Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Vitamin A, a critical micronutrient, plays a vital role in maintaining poultry health and maximizing productivity. Vitamin A deficiency can have severe consequences on poultry health, compromising growth, reproduction, immune competence, and overall productivity.
- vitamin A
- retinol
- supplementation
- poultry health
- requirement
1. Introduction
The well-being and productivity of poultry are closely tied to their nutritional status. Adequate nutrition is essential for maintaining optimal health, growth, and overall productivity in poultry flocks. Nutritional imbalances can lead to various health issues, reduced performance, and economic losses for poultry producers [1]. Among the many essential micronutrients, vitamins play a crucial role in supporting vital physiological functions. Understanding the significance of vitamins, particularly vitamin A (retinol), in poultry health and productivity is paramount for optimizing poultry management practices [2][3].
Vitamins are organic compounds that are required in small quantities but are essential for normal metabolic processes in domestic fowl [4]. They act as cofactors for various enzymatic reactions and are involved in critical physiological functions such as growth, reproduction, immune response, and vision. Among the different vitamins, vitamin A stands out as a key player in maintaining optimal health and productivity in animals [5]. It plays a vital role in vision, embryonic development, bone growth, immune function, and maintaining the integrity of epithelial tissues.
Exclusively animal-derived feeds such as fish liver oil and fish meal are the primary sources of vitamin A. However, there are certain plant pigments called carotenoids (e.g., β-carotene) that have the potential to exhibit vitamin A activity via metabolic conversion [2][6]. It is important to note that only a small portion of the carotenoids found in nature can be converted into vitamin A by mammals and birds [7]. The efficiency of this conversion depends on various factors such as the type and dietary concentration of protein and fat, total carotene load, animal species, and the bird’s vitamin A supply status [8][9]. Consequently, synthetic retinol, primarily in the form of retinyl acetate, is commonly applied in animal nutrition to meet the dietary requirement for vitamin A [3].
Vitamin A deficiency can have severe consequences on poultry health, compromising growth, reproduction, immune competence, and overall productivity [10]. Acute hypovitaminosis A is rare among poultry populations because retinol in the form of retinyl acetate is usually supplemented using premixes in domestic fowl feed [11][12]. However, subclinical vitamin deficiencies may occur more frequently [13]. Various factors contribute to the occurrence of hypovitaminosis A, such as incorrect premix and feed formulations, improper storage conditions for premixes and feeds, inadequate feed management practices, as well as contextual criteria. Poultry raised in intensive production systems may be particularly susceptible to suboptimal levels of micronutrients due to the presence of negative environmental factors and stressors [14][15]. These factors include high ambient temperature, high stocking density, microbial challenges, and hygienic issues. Under such conditions, the vitamin A requirement for poultry may increase [4][16].
2. The Crucial Contributions of Vitamin A: Metabolism, Growth, Immunity, Antioxidant Capacity, and Reproduction
Vitamin A plays a pivotal role in the intricate physiology of poultry, showcasing a myriad of essential functions. At the forefront, its three forms, known as retinol (the alcohol), retinal (the aldehyde), and retinoic acid (all-trans retinoic acid, ATRA) [17], assume a critical role in a range of metabolic processes, fostering growth, bolstering immunity, and even supporting the delicate realm of reproduction [18].
- (1)
-
Digestion and Metabolism:
Vitamin A digestion and metabolism in poultry are complex processes that ensure the efficient utilization of this essential micronutrient. In domestic fowl, vitamin A is obtained via the diet in the form of supplemented retinyl esters [3]. After ingestion, the feed undergoes enzymatic hydrolysis in the digestive system, where pancreatic enzymes such as esterases and lipases break down the retinyl esters into free retinol [19].
To facilitate absorption in the small intestine, the free retinol is incorporated into mixed micelles, which are small structures formed in the presence of bile salts and phospholipids [20]. These micelles aid in the absorption of retinol by the absorptive cells of the intestinal lining, known as enterocytes [7]. The enterocytes take up the retinol via specific transport proteins [21]. Once absorbed, retinol enters the bloodstream and is transported to the liver.
In the liver, retinol undergoes esterification, a process where it is conjugated with fatty acids to form retinyl esters. These esterified forms of vitamin A are stored in hepatic stellate cells within the liver, serving as a reservoir for the release of retinyl esters into circulation as needed [22].
Within target tissues, such as the eyes, skin, immune system, and reproductive organs, retinyl esters are hydrolyzed back into retinol [17]. This conversion is facilitated by cellular enzymes, and the released retinol can be further metabolized into its active form, ATRA [23]. ATRA acts as a ligand for nuclear receptors, regulating the expression of genes involved in various physiological processes, including vision, growth, reproduction, and immune function [24].
Any excess retinol that is not immediately needed can partly be eliminated via biliary excretion [25][26][27] or reesterified and stored in hepatic stellate cells [22]. The initial step of biliary excretion involves the secretion of retinol and its metabolites into the bile, enabling their eventual removal from the body via feces.
- (2)
-
Growth and Development:
ATRA plays a crucial role in regulating the ontogeny of poultry. It exerts its effects via various mechanisms that primarily focus on promoting growth and supporting organ development [28]. By binding to specific retinoic acid receptors (RARs) in target cells, ATRA acts as a transcriptional regulator, activating the transcription of genes involved in cell differentiation, proliferation, and tissue development [23]. This process ensures proper cell specialization and the formation of specific tissues and organs during embryonic development [29].
