2. Extended-Spectrum β-Lactamases (ESBL) and ESBL Producers
ESBL are hydrolyzing enzymes secreted by several Gram-negative bacteria of the family Enterobacteriaceae. They cause the inactivation of broad-spectrum oxyimino-cephalosporin (third- and fourth-generation) and monobactam (aztreonam) but not cephamycin (cefoxitin) or carbapenems (meropenem, imipenem, ertapenem, and doripenem) [
9,
10]. Generally, these enzymes are neutralized by β-lactamase inhibitors (BLIs) such as clavulanic acid, sulbactam, and tazobactam [
9]. Genes that encode ESBL are mostly found on transposons or insertion sequences of plasmids in association with other resistance genes. As a result, they can spread rapidly, causing resistance to multiple antimicrobials such as aminoglycosides, trimethoprim, sulphonamides, tetracyclines chloramphenicol, and fluoroquinolone [
11,
12,
13].
ESBL are produced by the nosocomial pathogens
E. coli,
Klebsiella pneumoniae,
Acinetobacter baumannii,
Pseudomonas aeruginosa, and
Enterobacter spp. [
14]. Among a wide range of Gram-negative bacterial species of different families harboring ESBL genes,
E. coli is the most common host, followed by
K. pneumoniae. Among the different variants of ESBL-producing
E. coli, the ST131 clone is the most dominant [
3].
The ESBL-encoding genes are highly diverse in nature and can be classified into many families with unique characteristics such as
blaTEM,
blaSHV, and
blaCTX-M. TEM 1, the first plasmid and transposon-mediated β-lactamase, was isolated from the blood culture of a named Temoniera in Greece in the early 1960s [
15]. It has spread worldwide and is now found in many species of the family
Enterobacteriaceae,
P. aeruginosa,
Hemophilus influenzae, and
Neisseria gonorrhoeae [
16]. The SHV-1 type is common in
Klebsiella spp. and
E. coli [
16]. CTX-M-type ESBL are predominant in
E. coli,
K. pneumoniae,
S. enterica serovar
Typhimurium, and
Shigella spp. [
17]. The plasmid-mediated OXA and AmpC-type ESBL were discovered in
P. aeruginosa and
K. pneumoniae isolates, respectively [
16,
18]. A series of
Salmonella serovars, including
S. enteritidis,
S. newport, and
S. paratyphi, have been characterized as ESBL producers that have been linked to serious foodborne gastroenteritis in humans [
19].
3. Classification and Evolution of ESBL
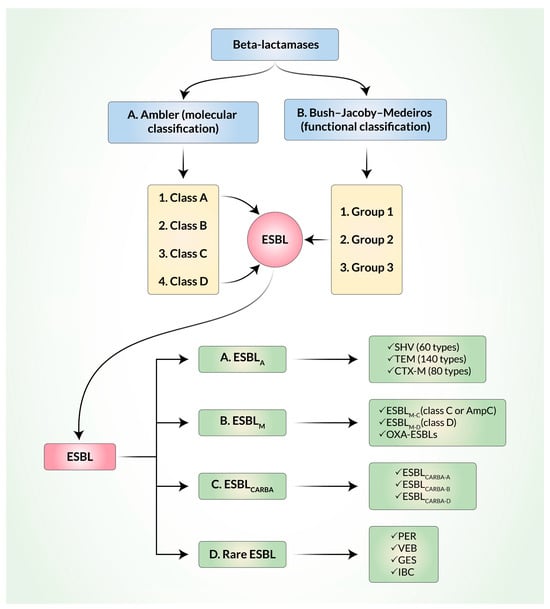
ESBL are structurally and functionally mutated versions of β-lactamases. It is noteworthy that β-lactamases can be defined and classified by the Ambler classification system on the basis of molecular structure [
20] and by the Bush–Jacoby–Medeiros classification system on the basis of function (
Figure 1). Among the four classes (A, B, C, and D) of the Ambler classification, ESBL belong to classes A and D where serine is used as an enzyme active center. According to the Bush–Jacoby–Medeiros system, β-lactamases are classified into groups 1 to 3, along with several subgroups, on the basis of lysis of β-lactam substrates and the effects of inhibitors. Ambler’s A and D classes of ESBL belong to group 2 in the Bush–Jacoby–Medeiros system. In order to keep track of the newly evolved β-lactamases, Bush and Jacoby later proposed an update to the original Bush–Jacoby–Medeiros functional classification system of β-lactamases [
11]. In both the original version and the updated 2009 version of the classification, ESBL belonged to group 2.
