Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Prostate cancer (PCa) remains a significant global health concern, being a major cause of cancer morbidity and mortality worldwide. Furthermore, profound understanding of the disease is needed. Prostate inflammation caused by external or genetic factors is a central player in prostate carcinogenesis.
- prostate cancer
- inflammation
- immune response
- leukocytes
1. Neutrophils
Neutrophils, originating from hematopoietic stem cells, are among the first immune cells recruited after an insult. They possess a short lifespan to prevent excessive tissue damage, owing to their high plasticity and robust effector response [150]. When recruited to a damaged area, neutrophils release proteases, including neutrophil elastase, neutrophil extracellular traps (NETs), and reactive oxygen species (ROS), which exacerbate damage and contribute to the development of chronic inflammation [151]. Under normal circumstances, neutrophils can shift their function towards immunosuppression, thus regulating the production of pro-inflammatory mediators. However, in disease states, this shift may not occur correctly, leading to the development of carcinogenesis [152]. Therefore, neutrophils serve as a crucial link between inflammation and cancer. A study has observed a correlation between low neutrophil counts and a positive PCa biopsy, while elevated neutrophil counts may indicate a benign prostate biopsy [153]. These results can predict the progression from an acute response, characterized by increased neutrophil levels, to a carcinogenic phenotype dominated by chronic inflammation [154]. Tumour associated neutrophils (TANs) have been reported in cancer-affected regions. TANs, along with regular neutrophils, secrete substantial amounts of matrix metalloproteinase (MMP)-9, which play a role in the degradation of the extracellular matrix and cancer progression [104].
TANs are a complex population in the tumour microenvironment, associated with poor outcomes in some PCa studies [72] and demonstrating antitumoral effects in others [155]. In vitro assays showed that coculture of human PCa cells in the presence of neutrophils leads to a reduction of cell growth via caspase activation [156]. These findings suggest that, as tumours progress, neutrophil cytotoxicity diminishes, allowing PCa to avoid neutrophil cytotoxic effects. Studies have linked neutrophils as crucial cells in PCa prevention. In bone metastatic PCa, there is an increased formation of neutrophils and NETs to limit the spread of infection and control metastasis [156]. The role of different inflammatory mediators produced by neutrophils and its role in cancer progression is summarized on Table 2.
Table 2. Involvement of different leukocytes and their associated cytokines in cancer and prostate cancer progression.
| Leukocyte | Inflammatory Mediator Produced |
Effect | Refs. |
|---|---|---|---|
| Neutrophils | MMP-9 | MMP-9 produced by TANs and neutrophils degrade ECM leading to cancer progression in human xenografts and Mmp9-knockout mice | [104] |
| GM-CSF and CXCL8 | KRAS stimulated the expression of GM-CSF and CXCL8 in neutrophils which modulates the tumour microenvironment towards cancer progression in mouse models of ovarian cancer | [105] | |
| IL-8/CXCR2 | Overexpression of CXCR2 in neutrophils promotes their attachment in lung cancer regions in a K-RAS mutant mouse model of lung cancer | [106] | |
| NDE, ROS, RNE | NDE, ROS, and RNE release from neutrophils lead to hMSH2-dependent G2/M checkpoint arrest and for the presence of replication errors in a co-culture model that mimics intestinal inflammation in ulcerative colitis | [107] | |
| ARG-1 | Release of ARG-1 from neutrophils inhibit CD3-mediated T cell activation and proliferation leading to cancer progression in classic Hodgkin Lymphoma patients | [108] | |
| NET | Increased neutrophil and NET formation intended attenuate the rate of metastatic PCa in bones in vitro and an in vivo mouse model | [71] | |
