Salinity stands as a significant environmental stressor, severely impacting crop productivity. Plants exposed to salt stress undergo physiological alterations that influence their growth and development. Meanwhile, plants have also evolved mechanisms to endure the detrimental effects of salinity-induced salt stress. Within plants, Calcineurin B-like (CBL) proteins act as vital Ca2+ sensors, binding to Ca2+ and subsequently transmitting signals to downstream response pathways. CBLs engage with CBL-interacting protein kinases (CIPKs), forming complexes that regulate a multitude of plant growth and developmental processes, notably ion homeostasis in response to salinity conditions.
- salinity
- salt stress
- calcineurin B-like proteins
- CBL-interacting protein kinases
- salt tolerance
1. Introduction
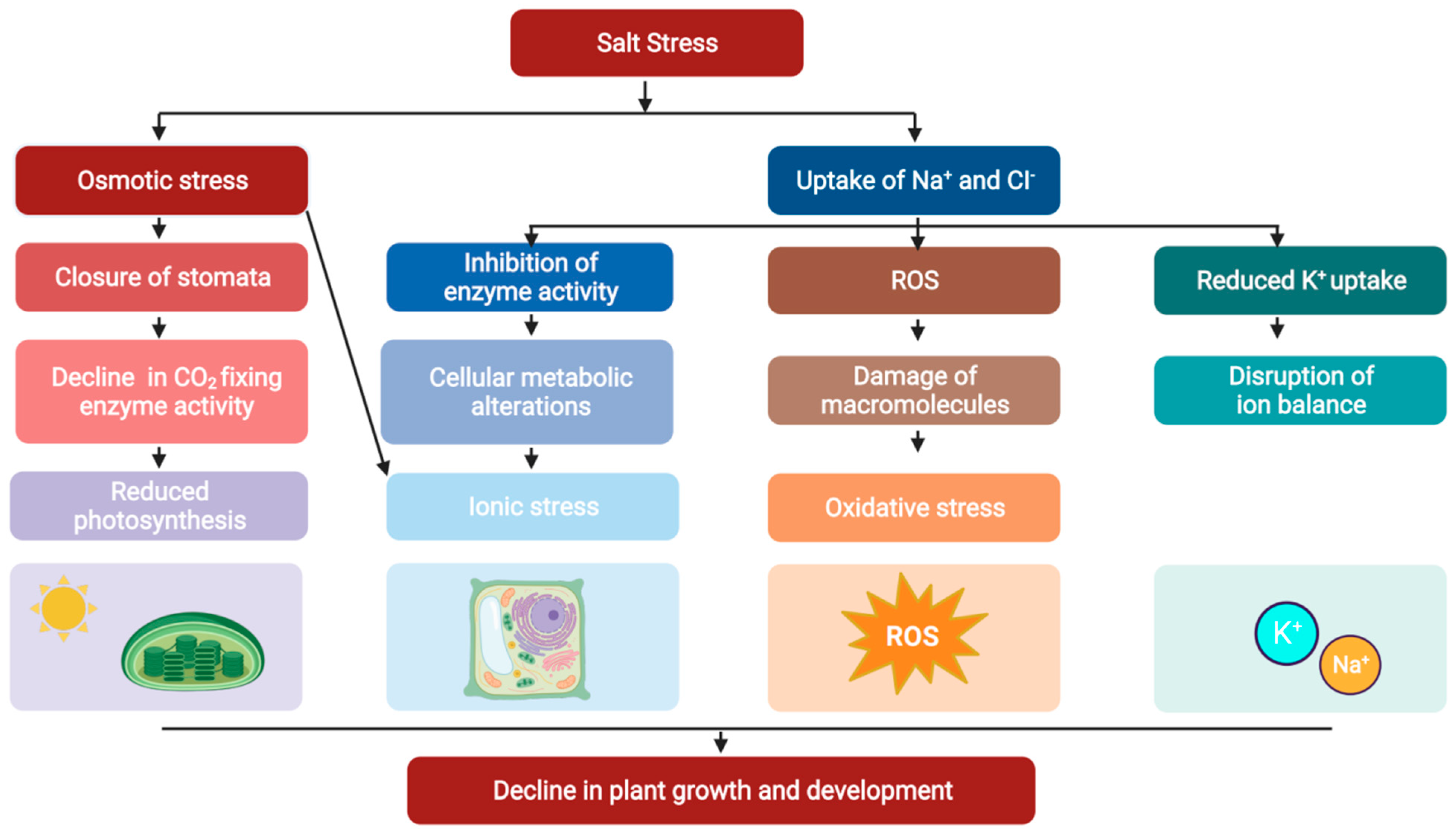
2. Effects of Salt Stress in Plants
2.1. Phenotypes of Salt Stress in Plants
2.2. Physiological Effects of Salt Stress in Plants
3. Plant Response to Salinity