Figure 1. Classification of extended-spectrum β-lactamases (ESBL). A. Ambler (molecular) classification. B. Bush–Jacoby–Medeiros classification.
More recently, ESBL have been classified into three main groups: Ambler class A ESBL (ESBL
A), miscellaneous ESBL (ESBL
M), and ESBL that degrade carbapenems (ESBL
CARBA) [
9]. Most ESBL in the world belong to the ESBL
A group, which includes several types of sulfhydryl reagent variable (SHV) β-lactamases, Temoniera (TEM) β-lactamases, and cefotaxime-M (CTX-M) β-lactamases [
21]. About 90% of TEM-1 harboring
E. coli can confer resistance to ampicillin, penicillin, and first-generation cephalosporins but not to oxyimino cephalosporin. Additionally, SHV-1 (68% similar to TEM on the basis of amino acid sequences) can provide resistance to penicillin, tigecycline, and piperacillin but not to oxyimino cephalosporin [
22]. During the 1980s, evolution of SHV-1 and TEM-1 from non-ESBL to ESBL in
K. pneumoniae and
E. coli strains, respectively, via specific amino acid substitutions, made them more capable of hydrolyzing oxyimino-cephalosporins [
13]. Among the 140 TEM and 60 SHV types identified, some are capable of inactivating third-generation cephalosporins and aztreonam [
22].
More recent outbreaks involving ESBL have been mediated by the CTX-M type rather than the TEM type or the SHV type [
23]. CTX-M-type ESBL (first reported in 1989 in Munich, Germany) preferentially hydrolyze cefotaxime over ceftazidime and are inhibited by tazobactam [
24]. They are distinct from TEM-type and SHV-type ESBL. The ESBL enzyme-encoded
bla genes originated from the chromosomes of
Kluyvera spp. (non-pathogenic
Enterobacteriaceae). CTX-M ESBL are grouped into six major types—CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, CTX-M-25, and KLUC—on the basis of ≥10% variance in amino acid sequence identity and several minor variants within the groups [
25].
More than 80 CTX-M types have been reported in both hospitals and communities as well as in food animals, fresh vegetables, water, and the environment [
22]. Mobile genetic elements (MGEs) such as IS
Ecp1 and IS
CR1 play an important role in transferring
blaCTX-M genes from the chromosomes of
Kluyvera spp. into the plasmids of
E. coli. The gene expression of
blaCTX-M is enhanced by several active promoter sequences encoded in some MGEs, resulting in increased cephalosporin resistance in
E. coli in hospital settings [
26]. While CTX-M-type ESBL are mainly detected in plasmid incompatibility groups, chromosomal integration was also reported [
25]. In humans, CTX-M-15 (CTX-M-1 group) and CTX-M-14 (CTX-M-9 group) are more prevalent, whereas CTX-M-1 (CTX-M-1 group) is more predominant in animals [
27]. Other CTX-M groups were reported in specific regions, such as the CTX-M-2 and CTX-M-8 groups in South America and the CTX-M-2 group in Japan [
25].
ESBL
M are further classified into ESBL
M-C (class C, plasmid-mediated AmpC) and ESBL
M-D (class D). The AmpC group confers resistance to penicillin, third- and fourth-generation cephalosporins, and, sometimes, to carbapenems. They are inhibited by cloxacillin and boronic acid. Some OXA-ESBL are also classified within the ESBL
M group. Carbapenem-resistant ESBL are also divided into ESBL
CARBA-A, ESBL
CARBA-B, and ESBL
CARBA-D [
28]. ESBL
CARBA can degrade all β-lactam antibiotics. They are inhibited by either ethylenediaminetetraacetic acid (EDTA) or dipicolinic acid (DPA), as in the cases of Metallo- β-lactamases (MBLs), boronic acid, or avibactam. Some OXA enzymes are also included in the ESBL
CARBA group. OXA-type β-lactamases that belong to Ambler class D are different from TEM and SHV, have the ability to hydrolyze oxacillin and cloxacillin, and are not inhibited by clavulanate acid. They have been mainly detected in
P. aeruginosa and a much lesser percentage (1–10%) have been detected in
E. coli. Other rarely found ESBL that are transmitted through plasmids are Pseudomonas extended resistant (PER), Vietnam ESBL (VEB), Guiana extended-spectrum (GES), and integron-borne cephalosporinase (IBC) [
3].
