Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Migraines display atypical age dependence, as the peak of their prevalence occurs between the ages of 20–40 years. With age, headache attacks occur less frequently and are characterized by a lower amplitude. However, both diagnosis and therapy of migraines in the elderly are challenging due to multiple comorbidities and polypharmacy. Dietary components and eating habits are migraine triggers; therefore, nutrition is a main target in migraine prevention. Several kinds of diets were proposed to prevent migraines, but none are commonly accepted due to inconsistent results obtained in different studies. The ketogenic diet is featured by very low-carbohydrate and high-fat contents. It may replace glucose with ketone bodies as the primary source of energy production. The ketogenic diet and the actions of ketone bodies are considered beneficial in several aspects of health, including migraine prevention, but studies on the ketogenic diet in migraines are not standardized and poorly evidenced. Apart from papers claiming beneficial effects of the ketogenic diet in migraines, several studies have reported that increased levels of ketone bodies may be associated with all-cause and incident heart failure mortality in older adults and are supported by research on mice showing that the ketogenic diets and diet supplementation with a human ketone body precursor may cause life span shortening. Therefore, despite reports showing a beneficial effect of the ketogenic diet in migraines, such a diet requires further studies, including clinical trials, to verify whether it should be recommended in older adults with migraines.
- migraine
- headache
- ketogenic diet
- elderly
- ketone bodies
- β-hydroxybutyrate
1. The Ketogenic Diet
Although historically, the ketogenic diet (keto diet) was aimed at treating epilepsy, nowadays it is thought to help weight loss and consequently fight obesity-related diseases, including type 2 diabetes mellitus, hyperlipidemia, heart disease, and cancer [11,40,41].
A ketogenic diet is basically characterized by a high intake of fat, moderate protein intake, and low intake of carbohydrates. Typically, it consists of 70–80% of fats, 15–20% of proteins, and 5–10% of carbohydrates [41].
The limitation in carbohydrate intake may result in decreased insulin secretion, a transition into a catabolic state, and the induction of gluconeogenesis and ketogenesis [42,43]. Gluconeogenesis leads to the internal production of glucose, mainly in the liver, from pyruvate, lactic acid, glycerol, and glucogenic amino acids, such as alanine, arginine, asparagine, cysteine, glutamine, aspartic acid, glutamic acid, and glycine [44]. However, if the internal production of glucose is not sufficient to provide the necessary amount of ATP, the metabolic pathway switches to ketogenesis, which produces ketone bodies to replace glucose as the main supply of energy. Ketone bodies, including β-hydroxybutyrate, acetoacetate, and acetone, are synthesized in the liver and then oxidized outside the liver (Figure 3). They are transformed into acetyl coenzyme A in mitochondria and enter the tricarboxylic acid cycle and oxidative phosphorylation to produce ATP [45].
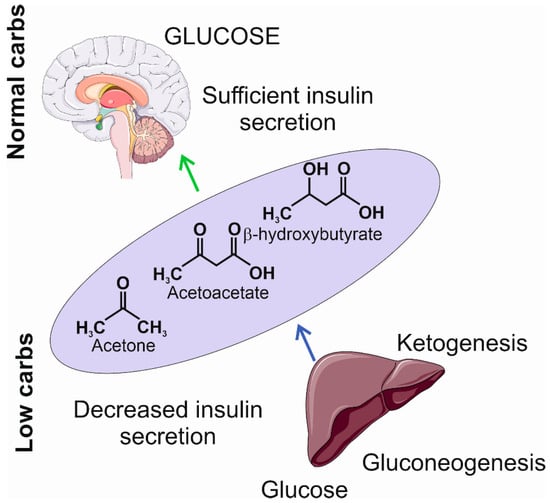
Figure 3. The insufficient levels of carbohydrates induce a decrease in insulin secretion and the activation of gluconeogenesis and ketogenesis. The former produces glucose at a low level; the latter produces ketogenic bodies, including acetone, acetoacetate, and β-hydroxybutyrate, that may become an additional source of energy in the brain. The light violet ellipse symbolizes circulation from which ketone bodies penetrate the brain as they can cross the blood–brain barrier. Parts of this figure were drawn using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/ (accessed on 1 November 2023)).