| IL-1 | IL-1RA from neutrophils leads to inhibition of senescence promoting cancer progression | [109] | |
| TNF-α | TNFR1 KO mice with depletion of TNF receptor 1 developed smaller tumours with attenuated proliferation and absence of metastasis | [110] | |
| Cathepsin G | Cathepsin G release from neutrophils increases cancer cell adhesion, and aggregation, and metastasis in breast cancer cells | [111] | |
| Basophils | IL-4 | Basophils from pancreatic ductal adenocarcinomas secrete IL-4 which induce GATA-3 expression in Th2 cells in patient samples and Mcpt8Cre mice | [112] |
| CCL3 | Basophils express CCL3 to negatively regulate the normal hematopoietic process in MCPT8-DTR mice and bone marrow samples from patients with CML | [113] | |
| CCL3/CCL4 | Basophil plays a role in tumour rejection by increasing CD8+ T cell infiltration promoted by CCL3 and CCL4 in HCmel12-, B16-, and 616-OVA-induced transgenic FOXP3.LuciDTR-4 mice melanoma | [114] | |
| VEGFA | Immunologic activation by VEGF-2 of basophils induced the release of VEGF-A which induce basophil chemotaxis | [115] | |
| HGF | HGF is expressed in CML basophils in KU812-induced CML cell line | [116] | |
| ANGPT | Basophils express ANGPT1 and ANGPT2 mRNAs | [117] | |
| Eosinophils | IL-2 | IL-2 activate Tregs and Th17 cells involved in the promotion of cancer in a mouse model of PCa, and fibrosarcoma and head and neck human cancer tissues | [118] |
| IL-4 | IL-4 production promotes tumour growth and interaction with TAMs in a pancreatic-induced cancer mouse model | [78] | |
| IL-6 | Increase of IL-6 correlated in patients with metastatic PCa compared with localized PCa | [119] | |
| IL-5 and CCL17 | Eosinophils increase after Sipuleucel-T treatment of patients with metastatic castration-resistant PCa correlated with increase of IL-5 and CCL17, survival and maximal T-cell proliferation responses | [120] | |
| IFN-γ | IFN-γ induced CD4+ T cells to eliminate MHC II-negative cancer cells | [121] | |
| TNF-α | TNF-α correlated with increased extension of PCa in samples from PCa patients | [119] | |
| TGF-α | Overexpression of TGF-α decreased latency, increased growth, and tumour size of bladder cancer rat model | [122] | |
| VEGF | VEGF associated with poor prognosis of human small-cell lung carcinoma | [123] | |
| GM-CSF | Expression of GM-CSF correlated with NF-κB activation in bone-metastatic tumour tissues from individuals with metastatic breast cancer | [124] | |
| Mast cells | Chymase | Chymase released from human mast cell release latent TGF-β-binding protein from the matrix | [125] |
| Histamine | Histamine inhibition from mast cells inactivate EMT and cholangiocarcinoma growth via inhibition of c-Kit signalling in Mz-ChA-1-induced cholangiocarcinoma mouse model and human Mz-ChA-1 cells | [126] | |
| TNF-α | TNF-α released from mast cells amplifies and activates the functionality of CD8+ dendritic cells in Mcpt5-CreTNFfl/fl mice | [127] | |
| IL-1β | Overexpression of IL-1β promoted tumour invasiveness and metastasis by inducing the expression of angiogenic genes and growth factors | [128] | |
| IL1, IL-4, IL-6 | Decreased cell growth and participates in tumour rejection in breast cancer cells | [129] | |
| IL-8, IL-10 | Mast cell-derived IL-8 and IL-10 act as tumour suppressors contributing to tumour cell growth | [130,131] | |
| PGD2 | PGD2 secretion from mast cells attenuates angiogenesis in a Lewis lung carcinoma mouse model | [132] | |
| Macrophages | IL-1β | IL-1β induced Snail stabilization in Snail/MCF7 cells and this effect was dependent on cell types and IL-1β concentration | [133] |
| IL-8 | IL-8 produced by macrophages induce EMT in hepatocellular carcinoma samples via JAK2/STAT3/Snail pathway | [134] | |
| TNF-α | TNF-α induces the stabilization of Snail in a non-phosphorylated, functional form and thus enhances cell migration and invasion dependent on NF-κB activation | [133] | |
