Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Non-targeted effects (NTE) are an intriguing phenomenon where the biological responses observed in cells or tissues are not directly exposed to a stressor (e.g., ionizing radiation or chemical agents). onventional radiation biology approaches have predominantly focused on the macroscopic effects of ionizing radiation, overlooking the quantum-scale interactions that may play a crucial role in NTE. Quantum biology (QB) offers a unique perspective to explore and understand the intricate and subtle processes underlying NTE.
- quantum biology
- non-targeted effects (NTE)
- Quantum superposition
- Quantum Entanglement
- Quantum tunneling
- Quantum coherence
- Quantum sensing
- Quantum information
1. Introduction
Non-targeted effects (NTE) are an intriguing phenomenon where the biological responses observed in cells or tissues are not directly exposed to a stressor (e.g., ionizing radiation or chemical agents). These effects can occur as a result of signaling and communication between exposed and non-exposed cells, leading to alterations in cellular behavior and function beyond the irradiated or exposed region [1]. NTE have implications for various fields, including radiation biology, toxicology, and environmental health, which demonstrates that it is essential to consider them in understanding the broader impact of stressors on biological systems [2][3]. Traditionally, the harmful effects of ionizing radiation have been attributed solely to the direct damage caused by energy deposition in the target cells [4][5]. However, research in recent years has revealed that radiation-induced effects extend beyond the directly irradiated cells, impacting neighboring and distant cells that are not exposed to radiation [6][7]. These NTE can result from signaling mechanisms, such as bystander effects and genomic instability, which trigger cellular responses in non-irradiated tissues [4]. They can also result from hormesis mechanisms, discussed later, which, in common with NTE, involve reactive oxygen species, bioelectric effects in mitochondria, and a number of epigenetic mechanisms, including genomic imprinting and methylation [8].
NTE have considerable significance in radiation exposure, including medical radiation therapy, environmental radiation exposure, and occupational exposures [9].
Understanding these effects takes center stage in the realm of optimizing radiation treatment strategies. The goal here is to strike a balance—maximizing the impact on cancer cells while minimizing any collateral damage to healthy tissues [10]. In environmental and occupational settings, the presence of NTE indicates that exposure to ionizing radiation or other potentially harmful agents can lead to effects not only at the site of exposure but also in distant and seemingly unexposed tissues [11]. Increasing evidence is also emerging for the interorganism and even interspecies communication of signals, leading to effects in unexposed individuals [12]. This potentially provides a bridge allowing population-level effects to be recorded. Current radiation protection regulations primarily focus on the direct effects of radiation on the target tissues [13][14]; however, the effects of radiation exposure are complex. Not all effects are harmful and there are many documented reports of low-dose hormesis and induced adaptive responses. Low-dose hypersensitivity has also been reported both in vivo and in vitro, meaning that it is critical to understand how and why a particular response dominates. Since NTE mechanisms and responses are so important in low-dose radiobiology, incorporating NTE into radiation protection models can lead to more comprehensive and accurate assessments of radiation risks to ensure better protection for individuals and populations [15][16].
Despite the significant progress made in unraveling the complexities of the non-targeted effects (NTE) induced by ionizing radiation, there are still several limitations in our current knowledge of the underlying mechanisms [16][17][18]. One major challenge is the multifaceted nature of NTE, which involve signaling pathways, cellular responses, and intercellular communication processes that extend beyond the directly irradiated cells [19]. The exact molecular events that trigger and propagate these non-targeted responses are becoming better understood and involve photon emission, the exosome-mediated transfer of information, the elevation of reactive oxygen species, and ion channel perturbations in the irradiated entities [20], while, in the recipients, TGF-beta, p53, and many other stress response and DNA damage response pathways are activated [21]. Mitochondria play a major role in the NTE processes (see the comprehensive review by Averbeck, 2023 [22]).
