Sugarcane (
Saccharum spp. hybrid) is an important member of the grass family Poaceae (Gramineae)
, subfamily Panicoideae, super tribe Andropogoneae, sub-tribe Saccharinae, and genus
Saccharum [
1]. Sugarcane is a C
4 perpetual plant and is well-cultivated commercially in at least 106 countries of tropical and subtropical areas, which are recognized by their hot and humid atmospheres and extremely productive lands suitable for sugarcane growth and development. Sugarcane is vital among crops because of its high sucrose content in cane [
2,
3]. Sugarcane has a significant capability for sucrose accumulation in stalks, chiefly in ripened internodes where its quantity drives aloft to 0.7 M [
4]. The main objective of sugarcane production is to yield sugar, which can be consumed in various categories of products. The sucrose is synthesized by photosynthesis in the green leaves of sugarcane plants and then transfers to sink organs, including consuming and storage sinks. In consuming sinks, sucrose is hydrolyzed to produce energy for growing roots, stems, and flowers while translocated to accumulate in the sink through phloem for storage purposes [
5,
6,
7]. Sucrose accretion in the parenchyma cells of sugarcane stalk is achieved via incessant cleavage and resynthesis [
8], whereas its metabolism is catalyzed by several key enzymes, such as sucrose phosphate synthase (SPS, EC:2.4.1.14), sucrose synthase (SuSy, EC 2.4.1.13), and invertase (INV, EC 3.2.1.26), including neutral invertase (NI), soluble acid invertase (SAI), and cell wall invertase (CWIN) [
9].
Plant hormones are the main regulatory tools involved in growth and development, which play a dominant role in integrating interior and exterior signals that temper development [
194]. So, it is assumed that internodal development, source-sink relationship and subsequently, cane yield and quality improvement in sugarcane are regulated by hormones. In this regard, five main plant growth hormones, auxin (IAA), gibberellin (GA), cytokinin (CTK), abscisic acid (ABA), and ethylene (ETH), are important, along with some recently identified growth regulators such as brassinosteroid (BR), jasmonates, salicylic acid, a peptide hormone, and strigolactone [
195,
196,
197].
2. IAA
Previous decades have witnessed several breakthroughs in recognizing a deceivingly simple transcriptional response pathway and cellular and molecular mechanisms of steering auxin transport [
198], synthesis and deactivation. At the same time, auxin function has been described in most growth and developmental progressions, interactions with other hormonal signaling pathways [
199], which is even valuable in pathogenic microorganisms and viruses [
200]. Abundant auxin-associated research has emphasized the activity of the leading naturally occurring auxin, indole-3-acetic acid (IAA). Numerous synthetic analogues, including 2,4-dichloro phenoxy acetic acid (2,4-D) and 1-naphthaleneacetic acid (NAA) have also been extensively utilized. Other naturally originating molecules also have auxin activity, for example, indole-3-butyric acid (IBA) has been the subject of vigorous examination for many years. IBA is closely alike to IAA with the exclusion of a supplementary CH
2 group, yet the function of IBA is somewhat more diverse than IAA. It is considered that IBA strongly controls auxin storage form, which permits spatiotemporal control of auxin levels through plant development, mainly in the elaboration of the root system [
201]. Auxin regulates various plant growth and development responses containing cellular elongation, expansion, and division in sugarcane. The basic auxin-response gene families comprise
auxin/indole-3-acetic acid,
auxin-response factor (
ARF) [
184],
Gretchen Hagen3 (
GH3),
small auxin-up RNAs (
SAUR) [
202], and
lateral organ boundaries (
LBD).
AUX/
IAA,
SAUR, and
GH3 genes can be stimulated instantly with auxin, leading to diverse cell and growth responses.
AUX/IAA is a vital gene family for plant growth regulation, and the process of auxin regulation is via the degradation of repressor genes
AUX/IAA and the modulation of gene expressions involved in multiple physiological processes.
ARF family contains important genes regulating the auxin-modulated gene expression [
203]. The
SAUR gene family contains early auxin-responsive genes that are significant for tissue elongation and can donate to biomass variances.
LBD is usually regulated by exogenous IAA and are involved in lateral organ development. The
GH3 gene family works in regulating and preserving endogenous auxin homeostasis [
204]. Much is known about the complex sucrose and auxin nexus that controls plant cell division and growth. Additional research into the complex network of sugar and auxin signaling pathways within plant tissues during specific growth stages or environmental conditions will improve our understanding of the regulatory systems underlying developmental processes and help develop new strategies to optimize sugarcane crop yield and stress tolerance.