| TGF-β | TGF-β induced EMT phenotypes in A549 cells, including changes in cell morphology and induction of mesenchymal marker expression in part by NF-κB signalling | [135] | |
| MMP-2, MMP-9 | Macrophage-derived MMP-9 and MMP-2 related with fibrous capsule leading led to the migration and invasion of hepatocellular carcinoma cells in human samples | [136] | |
| CHI3L1 | M2 macrophage-secreted CHI3L1 promoted metastasis of gastric and breast cancer cells in vitro and in vivo; CHI3L1 interaction with IL-13Rα2 upregulates MMPs | [137] | |
| IL-23/IL-17 | Upregulation of IL-23 leads to tumour growth and progression and development of a tumoral IL-17 response which promote tumorigenesis in a mouse model of colorectal cancer | [138] | |
| IL-6 | TAM-derived IL-6 highly expressed in Hepatocellular carcinoma patients, which is correlated with disease grades and tumour progression | [139] | |
| PDGF | PDGF release from macrophages mediates the recruitment of pericytes in human melanoma cell lines and OCM-1-induced melanoma mouse model | [140] | |
| T cells | IL17-A | Inhibition of IL17-A release by Th17 cells prevent development of microinvasive PCa in mouse models | [141] |
| IL-17 | IL-17-producing T cells can promote PCa progression by enhancing inflammation and angiogenesis | [69] | |
| PD-1 | A high percentage of CD8+ T cells express PD-1 in PCa samples, which impair an effective immune response by these cells | [44] | |
| IL-3 | IL-3 expressed by T cells increase the recruitment of basophils and immune cells into the tumour microenvironment, which is linked with a poor survival | [112] | |
| IFN-γ | IFN-γ can enhance antigen presentation and contribute to immune surveillance in PCa | [142] | |
| TNF-α | TNF-α produced by activated T cells regulated apoptosis, angiogenesis, and inflammation in PCa | [143] | |
| TGF-β | TGF-β produced by T cells can suppress and promote tumour growth in PCa depending on the signal it receives | [144] | |
| B cells | Lymphotoxin | Lymphotoxin lead to CXCL13/IKKa/STAT3/E2F1/BMI1 (RNF51) activation, ubiquitination of histones within PCa cell nuclei and proliferation of androgen-deprived PCa cells in castration-resistant PCa in mice | [145] |
| TGF-β | Secretion of TGF-β by B-cells leads to anergy of CD8+ T cells | [146] | |
| IL-2 | IL-2 and IL-4 produced by B cells regulate the Th2 memory responses to Heligmosomoides polygyrus (Hp) in chimeric mice lacking AID infected with Hp | [147] | |
| IL-6 | Chimera’s mice with B cell lack IL-6 have impaired Th1 and Th17 responses to Salmonella | [148] | |
| GABA | B cell-derived GABA promotes monocyte differentiation into anti-inflammatory macrophages that secrete IL-10 and inhibit CD8+ T cell killer function in mice | [149] |
ANGPT: Angiopoietin, ARG: Arginase, CCL: CC chemokine ligand, CML: Chronic myeloid leukaemia, CXCL: Chemokine (C-X-C motif) ligand, ECM: Extracellular matrix, EMT: Epithelial-mesenchymal transition, FOXP3: Forkhead box subfamily 3, GABA: γ-amino butyric acid, GM-CSF: Granulocyte-macrophage colony-stimulating factor, HGF: Hepatocyte growth factor, IFN-γ: Interferon-gamma, IL: Interleukin, IL-13Rα2: Interleukin-13 receptor α2 chain, IL-1RA: IL-1 receptor antagonist, MHC: Major histocompatibility complex, MMP: Matrix metalloproteinase, NDE: Neutrophil-derived elastase, NET: Neutrophil extracellular traps, PD: Programmed death, PDGF: Platelet-derived growth factor, PGD: Prostaglandin, RNE: Reactive nitrogen species, ROS: Reactive oxygen species, TAM: Tumour-associated macrophages, TAN: Tumour-associated neutrophils, TGF: Transforming growth factor, TNF: Tumour necrosis factor, VEGF: Vascular endothelial growth factor.
2. Basophils
Basophils constitute approximately 1% of circulating white blood cells and serve as protectors against allergens, pathogens, and parasites. In an inflammatory context, basophils can migrate to inflammatory regions and promote M2-like macrophage polarization, highlighting the disparity in function between circulating and resident basophils [157].