In addition, ATRA influences skeletal development in poultry by promoting the synthesis and mineralization of bone tissue. It regulates the expression of genes associated with bone growth and remodeling, resulting in the formation of a robust skeletal system [30]. Moreover, ATRA affects the development and function of the vital organs involved in growth. It stimulates the growth and maturation of intestinal villi, thereby enhancing nutrient absorption and digestion [31][32]. In the respiratory system, ATRA plays a role in lung development and surfactant production, facilitating efficient gas exchange [33][34].
Furthermore, ATRA supports growth and development by stimulating the production of growth factors such as insulin-like growth factor (IGF) and transforming growth factor-beta (TGF-β) [35]. These growth factors play crucial roles in tissue maturation, repair, and overall development.
- (3)
-
Immune Function:
Vitamin A, specifically in ATRA, plays a vital role in supporting a strong immune system in domestic fowl. ATRA binds to specific nuclear receptors RARs and retinoid X receptors (RXRs) present in the immune cells [36]. The binding of ATRA to these receptors triggers a sophisticated cascade of processes that regulates the transcription of genes and the synthesis of proteins, ultimately impacting immune function [37].
One of the primary ways in which vitamin A (via ATRA) regulates gene expression is by influencing the differentiation and maturation of immune cells [38]. Retinoic acid signaling facilitates the activation of cistrons, which are essential for lymphocyte development and triggering [39]. It plays a role in promoting the differentiation of T cells into various effector subsets, including helper T cells (such as Th1, Th2, and Th17) and regulatory T cells (Tregs) [40][41][42]. These subsets of T cells have critical functions in coordinating immune responses and maintaining immune balance. Additionally, ATRA aids in the maturation of B cells and enhances the production of specific antibodies by regulating the genes involved in antibody synthesis [43].
Furthermore, vitamin A plays a significant role in regulating the expression of genes associated with the integrity and barrier function of epithelial cells. ATRA signaling stimulates the production of proteins that maintain the structural resilience of epithelial cells, such as tight junction proteins and mucins [44][45][46][47]. These proteins contribute to the physical barrier that prevents the entry of pathogens into the body. By regulating the expression of cistrons involved in epithelial cell function, vitamin A helps fortify the first line of defense against infections.
Another important mechanism by which vitamin A impacts immune function is through its regulation of cytokine production. Cytokines are signaling molecules that coordinate immune responses and inflammation. ATRA influences the expression of the genes encoding cytokines and their receptors, thereby modulating the immune response [48][49]. For example, ATRA enhances the production of interleukin-10 (IL-10), an anti-inflammatory cytokine that helps mitigate excessive immune responses [50]. Moreover, vitamin A also promotes the expression of cistrons responsible for antimicrobial peptides, which are vital components of innate immunity [51][52].
- (4)
-
Antioxidant Capacity:
Antioxidants play a crucial role in maintaining the health of poultry, and vitamin A is particularly noteworthy due to its remarkable antioxidant properties [53][54].
Vitamin A exerts its antioxidant effects via various mechanisms. One of its principal functions is retinol’s ability to scavenge free radicals [55], although there is some controversy surrounding this mode of action [56]. Free radicals are highly reactive molecules that can cause damage to cell membranes, proteins, and DNA, resulting in cellular dysfunction and oxidative stress. By neutralizing these free radicals, retinol potentially helps protect the integrity of cellular components and maintain optimal cellular function [57].
Apart from its possible direct scavenging activity, vitamin A, in the form of ATRA, supports the activity of various antioxidant enzymes, including superoxide dismutase (SOD) and catalase (CAT), through its effect on gene expression [58]. When ATRA binds to RARs, it initiates a cascade of molecular events that ultimately lead to changes in cistron transcription. The ATRA–RAR complex recruits co-activators that facilitate the expression of nearby genes [59]. In the case of antioxidant enzymes such as SOD and CAT, ATRA enhances the transcription of their respective cistrons [60][61]. This increased gene expression results in the synthesis of higher levels of SOD and CAT proteins. SOD is an important enzyme that catalyzes the conversion of superoxide radicals (O2•−) into hydrogen peroxide (H2O2) [62], while CAT converts hydrogen peroxide into water and oxygen [63]. These enzymes play a crucial role in neutralizing harmful reactive oxygen species within the cells.
By enhancing the activity of SOD and CAT, vitamin A strengthens the antioxidant defense system of poultry birds [64]. Increased levels of SOD and CAT enable more efficient removal of reactive oxygen species, reducing redox imbalance and preventing damage to cellular components such as proteins, lipids, and DNA [65]. This additional layer of protection against oxidative stress contributes to the overall health and well-being of avian species.
- (5)
-
Reproduction:
Vitamin A plays a crucial role in the reproductive processes of domestic fowl. Its mechanism of action involves the regulation of gene expression, specifically through its effects on the retinoic acid signaling pathway. In the context of reproductive organs, ATRA influences the development and function of the testes, ovaries, and oviducts [66]. By binding to RARs and RXRs in these tissues, ATRA regulates the expression of genes involved in the growth, differentiation, and maturation of reproductive cells [67]. In males, ATRA promotes normal sperm production by stimulating the proliferation and differentiation of spermatogonia, which are the precursor cells of sperm [68]. It also plays a role in the maturation and motility of spermatozoa. In females, ATRA is essential for oocyte maturation and fertilization [69]. It helps regulate the production and release of mature oocytes from the ovaries, as well as the transport and viability of the oocytes within the oviducts.