Low insulin secretion during ketogenesis results in decreased fat and glucose accumulation, which, along with other changes, leads to increased breakdown of fat, resulting in fatty acid production [45]. When the ketogenic diet is applied, the accumulation of ketone bodies is sustained, and the organism reaches the state of nutritional ketosis, which is generally considered as safe and may be beneficial for the organism [41]. However, the overproduction of ketone bodies may lead to ketoacidosis, which is a life-threatening condition leading to blood acidosis [46]. Ketone bodies produce more ATP than equivalent amounts of glucose, e.g., 100 g of glucose yields 8.7 kg of ATP, while the same amount of acetoacetate, a primary product of fatty acid metabolites, yields 9.4 kg of ATP [47].
2. The Ketogenic Diet and Ketone Bodies in Migraines
The International Ketogenic Diet Study Group lists migraines as a neurological disease that can be potentially beneficially targeted via the ketogenic diet [59].
Migraines are associated with increased energy demands and decreased energy production by the migraine-affected brain [25]. Therefore, if the source of energy is limited, e.g., by low levels of carbohydrates typical for the ketogenic diet, this additionally deepens the energetic crisis typical for the brain in migraines. Therefore, how may the ketogenic diet exert a beneficial effect in migraines? The answer may not only lie in a switch between the utilization of glucose and ketone bodies as sources of energy but also signaling functions of ketone bodies.
However, independently of a source, energy production in the brain occurs in mitochondria, so they stand in a central position in the energetic aspect of migraine pathogenesis. The role of mitochondrial biogenesis and functioning in migraine pathogenesis has been debated for years, but so far, a definitive conclusion has not been drawn. Mitochondria, due to their role in generating energy and in the production of RONS, are involved in many physiological and pathological conditions; thus, attributing a significant role of mitochondria to migraine pathogenesis exclusively due to energy crises and oxidative stress would be a truism, important in many other pathologies. As mentioned, mitochondrial transmission may contribute to up to a three times higher prevalence of migraines in women than in men, although studies on migraine-specific loci in mtDNA are inconclusive [60].
The strongest support for the role of mitochondria in migraine pathogenesis likely comes from the association between mitochondrial diseases and migraines [61]. Stratification of migraine cases into specific types of mitochondrial diseases implied that migraines may not be just an association syndrome in a specific mitochondrial disease but, instead, might express a universal vulnerability of the CNS to some factors associated with mitochondrial dysfunction accompanying that disease. Riboflavin (vitamin B2) is a central component of flavin mononucleotide and flavin adenine dinucleotide, which are important in critical mitochondrial processes, including the metabolism of amino acids, purines, and fatty acids [62]. On the other hand, riboflavin was reported to show a preventive action against migraines in 8 randomized controlled clinical trials and 1 clinical trial with 673 subjects [63]. Riboflavin, similar to other B vitamins, is needed for the breaking down and processing of ketone bodies [64]. In turn, ketone bodies may improve mitochondrial functions through different mechanisms, including the attenuation of an increased level of H3_K27me2K36me1 and mitochondrial dysfunction followed by repressed peroxisome proliferator activator gamma coactivator 1 alpha (PGC-1α) downregulation [65]. In another study, a decreased level of mRNA expression of the PPARGCA1 gene encoding the PGC-1α protein along with fewer mtDNA copies were observed in a rat model of migraines [66]. Therefore, mitochondria can present an important link between the ketogenic diet and migraines, and PGC-1α may play a mechanistic role in this link.
Oxidative stress is involved in the pathogenesis of many disorders, but for a definite majority of them, the precise mechanism of that involvement is not known. The role of oxidative stress in migraine pathogenesis may be underlined by several mechanisms, including energy generation in the brain to amend energy deficits typical for migraine-affected brains, neuroinflammation, impaired DNA damage response, and others [67]. The ketogenic diet was reported to ameliorate oxidative stress and improve mitochondrial functions in rats with traumatic brain injury [68]. The former effect was mainly underlined by the overexpression of the antioxidant enzymes nicotinamide adenine dinucleotide (NADH), dehydrogenase quinone 1 (NQO1) and superoxide dismutase 1/2 (SOD1/2), whereas the latter effect was indicated by an increase in the activity of mitochondrial respiratory complexes II and III. In a later work, it was shown that the ketones β-hydroxybutyrate and acetoacetate exerted a beneficial effect on rat mitochondria isolated from neocortical neurons and exposed to a high concentration of calcium [69]. A combination of both ketone bodies decreased the death of the neurons and prevented alterations in the neur onal membrane induced by glutamate. Both ketones decreased the production of mitochondrial RONS and increased NADH oxidation in the mitochondrial respiratory chain. Therefore, ketone bodies may decrease RONS formation in neurons subjected to glutamate excitotoxicity by raising the NAD+/NADH ratio and increasing mitochondrial respiration in neocortical neurons. This mechanism may be important in migraine protection by ketones through the restoration of bioenergetic functions challenged by oxidative stress [25,70,71].