Elevated basophil and basophil-to-lymphocyte ratio were associated with a poor outcome in metastatic hormone sensitive PCa [158]. Epithelial-derived pro-inflammatory cytokines including interleukin (IL)-33, IL-18, granulocyte-macrophage colony-stimulating factor (GM-CSF), and growth factors including IL-3, IL-7, transforming growth factor-beta (TGF-β), vascular endothelial growth factor A (VEGF) promote activation of basophils [159]. Several studies demonstrated that activated basophils can secrete different cytokines involved in PCa including IL-4, which promotes tumour-promoting Th2 inflammation [112,160] and M2 macrophage polarization related to a poor prognosis [161], IL-13 [157], and tumour necrosis factor-alpha (TNF-α) [162]. Studies also suggested the role of basophils in angiogenesis. Basophils release high amount of VEGFA, a potent proangiogenic molecule [115]. Basophils are a source of hepatocyte growth factor (HGF), a powerful angiogenic factor in tumours [116]. Human basophils also express angiopoietins (ANGPT) 1 and ANGPT2 mRNAs which are involved in vascular permeability [117]. Other studies showed the protective role of basophils in cancer development [114]. Low levels of circulating basophils correlated with higher size and extend of the tumour, higher number of lymph nodes and poor survival in colorectal cancer patients [163]. The effects of different inflammatory molecules produced by basophils in cancer are described in Table 2.
While most data on the role of basophils in cancer progression pertains to cancers other than PCa, additional studies are needed to elucidate the mechanisms by which basophils influence PCa.
3. Eosinophils
Eosinophils constitute 1–4% of white blood cells and play a vital role in maintaining homeostasis and defending the host against infectious agents. They originate from multipotent CD34+ progenitors in the bone marrow [164]. Under normal conditions they are located in spleen, lymph nodes and thymus. When activated, they have the capacity to modulate the immune response, including the phenotype of T cells. [165]. The migration and recruitment of eosinophils to the tumour microenvironment are orchestrated by eotaxins, namely CC chemokine ligand (CCL)11, CCL24, CCL26, and CCL5 which activate the CCR3 receptor, highly expressed on eosinophils [166]. Eosinophils secrete cytotoxic granules including eosinophil cationic protein (ECP), major basic protein (MBP), eosinophil derived neurotoxin (EDN) and eosinophil peroxidase (EPO). Additionally, they release pro-inflammatory mediators such as IL-2, IL-4, IL-5, TGF-β, TNF-α, GM-CSF, and interferon-gamma (IFN-γ) [167]. IL-5 is a key mediator for eosinophil growth, differentiation, and activation [168]. Moreover, eosinophils express adhesion molecules CD11a/CD18, allowing them to interact with tumour cells, indicating their role in cancer progression [169]. Histological analysis of PCa samples revealed an increase in eosinophils compared to healthy controls in correlation with age and Gleason score [170].
On the other hand, activated eosinophils inhibited PCa cell growth through upregulation of E-cadherin, a metastasis suppressor molecule [171]. Evidence demonstrated that incubation of PCa cell lines with activated eosinophils inhibited cell growth [172]. Treatment of patients with metastatic castration-resistant PCa with Sipuleucel-T led to an increase in eosinophil counts, correlated with improved survival and enhanced maximal T-cell proliferation responses [120]. The role of different cytokines and chemokines produced by eosinophils are labelled in Table 2.
To advance the understanding of eosinophils in the tumour microenvironment and their interactions with other immune cells, it is crucial to improve the technological detection of eosinophils and discover novel biomarkers for defining eosinophil subpopulations. This will provide insights into their ability to modulate various cells in different cancers, including PCa, and their role in cancer progression.
