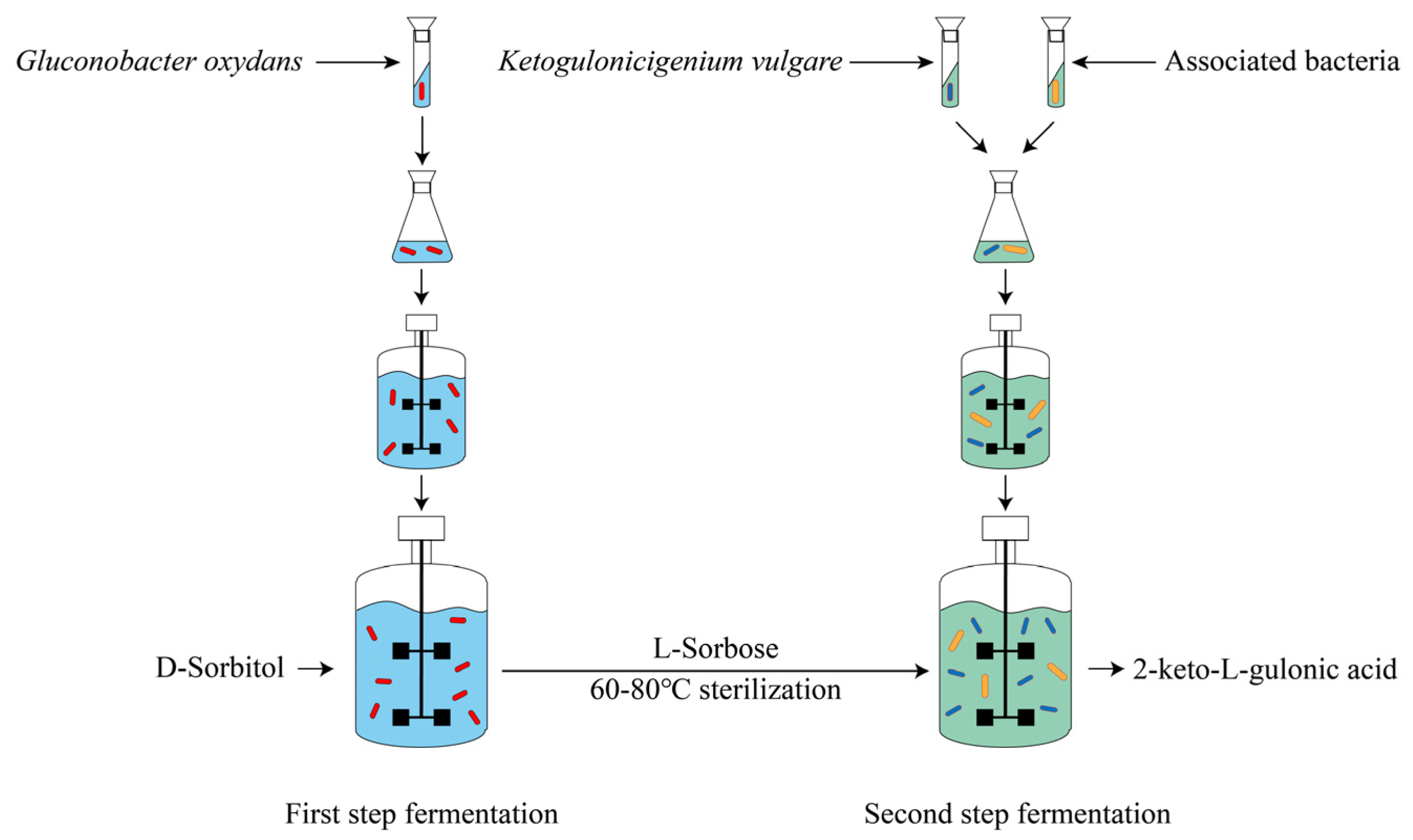
Vitamin C, a water-soluble vitamin with strong reducing power, cannot be synthesized by the human body and participates in a variety of important biochemical reactions. Vitamin C is widely used in the pharmaceutical, food, health care, beverage, cosmetics, and feed industries, with a huge market demand. The classical two-step fermentation method is the mainstream technology for vitamin C production. D-sorbitol is transformed into L-sorbose by Gluconobacter oxydans in the first step of fermentation; then, L-sorbose is transformed into 2-keto-L-gulonic acid (2-KGA) by a coculture system composed of Ketogulonicigenium vulgare and associated bacteria; and finally, 2-KGA is transformed into vitamin C through chemical transformation.