Living systems fundamentally rely on electrical processes (e.g., biochemical reactions or the flow of ions across cellular membranes) [23], and the energy transfer within these systems occurs through various mechanisms involving electromagnetic radiation (e.g., photons) and electric field fluctuations (e.g., electron interactions with photons) [24][25]. These processes encompass excitation and ionization, as well as rotational and vibrational transitions [26][27], and have the potential to induce damage and disrupt the normal functioning of molecules and biological processes, which may lead to various consequences, including the formation of free radicals, the activation of enzymes, protein malfunction or structural alterations, and changes in gene expression [28][29]. In the 1970s, David DeVault and Britton Chance made significant contributions to our understanding of these biological processes [24]. DeVault focused on molecular dynamics, electronic energy transfer, and reaction mechanisms in biological systems, especially electron transfer reactions in photosynthesis [24]. Britton Chance, on the other hand, concentrated on enzyme kinetics and pioneered the use of NADH as a marker for mitochondrial function. He developed non-invasive techniques to study mitochondrial redox states and advanced spectroscopic methods, expanding the applications of spectroscopy in enzyme kinetics and bioenergetics. Furthermore, for over a century, morphogenetic and bioelectric fields have been used to understand life processes [30][31]. Morphogenetic fields is believed to organize biological form development [32], while bioelectric fields result from ion movement across cell membranes and influence various cellular processes. These fields may be influenced by quantum effects [32], where entanglement facilitates long-distance communication between morphogenetic fields. This quantum phenomenon, coupled with tunneling activates signaling pathways within bioelectric fields. Fritz-Albert Popp significantly contributed to the study of biophotons [33][34], exemplified by his development of a photon counter, an instrument capable of quantifying biophoton emissions from living organisms. This helps us understand how biofields and biophotons are connected, revealing their quantum foundations. Popp’s investigations revealed that a variety of stimuli, including stress and radiation exposure, could stimulate an increase in biophoton emissions [35][36][37]. Biophotons, ultraweak photons emitted by living organisms, have been observed across a range of wavelengths, from ultraviolet (UV) to infrared (IR) [38], documented across various life forms, from bacteria to humans. While the exact mechanisms behind biophoton production are under ongoing research, they are believed to be associated with vital cellular processes such as metabolism, cell signaling, and DNA repair.
Conventional radiation biology approaches have predominantly focused on the macroscopic effects of ionizing radiation, overlooking the quantum-scale interactions that may play a crucial role in NTE [16][39][40]. Quantum biology (QB) offers a unique perspective to explore and understand the intricate and subtle processes underlying NTE [41][42]. In fact, the quantum effects hold the promise of understanding the mechanisms that govern NTE at the molecular and subcellular levels, offering tools that enable us to investigate how quantum processes shape interactions between molecules, signaling pathways, and cellular functions, as well as shedding light on the intricate dynamics underlying NTE responses and opening new avenues in radiation biology. As a result, more effective therapeutic approaches, improved radiation protection standards, and a better understanding of the effects of ionizing radiation on human health can be developed [39][43][44].
2. Quantum Biology and Non-Targeted Effects
2.1. Quantum Physics Meets Biology
Quantum physics, often referred to as quantum mechanics, explores the behavior of matter and energy at the most minuscule scales, encompassing the behavior of atoms and subatomic particles (e.g., electrons, protons, and photons) [45][46][47]. It stands apart from classical physics by allowing particles to exist in multiple states simultaneously—a concept known as wave–particle duality. This fundamental shift in perspective emerged in the early 20th century, addressing previously unexplained phenomena like the photoelectric effect and the blackbody spectrum [48][49][50][51]. Wave–particle duality, a central concept in quantum physics, reveals that particles can exhibit both wave-like and particle-like properties [52]. This duality is exemplified in the double-slit experiment [53][54], where electrons fired at a double slit create interference patterns akin to waves. While proposed a century ago, wave–particle duality remains crucial in understanding the behavior of matter at atomic and subatomic scales.