Cortical spreading depolarization (CSD) is a wave of neurons and neuroglia depolarization within the cerebral cortex and is attributed to migraine aura [72]. A protective effect of a ketogenic diet enriched with middle- and long-chain triglycerides on the CSD propagation was observed in rats, reflecting a decrease in brain cerebral excitability, typical for migraines [72].
There are several papers that have dealt with ketogenic therapies to prevent migraines or ameliorate headache attacks (reviewed in [59]). However, these ketogenic therapies are not standardized, and it is difficult to compare their results. The ketogenic diet we described in the previous sections can be considered as a “classical ketogenic diet”, typically with the 3–4:1 ketogenic ratio (3–4 mass units of fat per 1 mass unit of protein and carbohydrate). In addition to this, the low-calorie ketogenic diet, the modified Atkins diet, and a diet enriched with exogenous source(s) of β-hydroxybutyrate are considered dietary interventions that induce ketosis. Several randomized clinical trials evaluating the role of ketogenic interventions in migraines were performed. The first such trial by Di Lorenzo et al. included overweight migraine patients during a weight loss intervention [73]. Therefore, these results cannot be directly related to this review, as (1) migraines and overweight/obesity are important and autonomous issues, and (2) obesity in the elderly is a problem, but it is somehow overwhelmed by unexplained weight loss, which is typical for this group of patients. A recent review by Neri et al. presented clinical trials and other studies of migraine patients with ketogenic interventions resulting in the production of endogenous ketone bodies or the administration of exogenous ketone bodies [74]. That review also pointed at the reduction in the number of attacks and their severity in most clinical trials on the ketogenic diet in migraines [74]. Moreover, only a few studies reported mild side effects, but not all of them addressed that issue. However, the authors pointed that many studies were of moderate-to-low quality and reported inconsistent results, resulting in a poor recommendation strength. A low-to-moderate risk of bias was observed in most studies. So far, no clinical trials with a ketogenic intervention in elderly migraine patients have been performed.
3. The Ketogenic Diet and Ketone Bodies in Aging
There are conflicting opinions on the ability of older people to change their life-long habits to implement health recommendations. One says that they may have difficulties adapting to a new lifestyle; the other claims that they are more open to lifestyle modifications than their younger counterparts [75,76]. A study aimed to assess whether older patients, defined as 65 years of age or older, were able to adopt and maintain a ketogenic diet in a cohort of US citizens [77]. In 67% of patients, the ketogenic diet resulted in accomplishing its goals, concerning weight loss, glucose control, and anticancer effects. Adverse effects, mainly dyslipidemia and constipation, were reported by 15% of patients. These studies showed that older patients, who suffer from various health problems, may adopt to the ketogenic diet, and such a diet may effectively and positively change their health status. It should be underlined that most patients in that study had notable baseline morbidity.
Caloric restriction and resulting ketogenesis are the most consistently reported factors that may prolong life span [78]. It is important that these factors are reported to not only extend longevity but also health span in adult humans and mice [79,80]. However, both caloric restriction and the ketogenic diet are dietary interventions that may cause multiple metabolic changes, and it is difficult to judge about their mechanisms beyond their beneficial action [81].
Two human studies reported a harmful effect of ketone bodies. A positive correlation between circulating ketone bodies and all-cause mortality was observed in the general population-based cohort study Prevention of Renal and Vascular Endstage Disease Intervention (PREVEND) in individuals aged 54 ± 12 years [82]. This study also revealed that an increased all-cause mortality was associated with an increased fatty liver index, a proxy for non-alcoholic fatty liver disease, and this increase was partly mediated by circulating ketone bodies. A recent study reported a dose–response relationship of a 50% increase in all-cause mortality between the lowest and highest quintiles of ketone body concentrations in White and Black Americans [83]. Moreover, the levels of ketone bodies were associated with incident heart failure and higher all-cause mortality. These associations suggest a potential detrimental effect of ketone body metabolism in aging.