3.1. Physiological Responses and Adaptations of Plants to Salt Stress
3.2. Molecular Responses and Adaptations of Plants to Salt Stress
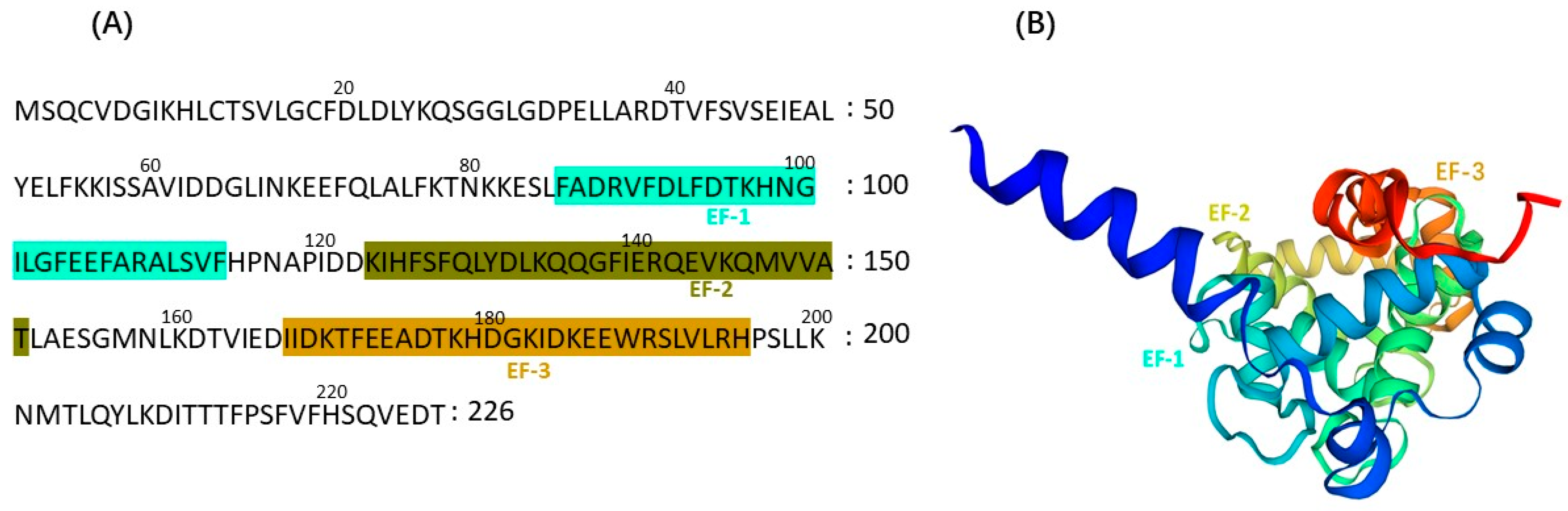
4. CBLs Function as Calcium Sensors in Plants
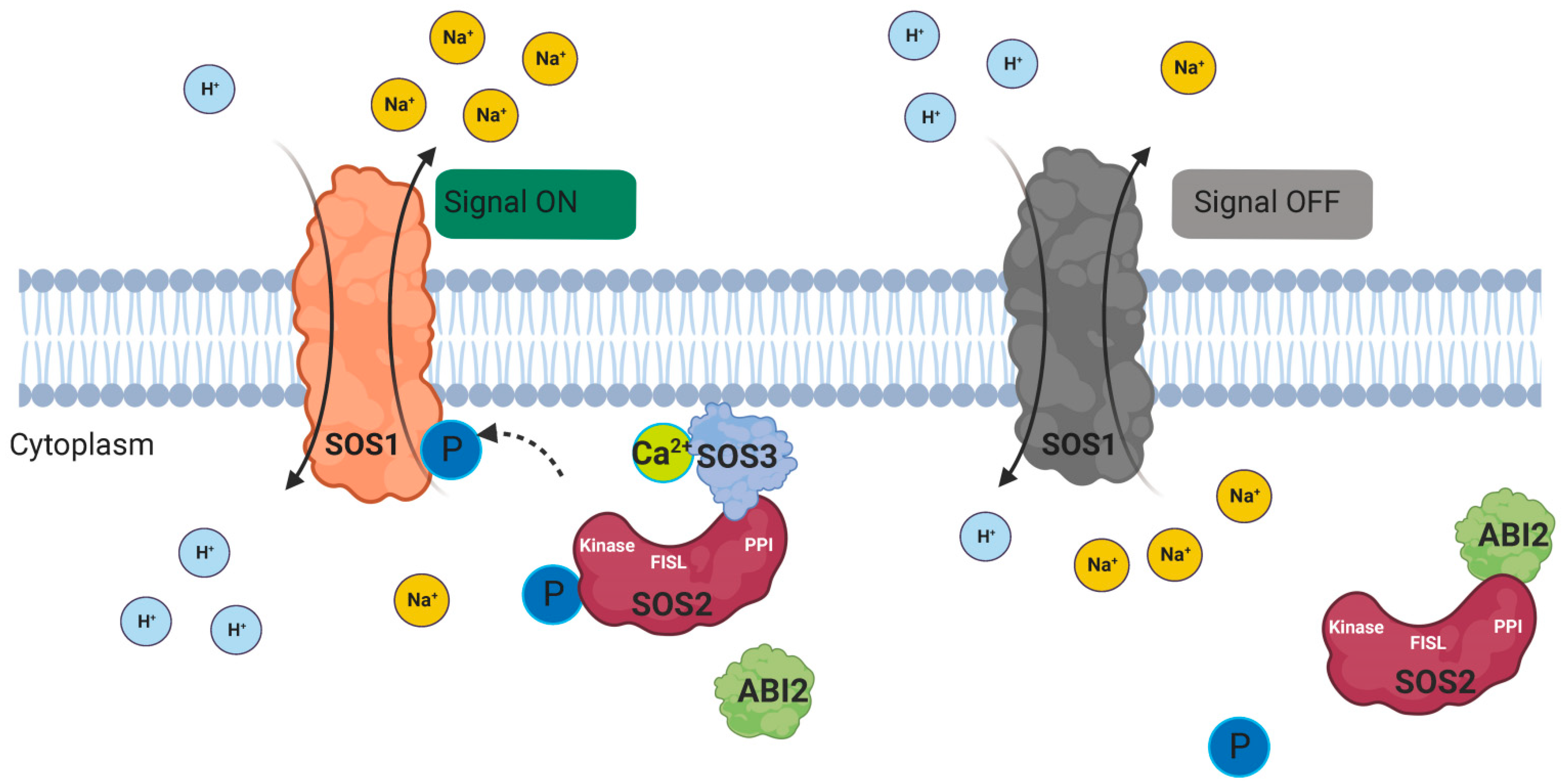
5. The CBL Family and Their Functions in Some Plants under Salt Stress
|
CBL Proteins |
Plant Sources |
Function |
CBL-CIPK Complex |
References |
|---|---|---|---|---|
|
CBL1 |
S. Japonica (Orchid of Nago) |
Rescues plant from salt hypersensitivity |
CBL1-CIPK1 |
[69] |
|
A. thaliana |
Increases salt tolerance |
- |
||
|
CBL2 |
A. thaliana |
Enhances salt tolerance |
CBL2-CIPK21 |
[72] |
|
CBL3 |
A. thaliana |
Enhances salt tolerance |
CBL3-CIPK21 |
[72] |
|
CBL4 |
Cucumis sativum (Cucumber) |
Enhances salt tolerance |
CBL4-CIPK6 |
[73] |
|
Brassica napus |
Enhances salt tolerance |
CBL4-CIPK24 |
[74] |
|
|
CBL5 |
S. italica (Foxtail millet) |
Maintains Na+ homeostasis and enhances salt tolerance |
CBL5-CIPK24 |
[75] |
|
N. tabacum (Common tobacco) |