3. ETH
ETH is a multifunctional hormone that controls plants’ growth and senescence [
205,
206]. Monitoring the ETH responses is a key commercial enterprise due to the widespread effects of ETH on plants of agronomic and horticultural value [
50]. ETH signaling can induce the biosynthesis of other hormones, for instance, rice ETH signaling encourages GA in deep water, which signals internode elongation, letting rice plants escape from whole submergence [
207]. ETH influences both growth and development of plants; in terms of growth, it is mostly allied with the regulation of cell size and generally limits cell elongation. However, it may also control cell division, while in the case of development, ethylene is normally associated with aging, sometimes required for processes like ripening, senescence, and abscission [
208]. These pathways depend deeply on negative regulation and post-translational control [
209]. ETH is also an important signaling hormone in various biological mechanisms of sugarcane [
202]. ETH not only increases sucrose accumulation but also increases biomass manufacturing and stress tolerance in sugarcane. Sugarcane ripening and sucrose accumulation have also been reported to be influenced by ETH [
210,
211]. The gene families of
reversion to ETH sensitivity (
RTE),
ETH receptor (
ETR),
ETH response sensor (
ERS),
ETH insensitive (
EIN)
ETP,
EIN2, targeting protein genes
EIL,
EIN3-
like, and
EBF (
EIN3-
F) are significantly mediating ETH signaling [
212,
213,
214]. ETH is a ripening hormone in plants that contributes to increasing sucrose storage in sugarcane [
211].
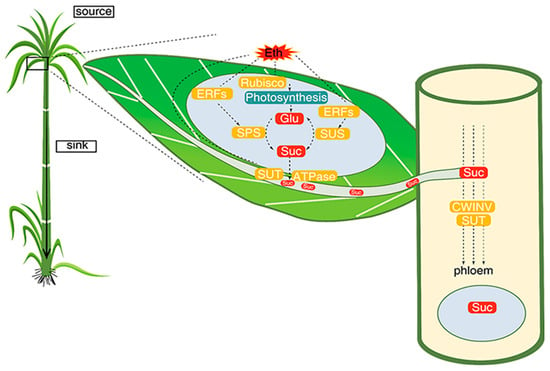
In sugarcane, ETH has been reported to play an important role in sugar synthesis (leaves), transportation, and storage in sink tissues. In a study [
210] (
Figure 1), sugarcane leaves were treated with ETH, which increased the activity of Rubisco, and genes associated with sucrose synthase, sucrose phosphate synthase and invertases, up-regulated by the action of ERFs which are known as sucrose synthesis stimulators. Sucrose and glucose seem to work as regulatory signals to regulate the photosynthesis process. Once sucrose is prepared in the leaves, it is transported to the phloem via plasmodesmata (symplastic-sugar transporters) and cell wall (apoplastic-wall invertase). Sugar movements from the leaves to the phloem generate gradient potential, which assists the biochemical equilibrium of sugar between the source and sinks in sugarcane. However, it needs further investigation to understand the exact molecular role of ETH in sugar production and storage.
Figure 1. Demonstration of ethylene contribution to sugar synthesis and transportation regulation in sugarcane.
4. GA
GA is jointly referred to as a group of diterpenoid acids, and some act as plant hormones and are crucial for normal plant growth and development [
215]. GA exploitation in agriculture is a usual practice, and the best-known involvement of GA manipulations is the insertion of dwarfing alleles into vital crops [
216]. This alteration resulted in one of the foundation stones of the so-called green revolution and directed a massive increase in global wheat and rice harvests. Documentation of the genes accountable for these traits presented that the encoded proteins are involved in the action or production of GA [
217]. More than 130 gibberellic acids (GAs) have been recognized in plants, fungi, and bacteria, and only a subclass of them, GA
1, GA
3, GA
4, and GA
7 are believed to function as bioactive hormones [
218]. Other forms of Gas that occur in plants are predecessors of the bioactive systems or deactivated metabolites [
219]. GA is the most studied signaling hormone in sugarcane [
220]. Family A of GA
3 is a commercially and scientifically well-known hormone [
221,
222]. Different isoforms of Gas play significant roles in the growth and development of plants, particularly leaf morphogenesis, floral development and fruit maturation [
223]. Alteration of interior Gas surges plant vegetative biomass, alters shoot structure, and regulates fruit and seed development [
185,
223,
224]. Variation in endogenous Gas in sugarcane via altering regulatory genes of its metabolism [
225], changing GA action modulator DELLA protein [
226], or exterior use of Gas meaningfully improves the stem growth and biomass [
227,
228]. GA
1, GA
3, GA
19, GA
20, and GA
29 were recognized in mature leaf and shoot apical meristem of flowering and nonflowering sugarcane in a study [
229]. The maximum rise in length and fresh weight of seven Hawaiian commercial sugarcane cultivars were observed when treated with a proper amount of GA
3 [
227]. Due to increased sink demand, the immature internodes exhibit a decrease in sucrose and an increase in hexose sugar levels over the shading period. GAs can thus be predicted to increase sucrose accumulation in sugarcane internodes by heightening sink demand, subsequently increasing cane yield [
230]. Further investigation is needed to find the role of GA associated with sucrose in sugarcane.