4. Mechanism of Resistance and Dissemination of Resistant Genes
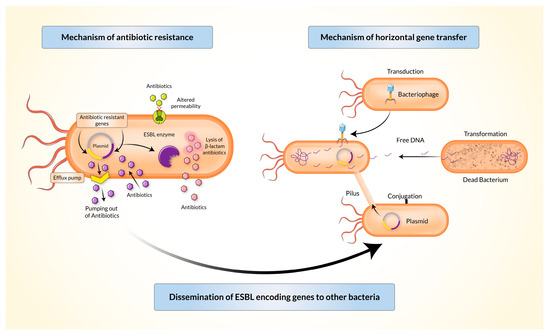
Gram-negative bacteria may inactivate β-lactam antibiotics (penicillin and cephalosporin) through several mechanisms (Figure 2). The periplasm of Gram-negative bacteria releases β-lactamase which has a higher affinity towards β-lactam antibiotics than the affinity of β-lactam antibiotics to their targets. The gene coding β-lactamase may be located in the immobile genetic chromosomes (in Enterobacter species) or extra-chromosomal MGEs such as a plasmid, integrin, or a transposon. The resistant genes evolve either gene-level mutations or acquisition of resistant genes from other bacteria of the same or different species.
Figure 2. Mechanisms of antibiotic resistance and horizontal gene transfer by extended-spectrum β-lactamase producing Enterobacteriaceae (ESBL-E).
Bacterial integrons, described at the end of the 1980s, act as a vehicle for the transmission (intraspecies or interspecies) of resistant genes by the acquisition of sequences present in transposons and/or conjugative plasmids through the process of horizontal gene transfer [
29]. This can happen through transformation, transduction, or conjugation (
Figure 2). Genes encoding TEM-type β-lactamases are mostly carried and disseminated by Tn1, Tn2, or Tn3-like transposons. Genes encoding SHV-type β-lactamases can be mediated by both chromosomes and plasmids. Conjugative transmission is most commonly observed in the CTX-M type [
3]. Five classes of integrons (
intI1,
intI2,
intI3,
intI4, and
intI5) were found to play major roles in the dissemination of antibiotic-resistance genes [
30].
Inhibitors used to block ESBL enzymes can help prevent the inactivation of β-lactam antibiotics. It is important to note that some β-lactamases may not be inactivated by some classical inhibitors such as clavulanate acid, sulbactam, and tazobactam [
31,
32]. Mechanisms of resistance in Gram-negative bacteria may also involve reduced membrane permeability through genomic mutations, decreased amounts of β-lactam antibiotics that can enter the cell, and a marked increase in antibiotic reflux from the periplasm to the exterior of the cell [
31].
5. Diagnostic Tools for Detection of ESBL
Routine screening along with rapid detection of ESBL-producing bacteria in laboratory and hospital settings is essential in the therapeutic approach and infection control to suppress any outbreaks. The Clinical and Laboratory Standards Institute (CLSI) recommends a two-step process for the detection of ESBL [
33]. The second part is only undertaken if the first step leads to a positive result. The first step involves a preliminary screening to detect sensitivity against some commonly used antibiotics such as cefotaxime, ceftriaxone, ceftazidime, or aztreonam. The second involves one of the available confirmatory tests to identify ESBL-producing organisms in the presence of β-lactamase inhibitor [
34]. Tests recommended by CLSI for the screening of ESBL include Kirby–Bauer disks and Vitek (sensitivity 92–93%). The confirmatory tests may be performed using a double-disk synergy test (DDST), combination disk method, or E-test ESBL strips. The combination disk method has a very high sensitivity (100%) for testing cefotaxime and cefepime, whereas the E-test has a comparatively lower sensitivity for testing cefotaxime and ceftazidime (71–73%) or cefepime (90%) [
22]. The phenotypic confirmatory method, double-disc synergy test, and E-test ESBL strip tests are easy to use in a laboratory setting, although none of these methods alone can identify all types of ESBL [
32]. It is worth mentioning that there are also guidelines set by the European Committee on Antimicrobial Susceptibility Test (EUCAST) for the detection of ESBL [
35].