Furthermore, ATRA influences embryonic development and hatchability in poultry [70][71]. During embryogenesis, ATRA controls the expression of genes involved in cell proliferation, differentiation, and morphogenesis [72]. It plays a crucial role in the development of various organ systems, including the cardiovascular system, nervous system, and reproductive system [73][74].
- (6)
-
Vision:
The essential role of vitamin A in poultry vision stems from its multiple important functions [2]. One of its primary roles is to support and maintain overall eye [75]. Vitamin A in the form of retinal is vital for the synthesis of rhodopsin, a light-sensitive pigment found in the retina, which is necessary for vision in low-light conditions [76]. On the other hand, ATRA promotes the development and differentiation of photoreceptor cells, allowing poultry to effectively perceive and process visual information [77]. Moreover, vitamin A helps protect the cornea and conjunctiva, preventing dryness and facilitating the production of a clear fluid by the lacrimal glands in birds [75][78]. This fluid helps maintain optimal vision clarity.
In conclusion, vitamin A is an essential micronutrient for avian species, serving critical functions in their physiology. It regulates gene expression, supports growth and organ formation, strengthens the immune system, and protects against oxidative damage. Additionally, it plays a pivotal role in reproductive processes, influencing organ development and contributing to successful embryonic development and hatchability. Overall, ensuring adequate vitamin A intake is crucial for the health and well-being of poultry.
3. Causes of Vitamin A Deficiency: Identifying Key Factors
Instances of severe vitamin A deficiency in poultry operations have significantly decreased in recent times, with only a few isolated cases observed [79]. This improvement can be attributed to increased awareness among farmers worldwide who are adopting scientific farming practices and implementing effective measures to address this issue. However, subclinical vitamin A deficiency can still be encountered [13].
Several key factors may contribute to vitamin A deficiency in poultry diets, with each factor playing a significant role in identifying the causes and implementing effective solutions. These key elements include:
- (1)
-
Inadequate feed formulation: One prominent factor contributing to vitamin A deficiency in poultry diets is inadequate feed formulation [80]. When the formulation of poultry feed does not include sufficient quality sources of preformed vitamin A, it fails to provide the necessary levels of this essential micronutrient. To address this issue, careful consideration should be given to the feed formulation process, including the use of least-cost feed formulation methods [81][82]. It is crucial to ensure the inclusion of stable and highly bioavailable commercial sources of vitamin A to prevent deficiencies.
- (2)
-