Overexpression causes necrotic lesions |
- |
[76] |
|
|
CBL6 |
T. dicoccoides (Wheat) |
Enhances salt tolerance |
- |
[77] |
|
CBL7 |
Beta vulgaris (Sugar beet) |
Gene expression significantly increased under salt stress |
- |
[42] |
|
CBL8 |
A. thaliana |
Enhances tolerance under high salt stress |
CBL8-CIPK24 |
[78] |
|
CBL9 |
T. halophilla |
Increases salt tolerance |
CBL9-CIPK23 |
[79] |
|
Zea mays (Maize) |
Rescues plants from salt hypersensitivity |
CBL9-CIPK23 |
[80] |
|
|
A. thaliana |
Increases salt tolerance |
CBL9-CIPK23 |
[81] |
|
|
CBL10 |
Solanum lycopersicum (Tomato) |
Protects growing tissues from salt stress |
- |
[67] |
|
A. thaliana |
Enhances salt stress tolerance |
CBL10-CIPK8 |
[68] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms242316958
References
- Chourasia, K.N.; More, S.J.; Kumar, A.; Kumar, D.; Singh, B.; Bhardwaj, V.; Kumar, A.; Das, S.K.; Singh, R.K.; Zinta, G.; et al. Salinity responses and tolerance mechanisms in underground vegetable crops: An integrative review. Planta 2022, 255, 68.