- Vitamin C
- 2-keto-L-gulonic acid
- mixed fermentation
1. Introduction
| Associated Bacteria | Fermentation Container | Time (h) |
L-Sorbose Concentration (g/L) | 2-KGA Concentration (g/L) |
Conversion Rate (%) |
References |
|---|---|---|---|---|---|---|
| Xanthomonas maltophilia IFO12692 | 3 L fermentor | 60 | 126 | 124.0 | - | [5] |
| Bacillus cereus 112 | Flask | 45 | 85 | 63.4 | - | [6] |
| Bacillus megaterium 116 | Flask | 45 | 85 | 64.5 | - | [6] |
| Bacillus megaterium 116 and Bacillus cereus 112 (1:3, v/v) | Flask | 45 | 85 | 69.0 | - | [6] |
| Bacillus cereus HB601 | Flask | 96 | 80 | - | 93.0 | [7] |
| Bacillus thuringiensis 320 | 260 m3 fermentor | 48 | 88–92 | 90.2 | 94.5 | [8] |
| Bacillus endophyticus Hbe603 | Flask | 72 | - | 70.0 | 93.0 | [9] |
| Bacillus subtilis A9 | Flask | 48 | 92.5 | 71.2 | - | [10] |
| Bacillus cereus 112 | Flask | 55 | 110 | 98.5 | 89.5 | [11] |
| Bacillus pumilus SH-B9 | Flask | 72 | 80 | 63.1 | - | [12] |
| Saccharomyces cerevisiae VTC2 | Flask | - | 20 | 13.2 | - | [13] |
2. Effect of Associated Bacteria on the Growth and 2-KGA Production of K. vulgare
2.1. Fermentation Process
2.2. Supplementation of Key Substance
2.3. Alleviation of Oxidative Pressure in Fermentation Systems
This entry is adapted from the peer-reviewed paper 10.3390/fermentation9121000
References
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615.
- Valdés, F. Vitamin C. Actas Dermo-Sifiliogr. 2006, 97, 557–568.
- Yang, W.; Xu, H. Industrial Fermentation of Vitamin C. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E., Revuelta, J.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 161–192.
- Giridhar, R.N.; Srivastava, A.K. Productivity improvement in L-sorbose biosynthesis by fedbatch cultivation of Gluconobacter oxydans. J. Biosci. Bioeng. 2002, 94, 34–38.
- Takagi, Y.; Sugisawa, T.; Hoshino, T. Continuous 2-Keto-l-gulonic acid fermentation by mixed culture of Ketogulonicigenium vulgare DSM 4025 and Bacillus megaterium or Xanthomonas maltophilia. Appl. Microbiol. Biotechnol. 2010, 86, 469–480.
- Mandlaa; Yang, W.C.; Han, L.T.; Wang, Z.Y.; Xu, H. Two-helper-strain co-culture system: A novel method for enhancement of 2-keto-L-gulonic acid production. Biotechnol. Lett. 2013, 35, 1853–1857.
- Zou, Y.; Hu, M.; Lv, Y.; Wang, Y.; Song, H.; Yuan, Y.J. Enhancement of 2-keto-gulonic acid yield by serial subcultivation of co-cultures of Bacillus cereus and Ketogulonicigenium vulgare. Bioresour. Technol. 2013, 132, 370–373.
- Yang, W.; Han, L.; Mandlaa, M.; Chen, H.; Jiang, M.; Zhang, Z.; Xu, H. Spaceflight-induced enhancement of 2-keto-L-gulonic acid production by a mixed culture of Ketogulonigenium vulgare and Bacillus thuringiensis. Lett. Appl. Microbiol. 2013, 57, 54–62.
- Jia, N.; Du, J.; Ding, M.Z.; Gao, F.; Yuan, Y.J. Genome Sequence of Bacillus endophyticus and Analysis of Its Companion Mechanism in the Ketogulonigenium vulgare-Bacillus Strain Consortium. PLoS ONE 2015, 10, 17.
- Yang, Y.; Gao, M.; Yu, X.D.; Zhang, Y.H.; Lyu, S.X. Optimization of medium composition for two-step fermentation of vitamin C based on artificial neural network-genetic algorithm techniques. Biotechnol. Biotechnol. Equip. 2015, 29, 1128–1134.
- Mandlaa; Sun, Z.Y.; Wang, R.G.; Han, X.D.; Xu, H.; Yang, W.C. Enhanced 2-keto-L-gulonic acid production by applying L-sorbose-tolerant helper strain in the co-culture system. AMB Express 2018, 8, 7.
- Zhang, Q.; Lin, Y.; Shen, G.; Zhang, H.; Lyu, S. Siderophores of Bacillus pumilus promote 2-keto-L-gulonic acid production in a vitamin C microbial fermentation system. J. Basic Microb. 2022, 62, 833–842.
- Wang, Y.; Li, H.C.; Liu, Y.; Zhou, M.Y.; Ding, M.Z.; Yuan, Y.J. Construction of synthetic microbial consortia for 2-keto-L-gulonic acid biosynthesis. Synth. Syst. Biotechnol. 2022, 7, 481–489.