4. Mast Cells
Mast cells derive from CD34+/CD117+ hematopoietic stem cells in the bone marrow and they undergo maturation within target tissues [173]. Besides KIT activation, which is essential for mast cell development, several cytokines, including IL-3, IL-4, IL-9, IL-10, IL-33, and TGF-β, influence their growth and survival [174]. Mast cells exhibit significant plasticity and can adopt various phenotypes depending on the host’s genetic background and local or systemic factors [175]. These cells are characterized by the presence of numerous granules rich in histamine and heparin. Upon activation, mast cells can degranulate and release inflammatory mediators to combat pathogens [176]. This response leads to the synthesis of specific cytokines, including anti-inflammatory TGF-β, IL-10, as well as the proinflammatory associated cytokines IL-4, IL-6, and IFN-γ [131]. Evidence demonstrated that mast cells are present in several tumours [177,178]. Zadvornyi et al. [179] demonstrated that increased mast cell infiltration and degranulation were associated with malignancy of PCa. Another study demonstrated the potential of mast cells to promote PCa cell proliferation and epithelial mesenchymal transition which is linked with invasion and metastasis [180]. A study dissected that infiltrating mast cells in PCa suppress androgen receptor-MMP signalling promoting PCa cell invasion [181]. Intratumoral mast cell tryptase+/chymase+/CD117+ phenotype was founded in malignant PCa samples [182]. Additionally, a high extratumoral mast cell count was linked with a high risk of biochemical recurrence and PCa metastasis [183].
Release of IL-1, IL-4 and IL-6 from mast cells was associated with elimination of tumour cells and rejection of tumours [129]. Other studies demonstrated that IL-1 is linked with tumour growth, angiogenesis, macrophage recruitment and metastasis [128]. Another study in human PCa samples associated higher mast cell infiltrates with a better PCa prognosis [184]. Moreover, mast cells can contribute to angiogenesis inhibition through secretion of prostaglandin D2 (PGD2) [132]. Table 2 dissects the role of each cytokine released by mast cells on cancer progression.
5. Macrophages
Macrophages are vital phagocytic cells integral to the innate immune response. The primary sources of macrophages are monocytes, which circulate in the blood, and tissue-resident macrophages originating from the yolk sac. These cells are recruited and activated by the specific microenvironment in which they operate. In the context of the tumour microenvironment, macrophage activation plays a significant role in influencing tumour development, progression, metastasis, immune regulation, and angiogenesis [79].
Activated macrophages can be classified into two main categories: M1-like macrophages, which promote inflammation to combat pathogen invasion and cancer, and M2-like macrophages, which are associated with tissue repair and support tumour progression [185]. M1-like macrophages secrete proinflammatory mediators including IL-12, TNF-α, chemokine (C-X-C motif) ligand (CXCL)-10, IFN-γ, and nitric oxide synthase (NOS), whereas M2-like macrophages produce anti-inflammatory IL-10, IL-13, and IL-4, arginase-1, the mannose receptor CD206, and scavenger receptors [186,187]. The polarization of macrophages into M1 or M2 phenotypes depends on the signals present in the microenvironment.
Research has demonstrated that tumour-associated macrophages (TAMs) often acquire a tumour-suppressive M2-like phenotype, contributing to the development of carcinogenesis [188]. The release of IL-1β, IL-8, TNF-α, TGF-β [133,134,135], MMP-2, and MMP-9 [136] by macrophages is involved in epithelial-mesenchymal transition (EMT), which promotes cancer cell invasion and metastasis. It is believed that metastatic processes could not be a late event in tumour progression. The primary tumours could prime the metastatic organ before tumour cell arrival. Macrophages are involved in the formation of this pre-metastatic niches. They are mobilized to bloodstream and are then clustered in these regions by CCL2, CSF-1, VEGF, platelet-derived growth factor (PDGF), TNF-α, and TGF-β [189,190]. The role of inflammatory mediators produced by macrophages in cancer is discussed in Table 2.
Given their ability to modulate the tumour microenvironment, the strategic targeting of macrophages has emerged as a promising approach in the development of new strategies for treating PCa. This approach holds the potential to provide novel and effective therapeutic alternatives for PCa patients.