Mishandling and improper storage of poultry premixes and feed can contribute to the deficiency of essential vitamins, including vitamin A. Over time, exposure to light, heat, and oxygen can lead to the degradation of vitamins in the premixes and feed [83]. For instance, some of the vitamin A sources available on the market, particularly those that are less stable, may lose their vitamin A activity completely if stored at elevated temperatures within a short period (Figure 1) [84]. Therefore, it is crucial to store premixes and feed in cool, dry, and dark conditions for the shortest possible time to maintain optimal levels of vitamin A. Regular quality checks and timely replenishment of premixes and feed stocks are also vital to preserve the potency of vitamin A and prevent its degradation. Furthermore, paying meticulous attention to the thermal treatment of feed is of the utmost importance [85]. Considering the specific example of pelleting, it is crucial to avoid subjecting the feed to excessively high temperatures for prolonged periods of time, as this can result in significant losses in vitamin A activity [86]. Under such conditions, implementing an additional safety margin for vitamin activity becomes sensible.
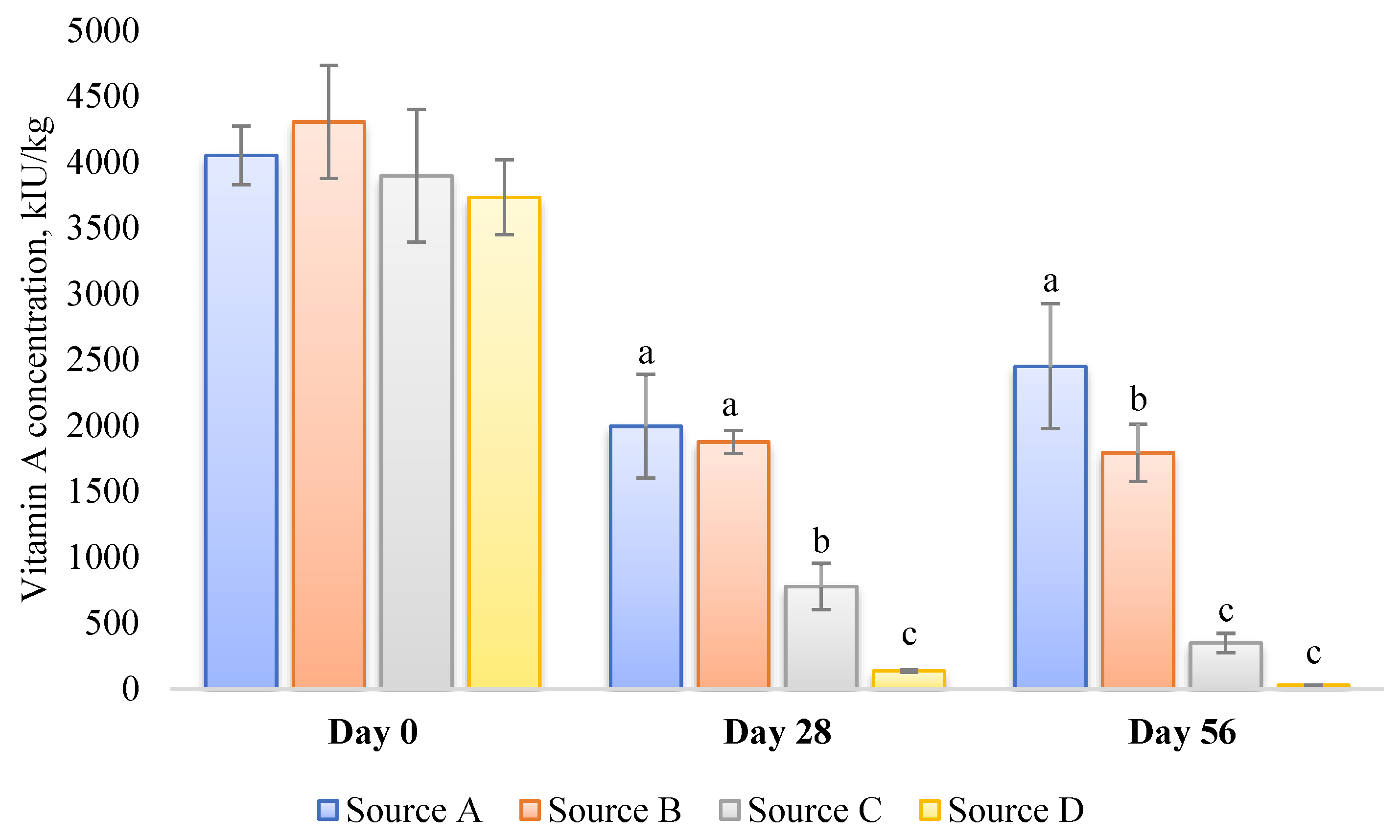 Figure 1. Stability of four different commercial vitamin A sources stored for up to 56 days in vitamin–mineral premix (incl., choline chloride) for broilers at 35 °C and 60–70% r.h. [84]. Values are presented as mean ± SD (n = 3). The premixes were prepared to contain 4.4 Mio IU vitamin A per kg. a–c Within a time frame (0, 28, or 56 d), values not sharing a common superscript letter are significantly different (p < 0.05).
Figure 1. Stability of four different commercial vitamin A sources stored for up to 56 days in vitamin–mineral premix (incl., choline chloride) for broilers at 35 °C and 60–70% r.h. [84]. Values are presented as mean ± SD (n = 3). The premixes were prepared to contain 4.4 Mio IU vitamin A per kg. a–c Within a time frame (0, 28, or 56 d), values not sharing a common superscript letter are significantly different (p < 0.05).
- (3)
-
Factors affecting absorption and utilization: Factors that affect the absorption and utilization of fat-soluble vitamins should not be overlooked [3]. Gut health disorders, parasitic infections, imbalances, or deficiencies of other nutrients (dietary fat, other fat-soluble vitamins etc.), stress, mycotoxins in feed, and certain diseases can impair the absorption of retinol in the digestive tract [87]. To mitigate these factors, implementing measures to promote good gut health, such as using intestinal health promoters or ensuring proper sanitation practices, is important [88]. These strategies optimize vitamin A absorption and utilization in poultry.
- (4)
-
Bioavailability: The bioavailability of vitamin A sources is often overlooked, but it plays a crucial role [89]. Unlike other vitamins, vitamin A in the form of retinyl acetate is commonly formulated in small solid beadlet particles by different suppliers (Figure 2). This can result in variations in stability and bioavailability among the vitamin A products available on the market [84]. Experienced formulators can easily create highly stable vitamin A products that can withstand challenging conditions with the help of special formulation aids. However, the main challenge lies in the digestive tract of poultry, where these stable formulations must release retinyl acetate in the intestinal lumen. If the formulations are too stable, they may have reduced or no biological value at all [90]. Therefore, it is essential to strike a delicate balance between the overall stability and bioavailability of a vitamin A commercial product. This balance ensures the product’s ability to withstand harsh storage conditions in premixes and high pelleting temperatures in feed, while also facilitating easy release in the digestive tract.