Quantum biology, which emerges at the nexus of quantum physics and biology, aims to explore the potential influence of quantum phenomena within biological processes, from the molecular to the organismal [55][56][57]. The terms “quantum effects”, “quantum behavior”, and “quantum phenomena” are related terms in quantum mechanics, describing the behavior of particles and systems at the subatomic level, such as electrons, photons, atoms, and molecules. Quantum effects manifest in various biological phenomena, affecting the timing and efficiency of essential biochemical reactions [58][59]. The extent of these quantum effects within the intricate realm of biology remains a challenging question [32][44]. Navigating quantum biological scales presents a formidable challenge in understanding the precise scope of quantum effects within biology. These effects are established at molecular and cellular scales, but evidence suggests their involvement even in the realm of organisms (e.g., intriguing research indicates that birds might leverage quantum effects in their remarkable navigation abilities) [60]. Quantum biology challenges long-held beliefs that limit quantum effects to microscopic scales within the warm and wet conditions of life and pushes the boundaries of our understanding of biology by examining how quantum effects, once thought to be relevant only at the microscopic scale, can play a role in complex, macroscopic biological systems [44]. Traditionally, physicists assumed that high temperatures, a low vacuum, or strong interactions in living cells limited the existence of quantum effects. A paradigm shift suggests that quantum effects can indeed operate within the complex fabric of biological systems [61][62]. However, a fundamental question persists—to what extent do these quantum effects stretch in time and space within the intricate tapestry of biology?
Classical and quantum variability, precisely the stochastic behaviors of their fluctuations, are crucial in understanding the interplay of biological systems exposed to radiation. Classical fluctuations are driven by various sources, including thermal motion, molecular interactions, and external factors such as radiation, where the stochastic behavior of these classical fluctuations arises from the random and chaotic movement of particles at the macroscopic level [23][63]. Due to their large-scale nature, classical fluctuations can often be described statistically, with behaviors following Gaussian or normal distributions. These fluctuations exhibit a degree of predictability when analyzed collectively but remain inherently stochastic at the individual particle level. In the context of radiation exposure, classical fluctuations can lead to unpredictable variations in tissue temperature, the generation of reactive oxygen species (ROS), which include molecules like superoxide (O2•−) and hydrogen peroxide (H2O2), and subsequent cellular responses, all of which are characterized by random and fluctuating patterns. Quantum fluctuations, on the other hand, are inherently stochastic at a fundamental level due to the Heisenberg Uncertainty Principle [64][65]. They are minute random fluctuations in the values of the fields that represent elementary particles, such as electric and magnetic fields, which represent the electromagnetic force carried by photons. This principle states that it is impossible to precisely determine both the position and momentum of a particle simultaneously. The energy of an electron in an atom is not constant, but rather fluctuates up and down slightly. As a result, quantum fluctuations arise due to the inherent uncertainty in particle behavior at the subatomic level. These fluctuations manifest as unpredictable variations in the positions, momenta, and energies of quantum entities. The stochastic behavior of quantum fluctuations is a fundamental aspect of quantum mechanics and is particularly pronounced in the microscopic world of biological molecules and cellular components. It introduces inherent randomness and indeterminacy into processes such as DNA repair, enzyme catalysis, and cellular signaling [61][66].
Biophotons are photons emitted by living organisms and can be classified into classical and quantum based on the processes through which they are emitted, absorbed, or transmitted. Classical biophotons are emitted by random, spontaneous transitions of biological molecules from one energy state to another and are not entangled or do not exhibit quantum coherence. Quantum biophotons, on the other hand, are emitted through quantum processes, such as resonant energy transfer or quantum tunneling, and can be entangled or exhibit quantum coherence [62]. The distinction between classical and quantum biophotons is not always clear-cut and, in many cases, biophotons may exhibit both classical and quantum properties (e.g., even if emitted through a quantum process, they may still exhibit random fluctuations in timing and intensity),whose significance for quantum fluctuations becomes particularly pronounced in the bystander effect (e.g., entangled biophotons emitted by a cell could convey information about radiation damage to other cells in the body, even if not directly targeted by radiation) [67]. Moreover, quantum fluctuations in biophotons may influence cellular responses to radiation damage and the potential for their quantum coherence is imbued with quantum-encoded information [68], which influence gene expression and various cellular processes, ultimately contributing to the bystander effect [69]. When emitted by irradiated cells, they may act as signaling agents, affecting gene expression and other cellular processes. Understanding the role of quantum effects is crucial in mediating the non-targeted effects of ionizing radiation on processes influenced by infrared radiation (IR) [70]—specifically, how quantum entanglement can facilitate IR-mediated signaling across long distances and how quantum tunneling can enable IR-mediated energy transfer even through barriers [71][72].