In addition to phenotypic confirmatory tests, genotypic confirmatory tests are performed to identify certain enzymes and their variants released by ESBL producers through methods that include polymerase chain reaction (PCR) and nucleotide sequencing [
22]. Other methods that can be used include the broth dilution method (BDM) [
36], isoelectric point determination, DNA probes, the oligotyping method, PCR with restriction fragment length polymorphism analysis (PCR-RFLP), PCR with single-strand conformational polymorphism analysis (PCR-SSCP), and real-time-PCR [
32]. The Cica Beta Test 1/HMRZ-86/Chromogenic cephalosporin is a rapid kit test (generates results within 15 min) that is used for detecting ESBL in Gram-negative rods from primary culture [
37]. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) is another diagnostic tool that has been successfully used to detect ESBL [
38]. Recently, the NG-Test CTX-M MULTI, a rapid immunochromatography technique (lateral flow), has proven to be useful for the detection of CTX-M-type enzymes (groups 1, 2, 8, 9, and 25), followed by the rapid identification of
Enterobacterales in blood or urine samples using MALDI-TOF MS and flow cytometry [
39,
40]. Moreover, for the detection of SHV-positive
K. pneumoniae, PCR with CRISPR-LbCas12a has demonstrated excellent sensitivity and specificity, and it is recommended for use in a hospital setting as it provides results in about two hours [
41].
The applicability of these detection methods in different situations can have limitations due to the frequent mutations that lead to changes in patterns of ESBL subtypes. This can make diagnosis more complex and difficult.
6. Risk Factors and Mode of Transmission of ESBL-Producing Bacteria
Throughout the recent decades, ESBL-producing bacteria have been increasingly detected in hospital and community settings and have thus emerged as a serious health problem for humans and animals [
42,
43]. Reduced treatment options, complex infections, high mortality, and costly treatments are some of the major concerns for people infected with ESBL-producing organisms [
2]. In the intensive care unit (ICU), ventilator-associated pneumonia by ESBL-producing bacteria has been detected in hospitalized patients [
44]. In the human population, risk factors for hospital-borne colonization and infection with ESBL producers include prolonged hospital stay, use of hemodialysis, and intravascular catheters [
45,
46]. Community-borne infections may be related to many factors, including international traveling [
47]. In veterinary medicine, cephalosporins are frequently used for the treatment of bacterial infections in farm animals and pet animals [
48]. In South Asia, excessive use of over-the-counter (OTC) cephalosporins may be a major cause for increasing ESBL-producing bacteria in the animal population, which can further cocirculate in the human population via the food chain.
ESBL-producing enteric bacteria, such as
E. coli, non-typhoidal
Salmonella spp., and
Campylobacter spp., are zoonotic pathogens spread to humans through the food chain and can transiently colonize the human gut. Resistant commensal
E. coli acts as a vehicle to transmit genetic resistance determinants in the gut or via milk and meat. Resistant pathogenic
E. coli may subsequently cause urinary tract infections in vulnerable patients [
49]. In food-producing animals and pet animals, cephalosporin-resistant
E. coli and
Salmonella spp. cause high levels of mortality and morbidity which pose a risk of spread to humans via improper handling and inadequate cooking of food [
50]. CTX-M-14 is predominant in Asian countries and has been detected in humans, pets, and poultry [
19]. The CTX-M-15-producing human ST15 and ST101
K. pneumoniae clones have been reported to be widely disseminated in pets and horses [
51]. The
blaCTX-M-1 encoding IncI1 plasmids were commonly identified in
E. coli isolates from animals and humans along with various sequence types (STs) of
E. coli [
52].
In addition to causing intestinal and urinary tract infections, ESBL-producing Gram-negative bacteria, such as
E. coli,
Proteus spp.,
Pseudomonas aeruginosa, and
Klebsiella spp., can also be responsible for diabetic foot ulcers in individuals with underlying health conditions, potentially leading to amputation and death [
53]. A high incidence of sternal wound infections caused by ESBL-producing
E. coli has also been reported among patients in postoperative care after cardiac surgery [
54].