- Zhang, Y.; Hou, K.; Qian, H.; Gao, Y.; Fang, Y.; Xiao, S.; Tang, S.; Zhang, Q.; Qu, W.; Ren, W. Characterization of soil salinization and its driving factors in a typical irrigation area of Northwest China. Sci. Total Environ. 2022, 837, 155808.
- Osman, K.T.; Osman, K.T. Saline and Sodic Soils. In Management of Soil Problems, 1st ed.; Springer International: Berlin/Heidelberg, Germany, 2018; pp. 255–298.
- Anwar, A.; Kim, J.K. Transgenic Breeding approaches for improving abiotic stress tolerance: Recent Progress and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 2695.
- Chen, J.; Mueller, V. Coastal climate change, soil salinity and human migration in Bangladesh. Nat. Clim. Chang. 2018, 8, 981–985.
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431.
- Shi, X.L.; Zhou, D.Y.; Guo, P.; Zhang, H.; Dong, J.L.; Ren, J.Y.; Jiang, C.J.; Zhong, C.; Zhao, X.H.; Yu, H.Q. External potassium mediates the response and tolerance to salt stress in peanut at the flowering and needling stages. Photosynthetica 2020, 58, 1141–1149.
- Huang, L.; He, B.; Han, L.; Liu, J.; Wang, H.; Chen, Z. A global examination of the response of ecosystem water-use efficiency to drought based on MODIS data. Sci. Total Environ. 2017, 601–602, 1097–1107.
- Sanyal, S.K.; Mahiwal, S.; Pandey, G.K. Calcium signaling: A communication network that regulates cellular processes. In Sensory Biology of Plants, Sopory, Sudhir; Springer: Singapore, 2019; pp. 279–309.
- Kundu, P.; Nehra, A.; Gill, R.; Tuteja, N.; Gill, S.S. Unraveling the importance of EF-hand-mediated calcium signaling in plants. S. Afr. J. Bot. 2022, 148, 615–633.
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053.
- Batool, N.; Shahzad, A.; Ilyas, N.; Noor, T. Plants and salt stress. Int. J. Agric. Crop Sci. 2014, 7, 582.
- Riaz, M.; Arif, M.S.; Ashraf, M.A.; Mahmood, R.; Yasmeen, T.; Shakoor, M.B.; Shahzad, S.M.; Ali, M.; Saleem, I.; Arif, M.; et al. A Comprehensive Review on Rice Responses and Tolerance to Salt Stress. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 133–158.
- Ji, X.; Tang, J.; Zhang, J. Effects of Salt Stress on the Morphology, Growth and Physiological Parameters of Juglansmicrocarpa L. Seedlings. Plants 2022, 11, 2381.
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes JJ, B.; Korte, A.; Haring, M.A.; de Boer, G.J.; et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 2017, 29, 3198–3213.
- Khalid, M.F.; Hussain, S.; Ahmad, S.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmed, N.; Anjum, M.A. Impacts of abiotic stresses on growth and development of plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–8.
- Urbanaviciute, L.; Bonfiglioli, L.; Pagnotta, M.A. Phenotypic and genotypic diversity of roots response to salt in durum wheat seedlings. Plants 2023, 2, 412.
- Baby, T.; Collins, C.; Tyerman, S.D.; Gilliham, M. Salinity negatively affects pollen tube growth and fruit set in grapevines and is not mitigated by silicon. AJEV 2016, 6, 218–228.