Quantum entities, such as electrons, protons, and photons, are fundamental to biological processes. They exhibit both particle-like and wave-like behaviors, famously experimented with the double-slit experiment. Electrons, despite their negative charge, are central to energy generation within cellular respiration and metabolic processes [73][74][75][76]. Their unique feature is the wave–particle duality, allowing them to simultaneously exist as both discrete particles, participating in chemical reactions, and as quantum entities exhibiting wave-like properties. This duality enables phenomena like quantum tunneling, which plays a pivotal role in energy and information transfer within living organisms. Protons, positively charged particles found in atomic nuclei, have precise roles in regulating the pH balance and enzymatic activity [77][78]. Just like electrons, they exhibit similar wave–particle duality in the quantum context, influencing energy transfer reactions and chemical interactions in biological systems [79][80]. Finally, photons are carriers of light and electromagnetic radiation, essential in various biological processes, including photosynthesis, vision, and medical imaging [81][82]. Furthermore, Marcus Arndt’s work in matter wave interferometry demonstrates that not only electrons and photons but also relatively larger molecules, such as those found in biological systems (e.g., protoporphyrins), can exhibit wave–particle duality effects and therefore display quantum behaviors, such as quantum tunneling, coherence, and entanglement, and these effects impact the biological domain (see Figure 1) [44][83].
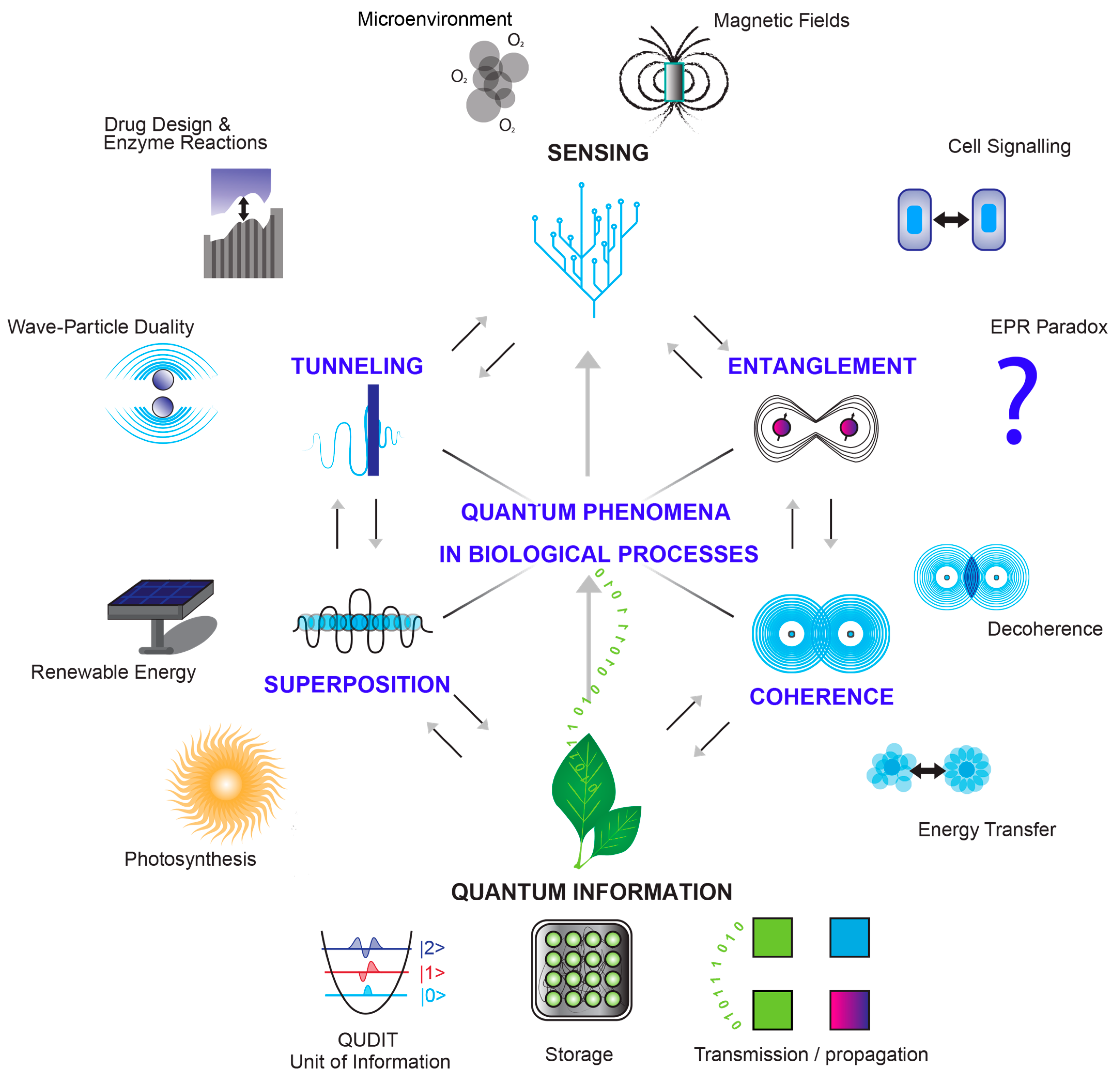
Figure 1. Quantum phenomena in biology. Quantum superposition—revealing simultaneous particle states: promising enhanced light harvesting in photosynthesis and pioneering renewable energy applications. Quantum entanglement—illustrating correlated states: significance in cellular signaling and the EPR paradox’s interconnected particles which raises deep questions (symbolized as an interrogation mark) about the nature of reality, the role of observation, and the fundamental principles of quantum mechanics. Quantum tunneling—visualizing barrier overcoming: enzymatic reaction applications and wave–particle duality. Quantum coherence—depicting maintained phase relationships: explore cellular energy transfer and function impact, and coherence vs. decoherence in quantum systems. Quantum sensing—demonstrating signal detection: encompasses cellular environment sensing and magnetic field detection in biology. Quantum information—unleashing biological data power: ‘Qdits’ in d-dimensional complex vector spaces for the representation of a complex unit of biological data that could be observed during manipulation, storage, and transmission/propagation.