- Saito, Y.; Ishii, Y.; Hayashi, H.; Imao, Y.; Akashi, T.; Yoshikawa, K.; Noguchi, Y.; Soeda, S.; Yoshida, M.; Niwa, M.; et al. Cloning of genes coding for L-sorbose and L-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-L-gulonate, a precursor of L-ascorbic acid, in a recombinant G. oxydans strain. Appl. Environ. Microbiol. 1997, 63, 454–460.
- Wang, E.X.; Ding, M.Z.; Ma, Q.; Dong, X.T.; Yuan, Y.J. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb. Cell. Fact. 2016, 15, 11.
- Li, G.; Li, D.; Zeng, W.; Qin, Z.; Chen, J.; Zhou, J. Efficient production of 2-keto-L-gulonic acid from D-glucose in Gluconobacter oxydans ATCC9937 by mining key enzyme and transporter. Bioresour. Technol. 2023, 384, 129316.
- Jiang, Y.; Wu, R.; Zhou, J.; He, A.; Xu, J.; Xin, F.; Zhang, W.; Ma, J.; Jiang, M.; Dong, W. Recent advances of biofuels and biochemicals production from sustainable resources using co-cultivation systems. Biotechnol. Biofuels. 2019, 12, 155.
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489.
- Agapakis, C.M.; Boyle, P.M.; Silver, P.A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 2012, 8, 527–535.
- Argyros, D.A.; Tripathi, S.A.; Barrett, T.F.; Rogers, S.R.; Feinberg, L.F.; Olson, D.G.; Foden, J.M.; Miller, B.B.; Lynd, L.R.; Hogsett, D.A.; et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl. Environ. Microbiol. 2011, 77, 8288–8294.
- Urbance, J.W.; Bratina, B.J.; Stoddard, S.F.; Schmidt, T.M.; Microbiology, E. Taxonomic characterization of Ketogulonigenium vulgare gen. nov., sp. nov. and Ketogulonigenium robustum sp. nov., which oxidize L-sorbose to 2-keto-L-gulonic acid. Int. J. Syst. Evol. Microbiol. 2001, 51, 1059–1070.
- Zou, W.; Liu, L.M.; Chen, J. Structure, mechanism and regulation of an artificial microbial ecosystem for vitamin C production. Crit. Rev. Microbiol. 2013, 39, 247–255.
- Ding, M.Z.; Song, H.; Wang, E.X.; Liu, Y.; Yuan, Y.J. Design and construction of synthetic microbial consortia in China. Synth. Syst. Biotechnol. 2016, 1, 230–235.
- Yang, W.C.; Sun, H.; Dong, D.; Ma, S.; Mandlaa; Wang, Z.X.; Xu, H. Enhanced 2-keto-L-gulonic acid production by a mixed culture of Ketogulonicigenium vulgare and Bacillus megaterium using three-stage temperature control strategy. Braz. J. Microbiol. 2021, 52, 257–265.
- Zhang, J.; Liu, J.; Shi, Z.P.; Liu, L.M.; Chen, J. Manipulation of B-megaterium growth for efficient 2-KLG production by K-vulgare. Process. Biochem. 2010, 45, 602–606.
- Wei, D.; Yuan, W.; Yin, G.; Yuan, Z.; Chen, M. Studies on kinetic model of vitamin C two-step fermentation process. Chin. J. Biotechnol. 1992, 8, 195–201.
- Zhang, Z.X.; Zhu, X.J.; Xie, P.; Sun, J.W.; Yuan, J.Q. Macrokinetic model for Gluconobacter oxydans in 2-keto-L-gulonic acid mixed culture. Biotechnol. Bioprocess Eng. 2012, 17, 1008–1017.
- Zhou, J.; Ma, Q.; Yi, H.; Wang, L.; Song, H.; Yuan, Y.J. Metabolome profiling reveals metabolic cooperation between Bacillus megaterium and Ketogulonicigenium vulgare during induced swarm motility. Appl. Environ. Microbiol. 2011, 77, 7023–7030.
- Zou, W.; Liu, L.M.; Zhang, J.; Yang, H.R.; Zhou, M.D.; Hua, Q.; Chen, J. Reconstruction and analysis of a genome-scale metabolic model of the vitamin C producing industrial strain Ketogulonicigenium vulgare WSH-001. J. Biotechnol. 2012, 161, 42–48.
- Jia, N.; Ding, M.Z.; Gao, F.; Yuan, Y.J. Comparative genomics analysis of the companion mechanisms of Bacillus thuringiensis Bc601 and Bacillus endophyticus Hbe603 in bacterial consortium. Sci. Rep. 2016, 6, 28794.