- Pushpavalli, R.; Quealy, J.; Colmer, T.D.; Turner, N.C.; Siddique KH, M.; Rao, M.V.; Vadez, V. Salt stress delayed flowering and reduced reproductive success of chickpea (Cicer arietinum L.), A response associated with Na+ accumulation in leaves. J. Agron. Crop Sci. 2016, 202, 125–138.
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429–430.
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433.
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120.
- Patro, L.; Mohapatra, P.K.; Biswal, U.C.; Biswal, B. Dehydration induced loss of photosynthesis in Arabidopsis leaves during senescence is accompanied by the reversible enhancement in the activity of cell wall beta-glucosidase. J. Photochem. Photobiol. B Biol. 2014, 137, 49–54.
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143.
- Naeem, M.; Abbas, A.; Ul-Allah, S.; Malik, W.; Baloch, F.S. Comparative genetic, biochemical and physiological analysis of sodium and chlorine in wheat. Mol. Biol. Rep. 2022, 49, 9715–9724.
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z.; Ahmad, P. Ion homeostasis for salinity tolerance in plants: A molecular approach. Physiol. Plant. 2021, 171, 578–594.
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804.
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxid. Act. 2022, 11, 225.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037.
- Corpas, F.J.; Del Río, L.A.; Palma, J.M. Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J. Integr. Plant Biol. 2019, 61, 803–816.
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 6, 1161–1173.
- Lotfi, R.; Ghassemi-Golezani, K.; Pessarakli, M. Salicylic acid regulates photosynthetic electron transfer and stomatal conductance of mung bean (Vigna radiata L.) under salinity stress. Biocatal. Agric. Biotechnol. 2020, 26, 101635.
- Chakraborty, K.; Basak, N.; Bhaduri, D.; Ray, S.; Vijayan, J.; Chattopadhyay, K.; Sarkar, R.K. Ionic Basis of Salt Tolerance in Plants: Nutrient, Homeostasis and Oxidative Stress Tolerance; Springer: Singapore, 2018; pp. 325–362.
- Hussain Wani, S.; Brajendra Singh, N.; Haribhushan, A.; Iqbal Mir, J. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165.
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86.
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2023; in press.
- Athar, H.U.R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058.
- Jian, G.; Mo, Y.; Hu, Y.; Huang, Y.; Ren, L.; Zhang, Y.; Hu, H.; Zhou, S.; Liu, G.; Guo, J.; et al. Variety-specific transcriptional and alternative splicing regulations modulate salt tolerance in rice from early stage of stress. Rice 2022, 15, 56.
- Santos, A.; Ferreira, L.; Oliveira, M. Concerted flexibility of chromatin tructure, methylome, and histone modifications along with plant stress responses. Biology 2017, 6, 3.
- Karle, S.B.; Guru, A.; Dwivedi, P.; Kumar, K. Insights into the role of gasotransmitters mediating salt stress responses in plants. J. Plant Growth Regul. 2021, 40, 2259–2275.
- Urbanaviciute, L.; Bonfiglioli, L.; Pagnotta, M.A. One hundred candidate genes and their roles in drought and salt tolerance in wheat. Int. J. Mol. Sci. 2021, 12, 6378.
- Geng, G.; Lv, C.; Stevanato, P.; Li, R.; Liu, H.; Yu, L.; Wang, Y. Transcriptome analysis of salt-sensitive and tolerant genotypes reveals salt-tolerance metabolic pathways in sugar beet. Int. J. Mol. Sci. 2019, 20, 5910.
- Sanyal, S.K.; Pandey, A.; Pandey, G.K. The CBL-CIPK signaling module in plants: A mechanistic perspective. Physiol. Plant. 2015, 155, 89–108.
- Beckmann, L.; Edel, K.H.; Batistič, O.; Kudla, J. A calcium sensor—protein kinase signaling module diversified in plants and is retained in all lineages of Bikonta species. Sci. Rep. 2016, 6, 31645.
- Mao, J.; Manik, S.; Shi, S.; Chao, J.; Jin, Y.; Wang, Q.; Liu, H. Mechanisms and physiological oles of the CBL-CIPK networking system in Arabidopsis thaliana. Genes 2016, 7, 62.
- Sanchez-Barrena, M.J.; Martinez-Ripoll, M.; Albert, A. Structural biology of a major signaling network that regulates plant abiotic stress: The CBL-CIPK mediated pathway. Int. J. Mol. Sci. 2013, 14, 5734–5749.