-
Quantum superposition
The concept of quantum superposition refers to the remarkable ability of tiny particles to exist in multiple states simultaneously [84], akin to simultaneously juggling multiple tasks [85][86][87]. This concept is relevant in various biological and physical processes, including the intriguing phenomenon of photosynthesis (e.g., how plants and bacteria utilize sunlight for energy production) [56][88]. In non-classical physics, particles have the potential to occupy different states simultaneously, much like plants capturing sunlight from multiple angles to drive energy production [56][88], which offers practical implications in enhancing renewable energy technologies (e.g., efficient solar panels by enabling them to capture more sunlight and convert it into energy more effectively) [89][90]. To grasp this concept more comprehensively, let us consider the notion of “quantum states”, where there are various ways in which particles can arrange themselves [91][92][93][94]. These states are described using “quantum numbers” [95], which act as labels explaining particle behavior. To illustrate, imagine a spinning top that can rotate in two directions—particles like electrons possess a spin, either “up” or “down”, [96][97][98][99], characterized using quantum numbers like “n” and “m”. When these quantum states interact, they give rise to new combinations, much like mixing colors to create new shades. This phenomenon can be described as particles behaving like waves, which produce different patterns as their states merge [100][101][102]. An analogy could be drawn from water waves overlapping to form intricate patterns. Particles have in fact intriguing wave characteristics when their quantum states come into contact, resulting in fascinating effects. This can be considered as putting together puzzle pieces to reveal the entire picture—the collective quantum states form the entire system, just like fitting puzzle pieces to create a complete image, where each observable quantum state is made up of smaller component states [91][92][103]. Finally, the Schrödinger equation, a mathematical tool, plays a pivotal role in elucidating how particles manifest wave-like properties [49][50][51], which enables us to simulate how particles respond when they interact with their environment [84].