- Zou, W.; Zhou, M.D.; Liu, L.M.; Chen, J. Reconstruction and analysis of the industrial strain Bacillus megaterium WSH002 genome-scale in silico metabolic model. J. Biotechnol. 2013, 164, 503–509.
- Ma, Q.; Zhou, J.; Zhang, W.; Meng, X.; Sun, J.; Yuan, Y.J. Integrated proteomic and metabolomic analysis of an artificial microbial community for two-step production of vitamin C. PLoS ONE 2011, 6, e26108.
- Liu, L.M.; Chen, K.J.; Zhang, J.; Liu, J.; Chen, J. Gelatin enhances 2-keto-L-gulonic acid production based on Ketogulonigenium vulgare genome annotation. J. Biotechnol. 2011, 156, 182–187.
- Xu, D.; Behr, J.; Geißler, A.J.; Bechtner, J.; Ludwig, C.; Vogel, R.F. Label-free quantitative proteomic analysis reveals the lifestyle of Lactobacillus hordei in the presence of Sacchromyces cerevisiae. Int. J. Food Microbiol. 2019, 294, 18–26.
- Szotkowski, M.; Holub, J.; Šimanský, S.; Hubačová, K.; Sikorová, P.; Mariničová, V.; Němcová, A.; Márová, I. Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress. Microorganisms 2021, 9, 1160.
- Yen, H.W.; Chen, P.W.; Chen, L.J. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour. Technol. 2015, 184, 148–152.
- Li, Q.; Diao, J.; Xiang, B.T.; Cao, Z. Studies on metabolism of nitrogen source in fermentation of 2-keto-gulonic acid. Acta Microbiol. Sinica. 1996, 36, 19–24.
- Messner, K.R.; Imlay, J.A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 1999, 274, 10119–10128.
- Kussmaul, L.; Hirst, J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA 2006, 103, 7607–7612.
- Massey, V.; Strickland, S.; Mayhew, S.G.; Howell, L.G.; Engel, P.C.; Matthews, R.G.; Schuman, M.; Sullivan, P.A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem. Biophys. Res. Commun. 1969, 36, 891–897.
- Grinblat, L.; Sreider, C.M.; Stoppani, A.O. Superoxide anion production by lipoamide dehydrogenase redox-cycling: Effect of enzyme modifiers. Biochem. Int. 1991, 23, 83–92.
- Geary, L.E.; Meister, A. On the mechanism of glutamine-dependent reductive amination of alpha-ketoglutarate catalyzed by glutamate synthase. J. Biol. Chem. 1977, 252, 3501–3508.
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585.
- Wang, P.P.; Zeng, W.Z.; Du, G.C.; Zhou, J.W.; Chen, J. Systematic characterization of sorbose/sorbosone dehydrogenases and sorbosone dehydrogenases from Ketogulonicigenium vulgare WSH-001. J. Biotechnol. 2019, 301, 24–34.
- Huang, M.; Zhang, Y.H.; Yao, S.; Ma, D.; Yu, X.D.; Zhang, Q.; Lyu, S.X. Antioxidant effect of glutathione on promoting 2-keto-l-gulonic acid production in vitamin C fermentation system. J. Appl. Microbiol. 2018, 125, 1383–1395.
- Zhang, Q.; Lyu, S. 2-Keto-L-gulonic acid inhibits the growth of Bacillus pumilus and Ketogulonicigenium vulgare. World J. Microbiol. Biotechnol. 2023, 39, 257.
- Fang, J.; Wan, H.; Zeng, W.; Li, J.; Chen, J.; Zhou, J. Transcriptome Analysis of Gluconobacter oxydans WSH-003 Exposed to Elevated 2-Keto-L-Gulonic Acid Reveals the Responses to Osmotic and Oxidative Stress. Appl. Biochem. Biotechnol. 2021, 193, 128–141.
- Kaur, G.; Asthir, B.J.B.P. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619.
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189.
- Zhu, Y.B.; Liu, J.; Du, G.C.; Zhou, J.W.; Chen, J. Sporulation and spore stability of Bacillus megaterium enhance Ketogulonigenium vulgare propagation and 2-keto-L-gulonic acid biosynthesis. Bioresour. Technol. 2012, 107, 399–404.
- Bosak, T.; Losick, R.M.; Pearson, A. A polycyclic terpenoid that alleviates oxidative stress. Proc. Natl. Acad. Sci. USA 2008, 105, 6725–6729.
- Kim, D.H.; Lee, D.; Monllor-Satoca, D.; Kim, K.; Lee, W.; Choi, W. Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr(VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates. J. Hazard. Mater. 2019, 372, 121–128.
- Górska, A.; Sloderbach, A.; Marszałł, M.P. Siderophore-drug complexes: Potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014, 35, 442–449.
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726.