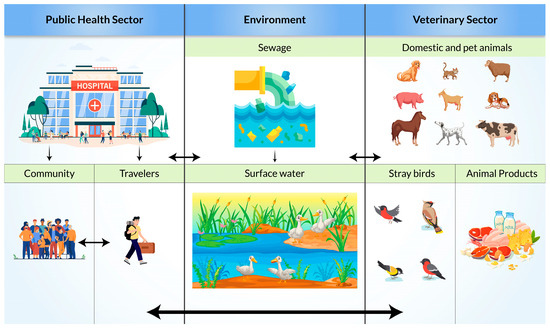
Resistance transmission routes for ESBL-producing bacteria are complex (
Figure 3). There are multiple direct and indirect transmission pathways from animal and inanimate sources to humans and from humans to animals and the environment [
55]. Extended-spectrum β-lactamase-producing enterobacterales isolates were reported in farmers and livestock (pig and poultry) [
56,
57]. Lower genomic ESBL diversity was also seen in farming communities than in the general and clinical populations. This can indicate a higher possibility of the exchange of ESBL genes between reservoirs in farming communities through close contact. Additionally, molecular similarities between human and environmental reservoirs may be an indication of transmission from human wastewater to surface water [
58]. Through the contaminated surface water, wild birds may get infected and act as vectors or even reservoirs for local dissemination [
59]. A high prevalence in migratory birds (17% in Pakistan, 17.3% to 38.18% in Bangladesh) is an indication that migratory birds can be a potential carrier for transmission in Asian countries [
60,
61,
62].
Figure 3. Possible transmission pathways of Extended-Spectrum β-Lactamase (ESBL)-producing bacteria.
7. Possible Therapeutic Options
Resistance towards certain commonly prescribed antibiotics, such as penicillin and cephalosporins, can make these drugs ineffective for treating infections. Carbapenems have been considered the main therapeutic option for the treatment of ESBL-E [
4]. The intravenous administration of carbapenem antibiotics is more efficient than its oral administration. However, injudicious overuse led to the emergence of carbapenem resistance.
Carbapenem-sparing strategies include the administration of non-carbapenem β-lactams (ceftolozane–tazobactam, ceftazidime–avibactam, temocillin, cephamycins, and cefepime) and non-β-lactams (aminoglycosides, quinolones, tigecycline, eravacycline, and fosfomycin).
For the non-carbapenem β-lactams, piperacillin–tazobactam (PTZ) combination is the most suitable alternative to carbapenems in the treatment of mild urinary tract infections (MIC ≤ 4 mg/L) [
63,
64]. Ceftolozane–tazobactam appears to be promising in the treatment of complicated intra-abdominal infections and complicated urinary tract infections [
65]. Tazobactam and Avibactam are β-lactamase inhibitors but tazobactam is affected by the inoculum effect [
63]. The effects of Tazobactam can be reduced by certain Gram-negative bacteria that are capable of releasing ESBL and AmpC beta-lactamases and can protect themselves through activation of efflux pumps and porin mutations. Avibactam has the ability to conserve the efficacy of ceftazidime against the highly prevalent β-lactamases, such as ESBL, and carbapenemases including OXA-48 and
K. pneumoniae carbapenemase (KPC). Hence, the ceftazidime–avibactam combination produces better results for the majority of MDR Gram-negative bacteria [
66]. Cephamycins include cefoxitin, cefotetan, moxalactam, cefmetazole, and flomoxef. Cephamycins are ineffective against AmpC cephalosporinases and porin mutations [
67]. Cefepime, a fourth-generation cephalosporin that is less hydrolyzed by AmpC lactamases and ESBL than other cephalosporins, could help against low-risk infections (MIC ≤ 2 mg/L) [
68]. However, there is a possible risk of mortality in some cases [
43]. Temocillin (b-a-methoxy-derivative of ticarcillin), a new drug, has a narrow spectrum that is limited only to Enterobacterales and is not easily degraded by various β-lactamases [
66].
For non-β-lactams, quinolones and aminoglycosides are good options. ESBL genes were shown to mediate quinolone resistance [
69]. The spreading of aminoglycoside-modifying enzymes can impact microbial susceptibility to aminoglycosides [
70]. Amikacin and the next-generation aminoglycoside plazomicin could be used for the treatment of urinary tract infections, including the treatment of acute pyelonephritis by plazomicin [
71,
72,
73]. Tigecycline has efficacy against ESBL-producing
E. coli and against multidrug-resistant (MDR) and extensively drug-resistant
Acinetobacter baumannii and
K. pneumoniae [
66,
74]. The tetracycline derivatives, Eravacycline and Omadacycline, have anti-ESBL activity that could be used to control Gram-negative bacteria [
75,
76]. Fosfomycin interferes with the synthesis of peptidoglycan by inhibiting phosphoenolpyruvate transferase and can be effective with urinary tract infections [
66]. Fosfomycin is efficient for the treatment of acute uncomplicated cystitis [
77]. Finally, monotherapy is generally less effective than combination therapy [
78].