5. ABA
ABA is a chief phytohormone that regulates plant growth, development, and stress responses. It plays an important role in various physiological processes of plants, such as stomatal closure, cuticular wax accumulation, leaf senescence, bud dormancy, seed germination, and osmotic regulation. During the last four decades, molecular genetics and biochemical methodologies have recognized the central components of ABA biogenesis and their signaling pathways. The genetic investigation of viviparous mutants in Arabidopsis and maize for fast screening of mutants insensitive to sugar, salt, and ABA during germination brought to the documentation of many components responsible for the biosynthesis of ABA and signaling. The initial isolates were found in the clade A PP2Cs like ABA insensitive ABI
1, ABI
2, ABI
3, ABI
4, and ABI
5 [
231,
232]. Biochemical exploration of the ABA stimulation of PTKs caused the recognition of AAPK, a homolog of the Arabidopsis soul PTKs, SnRK2s, in
Vicia faba [
233]. Because of strong functional severance, the ABA receptor pyrabactin resistance 1 (PYR1) and PYR1-like (PYL) proteins were not discovered before 2009 via chemical genetic isolation for mutants that are insensitive to the ABA homologous pyrabactin [
234]. At the same time, regulatory types of machinery of the ABA receptors were screened through the yeast two-hybrid screen method [
235]. The characteristics of the PYL/RCAR protein family were also established via in vitro reconstruction of the main ABA signaling pathway [
236] and later verified by extensive genetic and structural data [
231,
237]. ABA has dynamic regulatory and signaling regulation in plant physiological processes. During the past few years, ABA biosynthesis and signaling pathways were well-characterized in abiotic stress tolerance in several crops [
238,
239]. ABA concentration increases in response to drought, extreme temperature and high salinity to enhance tolerance [
240]. Foliar application of ABA in sugarcane enhanced the tolerance against water stress [
241]. In relation to stomatal physiology, ABA is one of the most important regulatory signaling molecules [
187,
226]. Besides its functions in the physiology of abscission, ABA acts in several stress-correlated responses, particularly root and stomatal responses to drought. ABA treatment increased the growth of leaf spindle, stalk, root, and Brix value in internodes of sugarcane reported in a study [
242]. ABA response to pathogens in sugarcane has also been reported [
158]. As it is evident from the literature that ABA is an important multifunction phytochrome, it should be further studied on the molecular level, particularly in sugarcane, to find its association with the synthesis, transportation, accumulation, and decomposition of sucrose. There are several important phytohormones, other genes and molecules, which have significant role in sugarcane sucrose metabolism, however their pinpoint function still need further investigation (
Table 1).
Table 1. List of major genes and molecules associated with sucrose metabolism in sugarcane.
| Sucrose phosphate synthase (SPS); SPS1, SPS2, SPS4, SPS5 |
| Sucrose synthase (SuSy); SuSy1, SuSy2, SuSy4 |
| Soluble acid invertase (SAI) |
| Cell wall invertase (CWI) |
| Neutral invertase (NI) |
| Sucrose transporter; SWEET1b, SWEET13c, SWEET4a/4b, SUT1, SUT4, SUT5, SUT6, ShPST2a, ShPST2b, ShSUT4. |
| SNF1-like kinases |
| Trehalose-phosphate synthase |
| Cellulose synthase (CeS); CesA1, CesA7, CesA9, bk2l3,CesA10, CesA11, CesA12 |
| Trehalose 6-phosphate (T6P) |
| Trehalose-6-phosphate phosphatase (TPP) |
| Transcription factors (TF); WRKY, MYB, NAC, AP2/ERF |
| Basic helix-loop-helix (bHLH) |
| ScFBHs and ScACS2 |
| Mitogen-activated PTK (MAPK) |
| Sucrose-nonfermentation1-related protein kinase1-2 (ScSnRK1-2) |
| BCL2 antagonist/killer 1 (ScBAK1) |
| Phytohormones; Auxin (IAA), AUX/IAA, Gibberellin (GA), Cytokinin (CTK), Abscisic acid (ABA), Ethylene (ETH). |
| Brassinosteroid (BR), jasmonates, salicylic acid, a peptide hormone, and strigolactone |
| Gretchen Hagen3 (GH3), small auxin-up RNAs (SAUR |
| Ethylene receptor (ETR), Ethylene response sensor (ERS), Ethylene insensitive (EIN) ETP, EIN2, Targeting protein genes EIL, EIN3 like, EBF (EIN3-F) |
| GA1, GA3, GA4 and GA7 A19, GA20 and GA29 |
| ABA receptor pyrabactin resistance 1 (PYR1), PYR1-like (PYL) |