6. T Cells
T cells play a crucial function in the adaptive immune response and were identified as key cells in the PCa tumour microenvironment [191]. These cells originate from the bone marrow and comprise various subtypes, including CD8+ T cells, CD4+ T cells, Th17 and regulatory T cells (Tregs). CD8+ T cells, known as cytotoxic T cells, exert their effects directly on infected cells. They predominantly secrete immune mediators such as IFN-γ, TNF-α, IL-2, granzyme, and perforin. In contrast, CD4+ T cells, or T helper cells, orchestrate immune responses by activating B cells and CD8+ T cells. They can be further categorized into Th1, Th2, Th17, and regulatory T cell (Treg) subsets. Th1 cells release proinflammatory cytokines, including IFN-γ, TNF-α, and IL-2, while Th2 cells secrete IL-4, IL-5, IL-13, IL-25, and IL-10, driving an anti-inflammatory response [192]. Th17 cells, characterized by secretion of IL-17A, IL-17F, IL-21, and IL-22, have been implicated in PCa metastasis. Studies have shown that the loss of Th17 function can hinder the development of microinvasive PCa in murine models [141]. Tregs were firstly defined as CD4+CD25high cells and were found to be increased in PCa patients [193]. These cells play a vital role in discriminating self from foreign antigens and can either activate or suppress immune responses. Tregs release immunosuppressive cytokines, including IL-10, TGF-β, IL-35, CD39, CD73, and indoleamine 2,3-dioxygenase (IDO) [194]. The immunosuppressive functions of Tregs favour tumour progression, and elevated Treg levels in PCa patients have been associated with poorer survival outcomes [80]. CD8+ T cells have been associated with a good prognosis and a study identified CD8+CD44+ population as important for reducing the tumour burden [195]. On the other hand, accumulation of CD4+ T cells in the PCa tumour microenvironment was associated with a poor survival [75]. In fact, this population is increased in PCa patients in comparison to healthy controls. An increase of CD4+ T cells was also associated with increased chemo-resistance to docetaxel in PCa cells [76]. Later, Kaur et al. [196] showed an association between increased transcription factor FOXP3+ Treg cells and risk of metastasis. Another study identified CD4+FOXP3+ Tregs and CD8+FOXP3+ Tregs increased in PCa samples and associated with increased risk of death [77,197].
Understanding the intricate roles of T cells, including their subtypes and cytokine profiles (Table 2), is essential for deciphering the complex immune landscape within the PCa microenvironment.
7. B Cells
B cells originate in the bone marrow and have the ability to migrate to the spleen and lymph nodes. Naïve B cells undergo activation into plasma cells in response to specific antigens during their development, leading to proliferation and differentiation. The maturation of B cells results in changes to their epitopes, and their characterization relies on CD markers such as CD19, CD20, CD21, CD40, and CD79b [198]. B cells can influence the tumour microenvironment through various mechanisms, including antibody presentation, antibody production, and cytokine secretion [199]. Studies have demonstrated that B cells activate CD4+ T cells, resulting in the accumulation of T cells in the tumour microenvironment and the differentiation of CD4+ and CD8+ T cells into distinct phenotypes [200]. Interactions between CD20+ B cells and T cells in the tumour microenvironment have been shown to impact the protective function of T cells [201]. Regulatory B cells (Bregs) are frequently found in advanced hepatocellular, gastric, and PCa, suggesting their potential influence on tumour development and progression [73,202,203]. Bregs are associated with an anti-immune function, as they release immunosuppressive molecules such as IL-10, IL-35, and TGF-β, which hinder the activity of T cells [146,204,205,206].
Literature data suggest that the infiltration of B cells increases the risk of adverse events in prostate carcinogenesis and malignancies. The effect of inflammatory modulators produced by B cells on tumour progression is described in Table 2.
8. Overall Remarks
Overall, IL-1, IL-6, IL-2, IL-4, IL-7, IL-8, IL-10, IL-17, IL-23, TNF-α, TGF-β, IFN-γ, VEGF, and GM-CSF are the main inflammatory mediators involved in PCa (Figure 3).