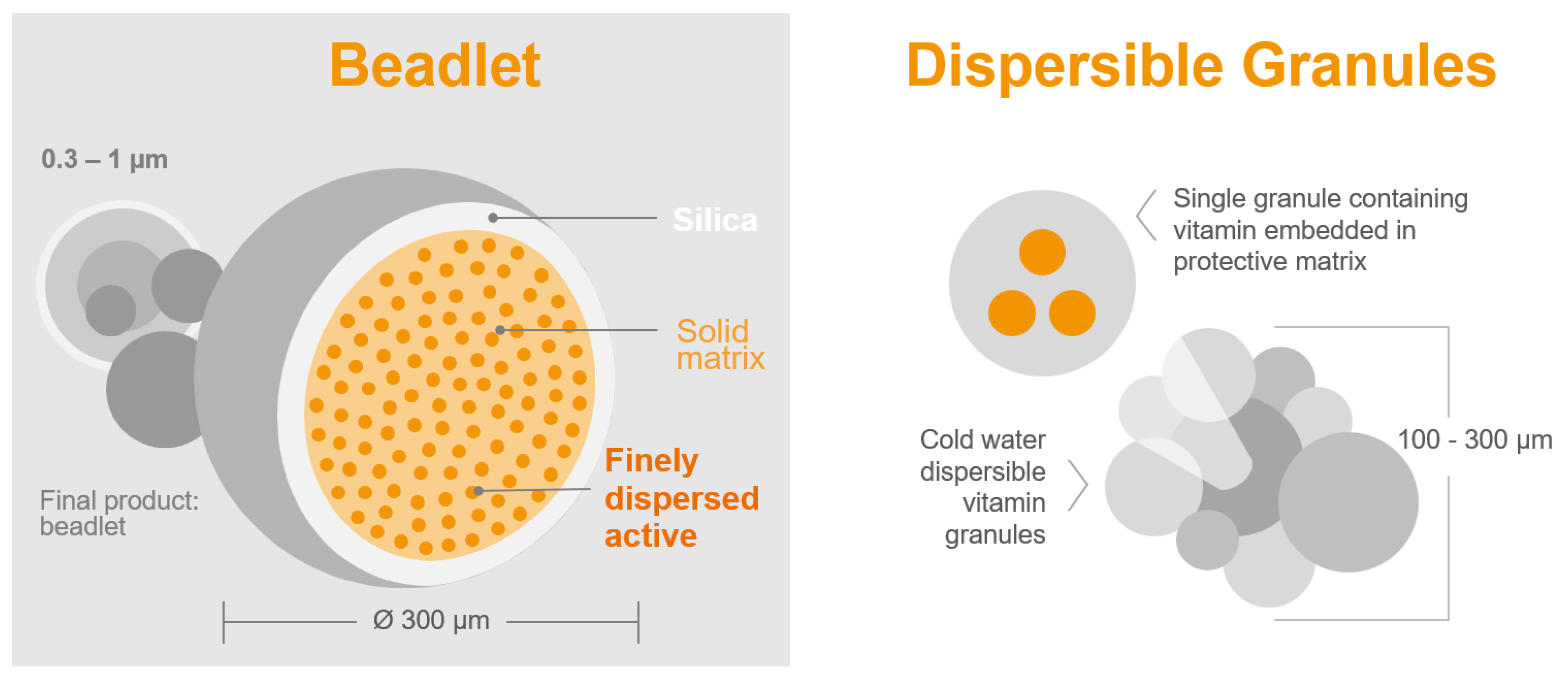 Figure 2. Microencapsulation: Delivering a diverse range of products tailored to target applications.
Figure 2. Microencapsulation: Delivering a diverse range of products tailored to target applications.
In conclusion, identifying the causes of vitamin A deficiency in poultry diets requires a comprehensive approach. Addressing inadequate feed formulation, ensuring proper storage and handling practices, sourcing high-quality feed ingredients, and mitigating factors that hinder absorption and utilization are all vital steps in preventing and managing vitamin A deficiency in domestic fowl.
This entry is adapted from the peer-reviewed paper 10.3390/poultry2040037
References
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Q. 2020, 41, 1–29.
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Puvača, N.; Laudadio, V.; Mazzei, D.; Seidavi, A.; Ayasan, T.; Tufarelli, V. Pros and Cons of Dietary Vitamin A and Its Precursors in Poultry Health and Production: A Comprehensive Review. Antioxidants 2023, 12, 1131.
- Shastak, Y.; Pelletier, W. Delving into Vitamin A Supplementation in Poultry Nutrition: Current Knowledge, Functional Effects, and Practical Implications. World's Poult. Sci. J. 2023, 79, 1–23.
- Shojadoost, B.; Yitbarek, A.; Alizadeh, M.; Kulkarni, R.R.; Astill, J.; Boodhoo, N.; Sharif, S. Centennial Review: Effects of vitamins A, D, E, and C on the chicken immune system. Poult. Sci. 2021, 100, 100930.
- McDowell, L.R. Vitamins in Animal and Human Nutrition; McDowell, L.R., Ed.; Iowa State University Press: Ames, IA, USA, 2000; pp. 15–90.
- Çalişlar, S. The Important of Beta Carotene on Poultry Nutrition. Selcuk. J. Agric. Food Sci. 2019, 33, 252–259.
- Moreno, J.A.; Díaz-Gómez, J.; Nogareda, C.; Angulo, E.; Sandmann, G.; Portero-Otin, M.; Serrano, J.C.E.; Twyman, R.M.; Capell, T.; Zhu, C.; et al. The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci. Rep. 2016, 6, 35346.
- Green, A.S.; Fascetti, A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016, 2016, 7393620.
- Surai, P. The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 1. World's Poult. Sci. J. 2012, 68, 465–476.
- Guo, S.; He, L.; Zhang, Y.; Niu, J.; Li, C.; Zhang, Z.; Li, P.; Ding, B. Effects of Vitamin A on Immune Responses and Vitamin A Metabolism in Broiler Chickens Challenged with Necrotic Enteritis. Life 2023, 13, 1122.
- Cortes, P.L.; Tiwary, A.K.; Puschner, B.; Crespo, R.M.; Chin, R.P.; Bland, M.; Shivaprasad, H.L. Vitamin A deficiency in turkey poults. J. Vet. Diagn. Investig. 2006, 18, 489–494.
- EFSA (European Food Safety Authority). Scientific opinion of the panel on additives and products or substances used in animal feed (FEEDAP) on a request from the European Commission on the consequences for the consumer of the use of vitamin A in animal nutrition. EFSA J. 2008, 873, 1–81.
- Rautenschlein, S.; Ryll, M. Nicht-infektiöse Erkrankungen und Veränderungen. In Erkrankungen des Nutzgeflügels; Eugen Ulmer Verlag: Stuttgart, Germany, 2014; pp. 210–220.
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264.
- Shah, A.S.R.; Çetingül, I.S. Nutritional advances in production performance and product quality of poultry husbandry under heat stress. Online J. Anim. Feed. Res. 2022, 12, 53–65.
