, 8–11 May 2007; pp. 251–257.
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343.
- Moore, P.H.; Ginoza, H. Gibberellin Studies with Sugarcane. III. Effects of Rate and Frequency of Gibberellic Acid Applications on Stalk Length and Fresh Weight 1. Crop Sci. 1980, 20, 78–82.
- Qiu, L.-H.; Chen, R.-F.; Luo, H.-M.; Fan, Y.-G.; Huang, X.; Liu, J.-X.; Xiong, F.-Q.; Zhou, H.-W.; Gan, C.-K.; Wu, J.-M. Effects of exogenous GA3 and DPC treatments on levels of endogenous hormone and expression of key gibberellin biosynthesis pathway genes during stem elongation in sugarcane. Sugar Tech 2019, 21, 936–948.
- Kuhnle, J.; Moore, P.; Haddon, W.; Fitch, M. Identification of gibberellins from sugarcane plants. J. Plant Growth Regul. 1983, 2, 59–71.
- Roopendra, K.; Sharma, A.; Chandra, A.; Saxena, S. Gibberellin-induced perturbation of source–sink communication promotes sucrose accumulation in sugarcane. 3 Biotech 2018, 8, 418.
- Gonzalez-Guzman, M.; Pizzio, G.A.; Antoni, R.; Vera-Sirera, F.; Merilo, E.; Bassel, G.W.; Fernández, M.A.; Holdsworth, M.J.; Perez-Amador, M.A.; Kollist, H. Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 2012, 24, 2483–2496.
- Laby, R.J.; Kincaid, M.S.; Kim, D.; Gibson, S.I. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000, 23, 587–596.
- Li, J.; Assmann, S.M. An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 1996, 8, 2359–2368.
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Tsz-fung, F.C. Abscisic acid inhibits PP2Cs via the PYR/PYL family of ABA-binding START proteins. Science 2009, 324, 1068.
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068.
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664.
- Zhao, Y.; Zhang, Z.; Gao, J.; Wang, P.; Hu, T.; Wang, Z.; Hou, Y.-J.; Wan, Y.; Liu, W.; Xie, S. Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity. Cell Rep. 2018, 23, 3340–3351.e3345.
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 2001, 410, 327–330.
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol. 2000, 3, 217–223.
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharmacol. Sin. 2014, 35, 567–584.
- Li, C.-N.; Yang, L.-T.; Srivastava, M.K.; Li, Y.-R. Foliar application of abscisic acid improves drought tolerance of sugarcane plant under severe water stress. Int. J. Agric. Innov. Res. 2014, 3, 101–107.
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125.
- Hayamichi, Y. Effects of abscisic acid treatment on the growth and sugar accumulation of sugarcane plant. Jpn. J. Trop. Agric. 1997, 41, 22–26.
- Zhang, X.; Chen, M.; Liang, Y.; Xing, Y.; Yang, L.; Chen, M.; Comstock, J.C.; Li, Y.; Yang, L. Morphological and physiological responses of sugarcane to Leifsonia xyli subsp. xyli infection. Plant Dis. 2016, 100, 2499–2506.
