Numerous environmental problems, which include global warming, acid rain, and stratospheric ozone depletion, have been brought on by the transformation, production, and consumption of energy. The quest for a substitute and more efficient sources of energy has been prompted by the potential scarcity of fossil fuels and concerns about the environment. The energy carrier hydrogen (H2) seems to have a lot of potential for significantly contributing to increased sustainability and environmental performance. Despite the fact that tackling future energy problems calls for a variety of strategies, many believe H2 will play a significant role, in part because it does not produce greenhouse gases when it is oxidised.
1. Steam Methane Reforming
Methane is a colourless, odourless, and highly inflammable compound which is obtained from both renewable (organic matter and animals) and non-renewable sources (coal production and fossil fuels). Steam methane reforming, one of the three hydrocarbon reforming technologies, is the least expensive and most widely used method for producing hydrogen from natural gas [
18,
19]. Partial oxidation and autothermal reforming are the other two methods used in this technology [
20]. Methane makes up a significant portion of natural gas and is one of the major contributors to global warming. Thus, generating hydrogen gas from methane provides a solution for slowing down its accumulation in the atmosphere. The process involves sulphur removal, steam reforming, shift reaction, and pressure swing absorption [
21]. The stages that characterise this process are the mixing of methane with steam under high temperature and pressure in the presence of a catalyst (Equation (1)) and a shift reaction in which carbon dioxide produced in the process undergoes additional reaction with steam to produce more hydrogen gas (Equation (2)). In Ref. [
22], the authors mention a temperature range of 700–900 °C and a pressure range of 1.5–3 MPa as favourable for the successful execution of the reaction in Equation (1).
This is a well-established technology with developed infrastructure and large-scale production capabilities. It is also cost-effective and makes use of natural gas infrastructure. However, this method is heavily dependent on fossil fuels leading to the emission of carbon dioxide into the atmosphere, thus increasing carbon dioxide levels.
2. Electrolysis
The ubiquity of water makes it a convenient resource for hydrogen production. Electrolysis is widely used in splitting the molecules of water into hydrogen and oxygen atoms; the dissociation process is given below [
24].
So far, three major electrolyte systems have been reported in the literature for water electrolysis operating under different conditions using different materials: proton exchange membranes (PEMs) [
25,
26], alkaline water electrolysis (AWE), and solid oxide electrolysis (SOE). In Ref. [
27], the authors mention a hybrid approach incorporating properties of PEM and AWE. T
AWE is the most developed of the three systems as the alkaline electrolysers have efficiencies ranging from 50 to 60% at a current density of 100–300 mA·cm
−2 [
28]. Different electrolytes reported for use in this system are potassium hydroxide (KOH), sodium hydroxide (NaOH), and sodium chloride (NaCl). Two non-platinum group metals, nickel (Ni) and iron (Fe), are used as electrodes in conjunction with the electrolyte and a diaphragm membrane. The diaphragm membrane distinguishes the cathode from the anode and is usually made of asbestos materials (which are currently outdated), limiting the operation temperature. There has been development of new materials to replace asbestos, which include ion inorganic membranes. Moreover, organic polymers, such as polypropylene, can be used in the construction of the diaphragm [
29]. Reactions at the anode and cathode are expressed as follows:
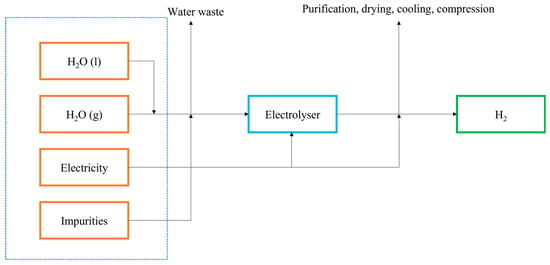
PEM electrolysers, another type of electrolysis device, are used for the production of hydrogen gas [
30]. These electrolysers deploy a proton exchange membrane as the electrolyte, an example being NafionTM. Electrolysis using PEM is depicted in
Figure 1, where water is split at the anode into proton and oxygen, after which the proton migrates to the cathode where it is reduced to hydrogen gas. The reactions are represented below:
Figure 1. Flow chart of hydrogen production using electrolysis.
Electrolysis through proton exchange membrane (PEM) technology offers distinct advantages, especially when integrated with renewable energy sources. One notable benefit is the rapid response of PEM electrolysers, which allows for quick adjustments to the fluctuating energy output of renewables like solar and wind. This responsiveness ensures efficient utilisation of the available energy, overcoming the intermittency challenge often associated with renewable sources. Moreover, the flexibility of PEM electrolysers enables them to be located in close proximity to renewable energy installations. By situating hydrogen generators near these sources, energy losses during transmission are minimised, enhancing overall efficiency. This proximity also facilitates the creation of localised, decentralised energy systems, promoting energy independence and reducing the need for extensive, centralised infrastructures. These factors underscore the significance of PEM electrolysis in harnessing renewable energy for sustainable hydrogen production, contributing to the advancement of clean and eco-friendly energy solutions [
31].
SOE electrolysers have been under development for some time now. Juxtaposed with AWEs and PEMs, these electrolysers can operate at very high temperatures of up to 1000 °C as their efficiency increases with increasing temperatures. They have the lowest specific system energy consumption of 4.5–7.0 kWh/Nm
2. The reactions, just like in the previous two at the anode and cathodes, are indicated below [
32]:
As evident in all three equations, different reactions occur at the anodes and cathodes based on the type of electrolyser used. Using electrolysis, hydrogen can be produced without any direct emissions, and it can incorporate the utilisation of other renewable energy sources: solar, wind, and geothermal, just to name a few. In comparison to other production methods, it requires a significant amount of electricity which may be readily available from non-renewable sources, hence being a double-edged sword.