-
Quantum Entanglement
Quantum entanglement is a mysterious phenomenon where two or more quantum systems are interconnected in such a way that they share the same properties, even when separated by vast distances. In this context, when two or more particles become entangled, their states become correlated, regardless of distance, which could potentially influence cellular signaling and communication processes. Einstein’s term, “spooky action at a distance”, captures its puzzling nature, as it seems to defy the laws of classical physics. Nevertheless, this phenomenon has been repeatedly confirmed through experiments. It refers to the interactions of particles whose states cannot be independently described accurately, even when separated by considerable distances [104][105][106]. This intriguing phenomenon has sparked debates due to its unique properties (e.g., measuring one entangled particle directly affects the others, leading to seemingly paradoxical effects), and the Einstein–Podolsky–Rosen (EPR) Paradox serves as an example of the complexity and implications of entanglement [107][108]. Interestingly, pairs of entangled electrons in “Posner molecules”, clusters of phosphate and calcium ions, have been shown to potentially transfer energy between cells. Moreover, in the development of the nervous system, the coordinated activity of neurons is crucial. Some models that use quantum-like concepts to describe neuron activity suggest that entanglement-like phenomena could play a role in the complex coordination required for nervous system development [109]. In terms of synaptic plasticity, which is the ability of synapses to strengthen or weaken over time, some studies have suggested a potential role for entanglement-like phenomena, which could potentially influence how our brains learn and adapt to new experiences [109]. Regarding brain damage repair, while there is no direct evidence linking entanglement to brain repair processes, the brain does have remarkable abilities to repair and reorganize itself, which involves processes such as neuroplasticity, where the brain forms new neural connections, and synaptic plasticity, where the strength of synapses changes. While interpretations regarding wave function collapse upon measurement and the speed of entangled particle influence may vary, physicists concur that entanglement creates correlations between measurements, carrying potential for technological applications [110][111][112][113], such as the creation of ghost images using parametric down-conversion (converting a blue photon into two red ones), which showcases the tangible significance of entanglement, highlighting its profound implications for technology and science.
-
Quantum tunneling
Quantum tunneling refers to the extraordinary ability of particles to transcend barriers that should be insurmountable according to the principles of classical physics. These effects have reshaped our understanding of chemistry on a microscopic scale and hold the potential to catalyze advances in medicine and enrich our comprehension of enzymatic functions [114]. To comprehend quantum tunneling, consider quantum particles as probability waves. They can exist at various points within their wavefunctions, just like a die has the possibility of landing on any side. Even though rolling a six is statistically less likely, it remains possible. Quantum tunneling is akin to this unpredictability. Despite a quantum particle’s apparent lack of energy to cross an energy barrier, there is still a possibility that it can tunnel through because its wavefunction extends to the other side of the barrier. To further comprehend this, imagine encountering an imposing hill obstructing a path and lacking the energy to ascend it. Within the realm of quantum physics, these minuscule particles exhibit the astonishing capability to breach such barriers, akin to traversing an imperceptible barrier. In a parallel narrative, consider playing a game where a ball can occasionally traverse a wall. Similarly, “wave–particle duality” characterizes the behavior of minuscule entities [115][116][117]. Electrons, for instance, exhibit both wave-like and particle-like traits. This duality extends to light, behaving as waves or photons. This duality’s consequence is the “Heisenberg Uncertainty Principle”, introducing a range between certainty and impossibility in particle movement (such as electrons, protons, and photons) [118][119]. Electrons might transcend barriers, albeit infrequently. Understanding this quantum puzzle and its role in cellular mechanics could yield novel medical strategies and insights into enzymatic functions [120][121][122]. Enzymes, akin to microscopic machinery within our bodies, orchestrate chemical reactions. Quantum tunneling catalyzes these reactions by providing an efficient shortcut where these particles hold a hidden path that expedites reactions. Quantum tunneling has the potential to facilitate the mobility of drugs and enhance their efficacy [123].