- Abd El-Hack, M.E.; Alagawany, M.; Mahrose, K.M.; Arif, M.; Saeed, M.; Arain, M.A.; Soomro, R.N.; Siyal, F.A.; Fazlani, F.A.; Fowler, J. Productive performance, egg quality, hematological parameters and serum chemistry of laying hens fed diets supplemented with certain fat-soluble vitamins, individually or combined, during summer season. Anim. Nutr. 2019, 5, 49–55.
- Carazo, A.; Macákova, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity. Nutrients 2021, 13, 1703.
- EFSA (European Food Safety Authority). Scientific Opinion on the Safety and Efficacy of Vitamin A (Retinyl Acetate, Retinyl Palmitate and Retinyl Propionate) as a Feed Additive for All Animal Species and Categories. EFSA J. 2013, 11, 3037.
- Harrison, E.H.; Hussain, M.M. Mechanisms Involved in the Intestinal Digestion and Absorption of Dietary Vitamin A. J. Nutr. 2001, 131, 1405–1408.
- Sideeg, R.M. Effect of Dietary Vitamin A and Nigella Sativa on the Performance of Broiler Chicks. Master’s Thesis, University of Khartoum, Khartoum, Sudan, 1996.
- Yin, H.D.; Tian, K.; Li, D.Y.; Gilbert, E.R.; Xiao, L.H.; Chen, S.Y.; Wang, Y.; Liu, Y.P.; Zhao, X.L.; Zhu, Q. Expression Profiles of Cellular Retinol-binding Protein, Type II (CRBP II) in Erlang Mountainous Chickens. Asian-Australas. J. Anim. Sci. 2014, 27, 310–315.
- Senoo, H.; Imai, K.; Mezaki, Y.; Miura, M.; Morii, M.; Fujiwara, M.; Blomhoff, R. Accumulation of vitamin A in the hepatic stellate cell of arctic top predators. Anat. Rec. 2012, 295, 1660–1668.
- Kim, D.H.; Lee, J.; Suh, Y.; Cressman, M.; Lee, K. Research Note: All-trans retinoic acids induce adipogenic differentiation of chicken embryonic fibroblasts and preadipocytes. Poult. Sci. 2020, 99, 7142–7146.
- Zhang, R.; Wang, Y.; Li, R.; Chen, G. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. Int. J. Mol. Sci. 2015, 16, 14210–14244.
- Sporn, M.B.; Roberts, A.B.; Goodman, D.S. The Retinoids; Academic Press: Orlando, FL, USA, 1984.
- Barua, A.B.; Olson, J.A. Retinoyl beta-glucuronide: An endogenous compound of human blood. Am. J. Clin. Nutr. 1986, 43, 481–485.
- Huan, J. Effect of Dietary Pyrrolizidine Alkaloids on Copper and Vitamin A Metabolism in the Chicken and Japanese Quail. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1991.
- Kam, R.K.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell. Biosci. 2012, 2, 11.
- Yu, M.; Guan, K.; Zhang, C. The promoting effect of retinoic acid on proliferation of chicken primordial germ cells by increased expression of cadherin and catenins. Amino Acids 2010, 40, 933–941.
- Underhill, T.M.; Weston, A.D. Retinoids and their receptors in skeletal development. Microsc. Res. Tech. 1998, 43, 137–155.
- Wang, J.L.; Swartz-Basile, D.A.; Rubin, D.C.; Levin, M.S. Retinoic acid stimulates early cellular proliferation in the adapting remnant rat small intestine after partial resection. J. Nutr. 1997, 127, 1297–1303.
- Seiler, K.M.; Waye, S.E.; Kong, W.; Kamimoto, K.; Bajinting, A.; Goo, W.H.; Onufer, E.J.; Courtney, C.; Guo, J.; Warner, B.W.; et al. Single-Cell Analysis Reveals Regional Reprogramming During Adaptation to Massive Small Bowel Resection in Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 407–426.
- Yan, C.; Ghaffari, M.; Whitsett, J.A.; Zeng, X.; Sever, Z.; Lin, S. Retinoic acid-receptor activation of SP-B gene transcription in respiratory epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 1998, 275, L239–L246.
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152.
- Danielpour, D.; Song, K. Cross-talk between IGF-I and TGF-beta signaling pathways. Cytokine Growth Factor Rev. 2006, 17, 59–74.
- Oliveira, L.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126.
- Halevy, O.; Arazi, Y.; Melamed, D.; Friedman, A.; Sklan, D. Retinoic acid receptor-alpha gene expression is modulated by dietary vitamin A and by retinoic acid in chicken T lymphocytes. J. Nutr. 1994, 124, 2139–2146.
- Liu, Z.-M.; Wang, K.-P.; Ma, J.; Zheng, S.G. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol. Immunol. 2015, 12, 553–557.
- Jiang, H.; Promchan, K.; Lin, B.R.; Lockett, S.; Chen, D.; Marshall, H.; Badralmaa, Y.; Natarajan, V. LZTFL1 Upregulated by All-Trans Retinoic Acid during CD4+ T Cell Activation Enhances IL-5 Production. J. Immunol. 2016, 196, 1081–1090.
- Jäger, A.; Kuchroo, V.K. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand. J. Immunol. 2010, 72, 173–184.