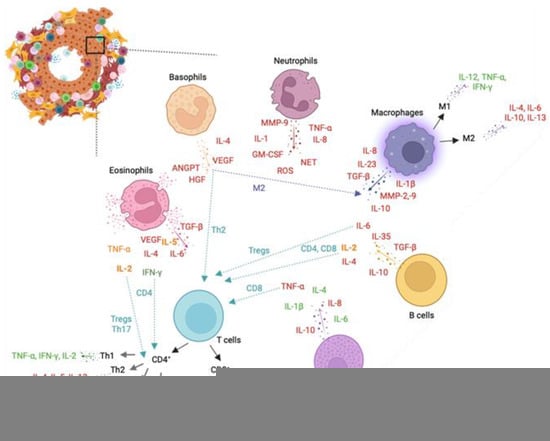
Figure 3. Leukocytes and leukocyte-mediated cytokines involved in prostate cancer (PCa). Representation of key cells in the prostate tumour microenvironment. Neutrophils mainly release matrix metalloproteinase (MMP)-9, interleukin (IL)-1, granulocyte-macrophage colony-stimulating factor (GM-CSF), reactive oxygen species (ROS), neutrophil extracellular traps (NETs), IL-8, and tumour necrosis factor (TNF)-α, all promoting PCa progression. Basophils promote activation of the M2-like phenotype in macrophages and Th2 response in T cells and release pro-tumour IL-4, VEGF, ANGPTs, and HGF cytokines. Eosinophils secrete pro-tumoral transforming growth factor (TGF)-β, IL-6, vascular endothelial growth factor (VEGF), and IL-4 cytokines and anti-tumoral interferon (IFN)-γ cytokine. Release of TNF-α, IL-2, and IL-5 from eosinophils can modulate a pro-tumoral or anti-tumoral activity depending on cell signals. IL-2 and IFN-γ stimulates activation of, respectively, Tregs/Th17 and CD4+ T cells. Mast cells secrete pro-tumoral TNF-α, IL-8, and IL-10 and anti-tumoral IL-1β, IL-4, and IL-6 cytokines. Tumour-associated macrophages secrete IL-1β, IL-8, IL-10, IL-23, MMP-2, MMP-9, and TGF-β, impacting tumour progression. Macrophages can shift to pro-inflammatory M1-like or anti-inflammatory M2-like phenotypes, influencing tumour outcomes. M1-like macrophages release IL-12, TNF-α, and IFN-γ, while M2-like macrophages secrete IL-4, IL-6, IL-10, and IL-13. T cells encompass CD4+ T cells (Th1, Th2, Th17, and Tregs) and CD8+ T cells, with Th1 having a pro-inflammatory response. Th2, Th17, and Tregs contribute to tumour progression. CD8+ T cells secrete IFN-γ, TNF-α, and IL-2, associated with a favourable prognosis in PCa. B cells release pro-tumoral IL-4, IL-6, IL-10, and TGF-β cytokines and the intermediate IL-2 cytokine. IL-2 and IL-4 stimulate CD4 and CD8 responses, while IL-6 activates Tregs in PCa. Green-coloured cytokines support tumour resolution and a positive prognosis. Red-coloured cytokines promote tumour growth, proliferation, and metastasis. Orange-coloured cytokines can trigger a pro- or anti-tumoral response in PCa. ANGPT: Angiopoietin, GM-CSF: Granulocyte-macrophage colony-stimulating factor, HGF: Hepatocyte growth factor, IFN: Interferon, IL- Interleukin, MMP: Matrix metalloproteinase, NETs: Neutrophil extracellular traps, PCa: Prostate cancer, ROS: Reactive oxygen species, TGF: Transforming growth factor, TNF: Tumour necrosis factor, Tregs: Regulatory T cells, VEGF: Vascular endothelial growth factor.
IL-1 and IL-6 promote cancer growth, proliferation, and progression [207,208]. IL-1 is increased in PCa and induces immunosuppressive function of mesenchymal stem cells [209,210]. IL-6 is increased in PCa, induces EMT and metastasis, increases the expression of androgen receptor, and induces infiltration of T cells into the tumour microenvironment [211,212]. IL-2 has been found to stimulate Tregs, with some studies associating it with tumour growth and progression, while others suggest its potential anti-tumour activity [213]. IL-4 increases the expression of androgens, activates the JNK pathway, and promotes tumour progression [214]. IL-7 induces EMT and cancer metastasis [215]. IL-8 stimulates proliferation of prostate stromal cells, regulates the expression of MMPs, promotes PCa progression, angiogenesis, and metastasis [216]. IL-10 inhibits anti-tumour responses and regulates androgen signalling, promoting cancer metastasis [217]. IL-17 promotes PCa growth, angiogenesis, and metastasis [218], increases the expression of programmed death-ligand 1 (PD-L1) and COX-2 and induces the release of IL-6 and IL-8 [219]. IL-23 regulates the androgen response and Th17 survival [217]. TNF-α and TGF-β are able to promote PCa progression and metastasis [220]. TNF-α upregulates the expression of PD-L1, and its control is indicative of tumour cell behaviour [219,221]. TGF-β induces EMT, inhibition of anti-tumour activity, reduces the expression of major histocompatibility complex (MHC)-I, regulates angiogenesis, the formation of the premetastatic niche, and metastasis in bone [222,223,224]. IFN-γ induces the release of IL-6 and IL-8 and promotes anti-tumour response [225]. VEGF contributes to angiogenesis, formation of premetastatic niche, tumour microenvironment remodelling, tumour invasion, and metastasis [224,226]. GM-CSF stimulates leukocytes and increases tumour antigen presentation to effector T cells [227,228].