- Li, R.; Zhang, J.; Wei, J.; Wang, H.; Wang, Y.; Ma, R. Functions and mechanisms of the CBL–CIPK signaling system in plant response to abiotic stress. Prog. Nat. Sci. 2009, 19, 667–676.
- Kolukisaoglu, U.; Weinl, S.; Blazevic, D.; Batistic, O.; Kudla, J. Calcium sensors and their interacting protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004, 134, 43–58.
- Mohanta, T.K.; Mohanta, N.; Mohanta, Y.K.; Parida, P.; Bae, H. Genome-wide identification of Calcineurin B-Like (CBL) gene family of plants reveals novel conserved motifs and evolutionary aspects in calcium signaling events. BMC Plant Biol. 2015, 15, 1–15.
- Du, W.; Yang, J.; Ma, L.; Su, Q.; Pang, Y. Identification and characterization of abiotic stress responsive CBL-CIPK family genes in medicago. Int. J. Mol. Sci. 2021, 22, 4634.
- Kanwar, P.; Sanyal, S.K.; Tokas, I.; Yadav, A.K.; Pandey, A.; Kapoor, S.; Pandey, G.K. Comprehensive structural, interaction and expression analysis of CBL and CIPK complement during abiotic stresses and development in rice. Cell Calcium 2014, 56, 81–95.
- Li, L.; Zhang, J.; Wei, J.; Wang, H.; Wang, Y.; Ma, R. A Ca2+ signaling pathway regulates a K(+) channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006, 103, 12625–12630.
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128.
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360.
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.S.; Zhu, J.K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734.
- Tang, R.J.; Wang, C.; Li, K.; Luan, S. The CBL-CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617.
- Abdula, S.E.; Lee, H.J.; Ryu, H.; Kang, K.K.; Nou, I.; Sorrells, M.E.; Cho, Y.-G. Overexpression of BrCIPK1 gene enhances abiotic stress tolerance by increasing proline biosynthesis in rice. Plant Mol. Biol. Rep. 2015, 34, 501–511.
- Wang, X.; Hao, L.; Zhu, B.; Jiang, Z. Plant calcium signaling in response to potassium deficiency. Int. J. Mol. Sci. 2018, 19, 3456.
- Kim, K.N.; Cheong, Y.H.; Gupta, R.; Luan, S. Interaction specificity of Arabidopsis calcineurin B-like calcium sensors and their target kinases. Plant Physiol. 2000, 124, 1844–1853.
- Ohta, M.; Guo, Y.; Halfter, U.; Zhu, J.K. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc. Natl. Acad. Sci. USA 2003, 100, 11771–11776.
- Sanyal, S.K.; Rao, S.; Mishra, L.K.; Sharma, M.; Pandey, G.K. Plant stress responses mediated by CBL-CIPK phosphorylation network. Enzymes 2016, 40, 31–64.
- Ma, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. The CBL–CIPK pathway in plant response to stress signals. Int. J. Mol. Sci. 2020, 21, 5668.
- Tang, X.; Li, Q.H.; Yu, Y.N.; Qiao, Y.M.; Haq, S.U.; Gong, Z.H. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437.
- Ishitani, M.; Liu, J.; Halfter, U.; Kim, C.S.; Shi, W.; Zhu, J.K. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 2000, 12, 1667–1678.
- Jiang, H.; Zhang, X.; Chen, X.; Aramsangtienchai, P.; Tong, Z.; Lin, H. Protein lipidation: Occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 2018, 118, 919–988.
- Batistic, O.; Kudla, J. Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 2004, 219, 915–924.
- Egea, I.; Pineda, B.; Ortiz-Atienza, A.; Plasencia, F.A.; Drevensek, S.; Garcia-Sogo, B.; Yuste-Lisbona, F.J.; Barrero-Gil, J.; Atares, A.; Flores, F.B.; et al. The SlCBL10 Calcineurin B-Like protein ensures plant growth under salt stress by regulating Na+ and Ca2+ homeostasis. Plant Physiol. 2018, 176, 1676–1693.
- Yin, X.; Xia, Y.; Xie, Q.; Cao, Y.; Wang, Z.; Hao, G.; Song, J.; Zhou, Y.; Jiang, X. The protein kinase complex CBL10-CIPK8-SOS1 functions in Arabidopsis to regulate salt tolerance. J. Exp. Bot. 2020, 71, 1801–1814.