The mechanism behind these observations in humans has been partly explained in an experiment with homozygotic mice with a mutation in the 3-hydroxy-3-methylglutaryl-CoA synthase 2 (HMGCS2) gene encoding for the protein critical for the second stage of ketogenesis [84,85]. These animals are incapable of endogenous ketogenesis. The HMGCS2−/− mice were given a normal diet, a ketogenic diet (comprising 4.5% carbohydrates, 80.8% fats, and 14.7% proteins), and a normal diet supplemented with 1,3-butanediol, a precursor of β-hydroxybutyrate, which showed that ketone bodies may exert a significant effect on the life span of animals in two periods of life: just after the birth and older age. Specifically, mice on a ketogenic diet displayed a higher level of mortality in old age, and mice on a diet supplemented with 1,3-butanediol exhibited higher mid-life mortality, but their mortality in old age was like that of control animals. An ad libitum ketogenic diet increased mortality, suggesting that not only the proportion of the main components of the ketogenic diet but also its rate of administration may determine its outcome.
Although traumatic headaches represent a different class of headaches than those typical for migraines, ketone bodies have therapeutic potential in traumatic brain injury, as shown in experimental animals [86]. However, the question of the age dependence of such a therapy belongs to the most important concerns associated with such a kind of therapy in humans.
Autophagy, a process of removing damaged and no longer needed cellular components with their possible recycling and reuse, plays a fundamental role in metabolism and is closely related to diet and aging [87]. Such a form of autophagy is associated with lysosomal degradation and is called degradative autophagy. However, several cellular components are not needed in their origin and are transported outside the cell in a process called secretory autophagy [88]. Normal autophagy is needed for homeostasis, and many disorders are associated with impaired autophagy [89]. In general, compromised autophagy is considered as a hallmark of aging [90]. On the other hand, several studies with long-lived organisms showed that delayed aging was associated with increased autophagy [91,92,93,94]. Recently, we suggested that an interplay between secretory autophagy in microglia and degradative autophagy in neurons may play a role in migraine pathogenesis with the involvement of brain-derived neurotrophic factor (BDNF) and the interaction of ATP with the purinergic receptor P2X7 (P2X7R) [95]. It was observed that a ketogenic diet upregulated hepatic autophagy in mice [96]. The same authors showed that the ketogenic diet upregulated the autophagosome-associated protein LC3-II in mouse hippocampal and cerebrocortical samples [97]. In general, these studies confirmed further research showing that ketosis may promote brain autophagy by activating Sirtuin 1 and hypoxia-inducible factor-1 [98]. Although vasodilation is no longer considered the sole cause of migraines, the ability of vasoactive substances to induce migraines, the efficacy of drugs targeting vasoactive sites in the brain, and the positive correlation of migraines with cardiovascular diseases make it a causative component in the pathogenesis of migraines [99]. It was shown that mice fed with a high-fat diet exhibited defects in metabolism and autophagy, which were ameliorated with a ketogenic amino acid (KAA) replacement diet [100]. That study showed that the biosynthesis of β-hydroxybutyrate could be impaired via the inhibition of autophagy. Consequently, β-hydroxybutyrate induced a potent vasodilator effect via potassium channels. Finally, it was observed that the prolonged consumption of a high-salt diet negatively regulated both β-hydroxybutyrate biosynthesis and hepatic autophagy, and that resuming of β-hydroxybutyrate bioavailability inhibited high-salt diet-induced endothelial dysfunction. Therefore, these studies suggest a direct mechanism by which ketogenic dietary interventions ameliorate vascular health, which may play a role in the beneficial effect of a ketogenic diet on migraines. The relationship between autophagy and aging suggests that ketogenic interventions in migraines in the elderly may not be sufficient.
In light of recent research, supplementation of the diet with ketone bodies may result in different effects depending on the timing and the method of such supplementation. The next issue that could be addressed is whether the supplementation occurs in normal or damaged organisms, as the ketogenic diet mat play an important role in tissue repair [82,101]. Another factor that should be included in the consideration of the role of ketogenesis in aging is sex, as it was shown that the difference in quintiles of ketone bodies between women and men was at the border of significance [81]. The ketogenic diet as a dietary intervention should be controlled both quantitively and qualitatively, as its ad libitum form may lead to an uncontrolled rise in ketone bodies.
This entry is adapted from the peer-reviewed paper 10.3390/nu15234998
This entry is offline, you can click here to edit this entry!