- Brown, C.C.; Esterhazy, D.; Sarde, A.; London, M.; Pullabhatla, V.; Osma-Garcia, I.; Al-Bader, R.; Ortiz, C.; Elgueta, R.; Arno, M.; et al. Retinoic acid is essential for Th1 cell lineage stability and prevents transition to a Th17 cell program. Immunity 2015, 42, 499–511.
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349.
- Marks, E.; Ortiz, C.; Pantazi, E.; Bailey, C.S.; Lord, G.M.; Waldschmidt, T.J.; Noelle, R.J.; Elgueta, R. Retinoic Acid Signaling in B Cells Is Required for the Generation of an Effective T-Independent Immune Response. Front. Immunol. 2016, 7, 643.
- Elias, P.M.; Friend, D.S. Vitamin-A-induced mucous metaplasia. An in vitro system for modulating tight and gap junction differentiation. J. Cell Biol. J. Cell Biol. 1976, 68, 173–188.
- Fan, X.; Liu, S.; Liu, G.; Zhao, J.; Jiao, H.; Wang, X.; Song, Z.; Lin, H. Vitamin A Deficiency Impairs Mucin Expression and Suppresses the Mucosal Immune Function of the Respiratory Tract in Chicks. PLoS ONE. 2015, 10, e0139131.
- Abdelhamid, L.; Luo, X.M. Retinoic Acid, Leaky Gut, and Autoimmune Diseases. Nutrients 2018, 10, 1016.
- Lochbaum, R.; Schilpp, C.; Nonnenmacher, L.; Frick, M.; Dietl, P.; Wittekindt, O.H. Retinoic acid signalling adjusts tight junction permeability in response to air-liquid interface conditions. Cell. Signal 2020, 65, 109421.
- Chung, Y.; Chang, S.H.; Martinez, G.J.; Yang, X.O.; Nurieva, R.; Kang, H.S.; Ma, L.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009, 30, 576–587.
- Reid, W.D.; Close, A.J.; Humphrey, S.; Chaloner, G.; Lacharme-Lora, L.; Rothwell, L.; Kaiser, P.; Williams, N.J.; Humphrey, T.J.; Wigley, P.; et al. Cytokine responses in birds challenged with the human food-borne pathogen Campylobacter jejuni implies a Th17 response. R. Soc. Open Sci. 2016, 3, 150541.
- Wang, X.; Allen, C.; Ballow, M. Retinoic acid enhances the production of IL-10 while reducing the synthesis of IL-12 and TNF-alpha from LPS-stimulated monocytes/macrophages. J. Clin. Immunol. 2007, 27, 193–200.
- Wu, H.; Zhang, G.; Minton, J.E.; Ross, C.R.; Blecha, F. Regulation of cathelicidin gene expression: Induction by lipopolysaccharide, interleukin-6, retinoic acid, and Salmonella enterica serovar typhimurium infection. Infect. Immun. 2000, 68, 5552–5558.
- Elloumi, H.Z.; Holland, S.M. Complex regulation of human cathelicidin gene expression: Novel splice variants and 5′UTR negative regulatory element. Mol. Immunol. 2008, 45, 204–217.
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free. Radic. Biol. Med. 1999, 26, 746–761.
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553.
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721.
- Bohn, T.; Böhm, V.; Dulińska-Litewka, J.; Landrier, J.-F.; Bánáti, D.; Kucuk, O.; Borel, P.; Canas, J.A.; Rühl, R. Is vitamin A an antioxidant? Int. J. Vitam. Nutr. Res. 2022, a000752.
- Tesoriere, L.; Ciaccio, M.; Bongiorno, A.; Riccio, A.; Pintaudi, A.; Livrea, M. antioxidant activity of all-trans-retinol in homogeneous solution and in phosphatidylcholine liposomes. Arch. Biochem. Biophys. 1993, 307, 217–223.
- Ahlemeyer, B.; Bauerbach, E.; Plath, M.; Steuber, M.; Heers, C.; Tegtmeier, F.; Krieglstein, J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free. Radic. Biol. Med. 2001, 30, 1067–1077.
- Szymański, L.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660.
- Gad, A.; Abu Hamed, S.; Khalifa, M.; Amin, A.; El-Sayed, A.; Swiefy, S.A.; El-Assal, S. Retinoic acid improves maturation rate and upregulates the expression of antioxidant-related genes in in vitro matured buffalo (Bubalus bubalis) oocytes. Int. J. Veter-Sci. Med. 2018, 6, 279–285.
- Pu, J.; Chen, D.; Tian, G.; He, J.; Huang, Z.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Luo, Y.; et al. All-Trans Retinoic Acid Attenuates Transmissible Gastroenteritis Virus-Induced Apoptosis in IPEC-J2 Cells via Inhibiting ROS-Mediated P38MAPK Signaling Pathway. Antioxidants 2022, 11, 345.
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928.
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274.
- Mishra, B.; Jha, R. Oxidative Stress in the Poultry Gut: Potential Challenges and Interventions. Front. Veter. Sci. 2019, 6, 60.
- Surai, P.F. Antioxidant Systems in Poultry Biology: Superoxide Dismutase. J. Anim. Res. Nutr. 2015, 1, 8.
- Fu, Z.; Kato, H.; Sugahara, K.; Kubo, T. Retinoic acid accelerates the development of reproductive organs and egg production in Japanese quail (Coturnix coturnix japonica). Biol. Reprod. 2000, 63, 1795–1800.
- Endo, T.; Mikedis, M.M.; Nicholls, P.K.; Page, D.C.; de Rooij, D.G. Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules 2019, 9, 775.