- Cho, J.H.; Choi, M.N.; Yoon, K.H.; Kim, K.N. Ectopic Expression of SjCBL1, Calcineurin B-Like 1 gene from Sedirea japonica, rescues the salt and osmotic stress hypersensitivity in Arabidopsis cbl1 Mutant. Front. Plant Sci. 2018, 9, 1188.
- Albrecht, V.; Weinl, S.; Blazevic, D.; D’Angelo, C.; Batistic, O.; Kolukisaoglu, U.; Bock, R.; Schulz, B.; Harter, K.; Kudla, J. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J. 2003, 36, 457–470.
- Cheong, Y.H.; Kim, K.N.; Pandey, G.K.; Gupta, R.; Grant, J.J.; Luan, S. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 2003, 15, 1833–1845.
- Pandey, G.K.; Kanwar, P.; Singh, A.; Steinhorst, L.; Pandey, A.; Yadav, A.K.; Tokas, I.; Sanyal, S.K.; Kim, B.G.; Lee, S.C.; et al. Calcineurin B-Like protein-Interacting Protein Kinase CIPK21 regulates osmotic and salt Stress responses in Arabidopsis. Plant Physiol. 2015, 169, 780–792.
- Wang, M.; Yang, S.; Sun, L.; Feng, Z.; Gao, Y.; Zhai, X.; Dong, Y.; Wu, H.; Cui, Y.; Li, S.; et al. A CBL4-CIPK6 module confers salt tolerance in cucumber. Veg. Res. 2022, 2, 1–10.
- Liu, W.Z.; Deng, M.; Li, L.; Yang, B.; Li, H.; Deng, H.; Jiang, Y.Q. Rapeseed calcineurin B-like protein CBL4, interacting with CBL-interacting protein kinase CIPK24, modulates salt tolerance in plants. Biochem. Biophys. Res. Commun. 2015, 467, 467–471.
- Yan, J.; Yang, L.; Liu, Y.; Zhao, Y.; Han, T.; Miao, X.; Zhang, A. Calcineurin B-like protein 5 (SiCBL5) in Setaria italica enhances salt tolerance by regulating Na+ homeostasis. Crop J. 2022, 10, 234–242.
- Mao, J.; Yuan, J.; Mo, Z.; An, L.; Shi, S.; Visser, R.G.F.; Bai, Y.; Sun, Y.; Liu, G.; Liu, H.; et al. Overexpression of NtCBL5A leads to necrotic lesions by enhancing Na+ sensitivity of tobacco leaves under salt stress. Front. Plant Sci. 2021, 12, 740976.
- Chen, L.; Ren, J.; Shi, H.; Zhang, Y.; You, Y.; Fan, J.; Chen, K.; Liu, S.; Nevo, E.; Fu, J.; et al. TdCBL6, a calcineurin B-like gene from wild emmer wheat (Triticum dicoccoides), is involved in response to salt and low-K+ stresses. Mol. Breed. 2015, 35, 50.
- Steinhorst, L.; He, G.; Moore, L.K.; Schultke, S.; Schmitz-Thom, I.; Cao, Y.; Hashimoto, K.; Andres, Z.; Piepenburg, K.; Ragel, P.; et al. A Ca2+-sensor switch for tolerance to elevated salt stress in Arabidopsis. Dev. Cell 2022, 57, 2081–2094.e7.
- Sun, Z.; Qi, X.; Li, P.; Wu, C.; Zhao, Y.; Zhang, H.; Wang, Z. Overexpression of a Thellungiella halophila CBl9 homolog, ThCBL9, confers salt and osmotic tolerances in transgenic Arabidopsis thaliana. J. Plant Biol. 2008, 51, 25–34.
- Zhang, F.; Li, L.; Jiao, Z.; Chen, Y.; Liu, H.; Chen, X.; Fu, J.; Wang, G.; Zheng, J. Characterization of the calcineurin B-Like (CBL) gene family in maize and functional analysis of ZmCBL9 under abscisic acid and abiotic stress treatments. Plant Sci. J. 2016, 253, 118–129.
- Nath, M.; Bhatt, D.; Jain, A.; Saxena, S.C.; Saifi, S.K.; Yadav, S.; Negi, M.; Prasad, R.; Tuteja, N. Salt stress triggers augmented levels of Na+, Ca2+ and ROS and alter stress-responsive gene expression in roots of CBL9 and CIPK23 knockout mutants of Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 265–276.