3. Biomass Gasification
Gasification can be explained as a thermochemical reaction between an organic substance and a gasifier (oxygen, steam, air, carbon dioxide). Organic matter can be obtained from both renewable and non-renewable sources, such as agricultural waste and coal respectively. Gasification of materials from renewable and non-renewable sources both have different implications for the environment with the latter considered a more sustainable option. This section focuses on the gasification of biomass. Biomass can take various forms: wood and forestry residues, agricultural residues, and algae, to name a few. Biomass gasification involves the conversion of these materials into synthetic gas (syngas). This is a reaction that takes place at high temperatures dictated by a partial oxidation process to release hydrogen, carbon monoxide, methane, and other trace gases [
35]. Biomass gasification occurs at a high temperature range of 700–1200 °C [
36].
This process can be integrated with existing biomass or waste processing systems; it can contribute to a more efficient waste management practice. It reduces reliance on non-renewable sources; however, biomass availability can be challenging, and the process can be challenging in terms of control and optimisation. Additionally, incomplete combustion of biomass feedstocks can lead to carbon emissions.
4. Photoelectrochemical Water Splitting
Photoelectrochemical water splitting is a way of leveraging sunlight to break water down into hydrogen and oxygen using a semiconductor. The overall reaction process is illustrated below as
hv represents an incident photon:
The half equations for the overall reaction are represented by:
A specialised device, a photoelectrochemical cell, consisting of a photoelectrode, and electrolyte, and a counter electrode is used to achieve this purpose. Advantages of producing hydrogen gas using this approach include direct usage of sunlight, which is a renewable resource, and the scalability and suitability of the method. The downsides of this method, however, are evident in its low efficiency, sporadicity of solar energy, and stability challenges for efficient photoelectrodes.
5. Thermochemical Water Splitting
Thermochemical water splitting, also referred to as thermolysis, involves the decomposition of water into hydrogen and oxygen using heat. It is considered the most environmentally friendly method for producing hydrogen gas with respect to CO
2 emissions and acidification potential. It usually involves a series of thermochemical cycles, where materials undergo chemical transformations at high temperatures. The sulphur–iodine chemical cycle is one of the most common cycles used in depicting this thermochemical reaction. It is worth noting that the sulphur–iodine cycle is just one example of a thermochemical water-splitting process. There are other thermochemical cycles with different sets of reactions and operating conditions. It begins with the decomposition of sulphuric acid at 300 °C to 500 °C to release water without a catalyst. SO
3 is then separated at 800 °C to 900 °C to produce oxygen, after which sulphuric acid is produced from the next reaction, and finally hydrogen is produced from iodine decomposition [
39].
With respect to ongoing research and developments in the field of the above-mentioned hydrogen methods, current work in SMR focuses on carbon capture and utilisation techniques to mitigate greenhouse gas emissions associated with this process [
40,
41]. Scientists are exploring advanced materials and catalysts that can enhance the efficiency of carbon capture and storage, making SMR a more environmentally friendly option. Additionally, efforts are directed towards optimising the process parameters and integrating SMR with carbon capture and storage technologies, aiming to reduce the overall environmental impact of hydrogen production from natural gas.
Current research in electrolysis is geared towards improving the efficiency and durability of electrolysers [
42,
43]. Scientists are exploring novel electrolyte materials for both proton exchange membrane and solid oxide electrolysers, aiming to enhance conductivity and reduce degradation. Moreover, research efforts are focused on developing low-cost, highly efficient catalysts for the hydrogen evolution and oxygen evolution reactions, making electrolysis a more economically viable and sustainable method for hydrogen production, especially when coupled with renewable energy sources [
44].
Recent developments in biomass gasification involve advancements in reactor design and gas cleaning technologies. Researchers are working on innovative gasifier designs that enhance the conversion efficiency of biomass into syngas [
45,
46]. Additionally, efforts are directed towards developing effective methods for removing impurities from the syngas, ensuring the production of high-purity hydrogen. Integrating biomass gasification with carbon capture and storage techniques also holds promise in creating a carbon-negative hydrogen production process, further reducing its environmental impact.
Research in photoelectrochemical water splitting centres around the development of efficient and stable photoelectrodes. Scientists are exploring new materials, such as metal oxides, perovskites, and quantum dots, for use in photoelectrodes to enhance light absorption and charge separation [
47,
48]. Additionally, efforts are aimed at improving the stability of these materials under harsh electrolytic conditions. Integration with advanced solar cell technologies, such as tandem solar cells, is also a focus, aiming to increase the overall efficiency of photoelectrochemical water splitting and make it a viable option for large-scale hydrogen production powered by solar energy.
Ongoing research in thermochemical water splitting is focused on the development of high-temperature thermochemical cycles that enable efficient water splitting at elevated temperatures. Scientists are investigating various redox materials and reaction pathways to identify thermochemical cycles with high efficiency and low energy input [
49]. Furthermore, efforts are directed towards reactor design and heat management systems, aiming to optimise the overall process and reduce heat losses. The integration of renewable heat sources, such as concentrated solar power, into thermochemical water-splitting processes is also a promising area of research, enabling sustainable and efficient hydrogen production through thermochemical pathways [
50,
51].
This entry is adapted from the peer-reviewed paper 10.3390/cleantechnol5040067