- Busada, J.T.; Geyer, C.B. The Role of Retinoic Acid (RA) in Spermatogonial Differentiation. Biol. Reprod. 2016, 94, 10.
- Abdelnour, S.A.; Abd El-Hack, M.E.; Swelum, A.A.; Saadeldin, I.M.; Noreldin, A.E.; Khafaga, A.F.; Al-Mutary, M.G.; Arif, M.; Hussein, E.O.S. The Usefulness of Retinoic Acid Supplementation during In Vitro Oocyte Maturation for the In Vitro Embryo Production of Livestock: A Review. Animals 2019, 9, 561.
- Wang, W.-D.; Hsu, H.-J.; Li, Y.-F.; Wu, C.-Y. Retinoic Acid Protects and Rescues the Development of Zebrafish Embryonic Retinal Photoreceptor Cells from Exposure to Paclobutrazol. Int. J. Mol. Sci. 2017, 18, 130.
- Kim, D.H.; Lee, J.; Kim, S.; Lillehoj, H.S.; Lee, K. Hypertrophy of Adipose Tissues in Quail Embryos by in ovo Injection of All-Trans Retinoic Acid. Front. Physiol. 2021, 12, 681562.
- Mark, M.; Ghyselinck, N.B.; Chambon, P. Function of retinoic acid receptors during embryonic development. Nucl. Recept. Signal. 2009, 7, e002.
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931.
- Gur, M.; Bendelac-Kapon, L.; Shabtai, Y.; Pillemer, G.; Fainsod, A. Reduced Retinoic Acid Signaling During Gastrulation Induces Developmental Microcephaly. Front. Cell Dev. Biol. 2022, 10, 844619.
- Aydelotte, M.B. Vitamin A deficiency in chickens. Br. J. Nutr. 1963, 17, 205–210.
- Bridges, C.D.; Alvarez, R.A.; Fong, S.L.; Liou, G.I.; Ulshafer, R.J. Rhodopsin, vitamin A, and interstitial retinol-binding protein in the rd chicken. Invest. Ophthalmol. Vis. Sci. 1987, 28, 613–617.
- Kelley, M.W.; Turner, J.K.; Reh, T.A. Retinoic acid promotes differentiation of photoreceptors in vitro. Development 1994, 120, 2091–2102.
- Lima, H.; Souza, L. Vitamin A in the diet of laying hens: Enrichment of table eggs to prevent nutritional deficiencies in humans. World's Poult. Sci. J. 2018, 74, 619–626.
- Randhawa, S.R.; Deshmukh, S.; Banga, H.S.; Singh, N. Concomitant Vitamin A Deficiency Following Suspected Fowl pox virus Infection Leading to Esophageal Gland Metaplasia in a Layer Flock. J. Anim. Res. 2018, 8, 1087–1090.
- Kleyn, R.; Chrystal, P. Vitamins. In Broiler Nutrition: Masterclass; Context Products Ltd.: Leicestershire, UK, 2020; pp. 129–142.
- Al-Deseit, B. Least-Cost Broiler Ration Formulation Using Linear Programming Technique. J. Anim. Vet. Advan. 2009, 8, 1274–1278.
- Mallick, P.; Muduli, K.; Biswal, J.N.; Pumwa, J. Broiler Poultry Feed Cost Optimization Using Linear Programming Technique. J. Oper. Strat. Plan. 2020, 3, 31–57.
- Yang, P.; Wang, H.K.; Zhu, M.; Li, L.X.; Ma, Y.X. Degradation kinetics of vitamins in premixes for pig: Effects of choline, high concentrations of copper and zinc, and storage time. Anim. Biosci. 2021, 34, 701–713.
- Hirai, R.A.; De Leon, D.; Randig-Biar, M.; Silva, A.; Sanchez, E.; McElroy, A.P.; Bailey, C.A.; Martinez, N.; Sokale, A.; Music, L. Evaluation of the stability of vitamin A acetate concentrates mixed in a vitamin-trace mineral premix over a 56-day high temperature and humidity storage stress. In Proceedings of the 2023 The International Poultry Scientific Forum, Atlanta, GA, USA, 23–24 January 2023; p. 112.
- Abdollahi, M.R.; Ravindran, V.; Svihus, B. Pelleting of broiler diets: An overview with emphasis on pellet quality and nutritional value. Anim. Feed. Sci. Technol. 2013, 179, 1–23.
- Baker, D.H. Bioavailability of minerals and vitamins. In Swine Nutrition, 2nd ed.; Lewisand, A.J., Southern, L.L., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2001; pp. 357–379.
- Leeson, S.; Summers, J.D. Commercial Poultry Nutrition. In Scott’s Nutrition of the Chicken, 3rd ed.; Leeson, S., Ed.; Nottingham University Press: Nottingham, UK, 2001; p. 398.
- Oviedo-Rondón, E.O. Holistic view of intestinal health in poultry. Anim. Feed. Sci. Technol. 2019, 250, 1–8.
- Baker, D.H.; Stein, H.H. Bioavailability of Minerals and Vitamins in Feedstuffs. In Sustainable Swine Nutrition; Chiba, L.I., Ed.; Wiley-Blackwell Press: Ames, IA, USA, 2013; pp. 341–364.
- Teleki, A.; Hitzfeld, A.; Eggersdorfer, M. 100 Years of Vitamins: The Science of Formulation is the Key to Functionality. KONA Powder Part. J. 2013, 30, 144–163.
This entry is offline, you can click here to edit this entry!