The activity of interleukins is primarily modulated through the Janus Kinase/signal transducers and activators of transcription (JAK/STAT) pathway. This signalling pathway is integral to normal development, cellular homeostasis, cell proliferation, differentiation, and apoptosis [229]. Ligand binding initiates the multimerization of receptor subunits, leading to the activation of the JAK/STAT pathway and the transmission of signals through the phosphorylation of receptor-associated JAK tyrosine kinases. Consequently, activated JAKs induce the phosphorylation and activation of STATs. This phosphorylation prompts the dimerization of STATs via their conserved SH2 domain, subsequently allowing them to enter the nucleus. Within the nucleus, STATs bind to specific DNA sequences, either stimulating or suppressing the transcription of target genes [230]. It was reported that JAK/STAT3 inhibition suppress PCa cell growth and increases apoptosis [231]. BRCA1 via JAK1/2 and STAT3 phosphorylation can induce cell proliferation and inhibit cancer cell death [232]. The androgen receptor could also activate JAK/STAT3 and stimulate cell proliferation and antiapoptotic effect increasing tumour invasion [233,234].
NF-κB is a transcription factor predominantly activated by cytokines such as TNF-α in PCa. In androgen-dependent PCa, IL-6 and VEGF stimulates the increase of the expression of NF-κB [235]. NF-κB targets a transcription regulatory element of PSA and correlates with cancer progression, chemoresistance, and PSA recurrence [236].
Growth factors including VEGF, epidermal growth factor (EGF), insulin-like growth factor (IGF)-1, HGF, and TGF-β are key players in the receptor tyrosine kinase (RTK) signalling pathway. These growth factors activate the extracellular signal-regulated kinases (ERK)/MAPK or PI3K/AKT/mTOR mechanisms [237]. Growth factor receptors possess RTK activity, and their binding to ligands leads to the activation of transcription factors, resulting in the altered expression of genes associated with cell growth, proliferation, and survival [238]. IGF-1 functions as a positive growth-promoting signal transduction pathway, while FGF plays a dual role as a positive growth factor and an angiogenic growth factor. On the other hand, TGF-β serves as a negative growth factor, regulating cell differentiation and proliferation [239]. Studies demonstrated that alterations on the expression of TGF-β, EGF and their receptors correlates with PCa progression and biochemical recurrence [240,241]. The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway is often upregulated due to the loss of the tumour suppressor PTEN, which negatively regulates the PI3K/AKT pathway [242]. It has been demonstrated that the aberrant PI3K/AKT pathway disturbs the action of ERKs, thereby supporting androgen receptor-independent growth in PCa [243]. Overexpression of growth factors promotes the activation of Ras and MAPK pathways [244]. Upon activation, MAPKs phosphorylate transcription factors such as c-Jun, c-Fos, ATF2, and p53. Additionally, ERK or p38 MAPKs can activate MAPK interacting protein kinases 1 and 2 (MNK1 and MNK2), which controls signals involved in mRNA translation [245]. Interestingly, MNKs have been found to be overexpressed in PCa [246].
Inflammatory signalling plays a significant role in the development and progression of PCa. Considering these findings, therapeutic strategies targeting inflammatory signalling pathways in PCa may help manage the disease and potentially improve outcomes.
Ongoing research is exploring new treatments and strategies, especially those utilizing natural bioactive compounds, to mitigate the severe side effects, radiotherapy resistance, and recurrence of PCa.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11123140
This entry is offline, you can click here to edit this entry!