-
Quantum coherence
Quantum coherence refers to the ability of quantum systems to maintain phase relationships [106] and could play a role in various biological processes, including energy transfer within cells, potentially impacting cellular functions. These effects could facilitate energy transfer and information processing in biological systems, enhancing their adaptability and survival in changing environments [124][125][126]. However, other studies argue that coherence is fragile in warm and noisy biological conditions, and classical mechanisms can explain observed phenomena without invoking quantum effects [106][127]. Entanglement is in fact related to the ideas of quantum coherence and decoherence [105]. In classical physics, two waves are coherent if their properties produce stationary interference, but the same applies to wave functions. This can be illustrated in the already mentioned double-slit experiment, where electrons pass through both slits and form an interference pattern on a target. The coherence of the waves allows the electrons to interfere with each other [108], and, when quantum systems interact with the environment, this coherence becomes shared and lost over time, known as quantum decoherence, resulting in the loss of quantum behavior [106][128]. Identifying quantum coherence and dynamics efficiently, given limited system access, is essential for reliable quantum applications [129][130][131]. Moreover, the question of whether quantum coherence can exist in biological organisms in vivo, such as in photosynthetic complexes or avian chemical compasses, surrounded by hot and wet environments, has sparked interest in understanding the relationship between quantum coherence and biological function [132][133]. In such cases, full-system access is often limited, and the detection of the signatures of quantum coherence is often indirect.
-
Quantum sensing
Quantum sensors have the potential to revolutionize our understanding of radiation-induced effects on biological systems [134][135] by providing more accurate and sensitive measurements of ionizing radiation, leading to safer and more effective treatments in medical radiology and radiation therapy [136]. Quantum sensors can also detect early cellular changes caused by radiation exposure, enabling the better monitoring of radiation-related health risks. Quantum-enhanced medical imaging techniques, such as quantum MRI and MEG, can significantly improve the quality and precision of medical imaging, providing insights into radiation-induced effects on tissues and organs [137]. Additionally, quantum biosensors can be used to detect biomarkers related to radiation exposure and its effects, such as DNA damage and repair [138]. This information can be invaluable in radiation research and occupational health monitoring. Finally, quantum-enhanced microscopy techniques [139][140] can enable the observation of biological samples exposed to radiation at the molecular and cellular levels with unprecedented detail, providing insights into the mechanisms of radiation-induced damage and repair processes in biological systems.
-
Quantum information in quantum biology and radiation effects
Classical information is encoded in binary bits, typically represented as 0 or 1, while quantum information thrives on the unique property of quantum states that can exist in superpositions. Quantum information is found where the intricate behaviors of waves and particles governed by quantum mechanics are used to encode, process, and propagate data [141][142][143] or when itis associated with secure communication, precise sensing, and advanced computing, and now for potential applications in quantum biology, environmental interactions, and non-targeted effects (NTE) [56][144]. Its use in biology reveals the behavior of biomolecules and cellular systems and functions by harnessing their particle quantum properties (e.g., superposition, entanglement, coherence, tunneling, and efficient quantum state propagation). This distinct property enables novel forms of communication and processing, offering promising avenues for the detection and measurement of the non-targeted effects of ionizing radiation. In NTE, quantum phenomena are believed to play a role in the complex interplay between external stressors and biological systems, leading to unexpected responses [41]. The spread of NTE effects through biophotons and other particles is particularly significant in biological communication, influencing how living organisms interact and exchange information [145][146][147][148]. Furthermore, applying quantum information principles to environmental research has the potential to significantly improve our ability to monitor and analyze ecosystems with the use of quantum-enhanced sensing techniques, which enable us to detect even the smallest changes in environmental factors with unrivalled precision and allow us to detect ecological disruptions and potential threats early on [149][150]. The intricate interplay between ecosystems and external influences, including pollutants, climate variations, and habitat modifications, can be investigated, protected, and promoted with unprecedented precision and understanding using quantum information tools.
2.2. Areas Where Quantum Effects May Occur
Quantum effects have the potential to manifest in situations involving physical energy transduction, transition, or operative gradients. In essence, these effects could play a role in processes driven by electromagnetic gradients or where energy undergoes transformation, transduction, or capture (see Figure 2) [32]. Quantum effects may also influence phenomena at the intersection of morphogenetic and bioelectric fields—for instance, within the rhythmic changes at the level of gene expression, as well as protein quantities and subcellular distribution, which confer temporal features to the molecular platforms hosting electrochemical processes and non-trivial quantum phenomena [44].
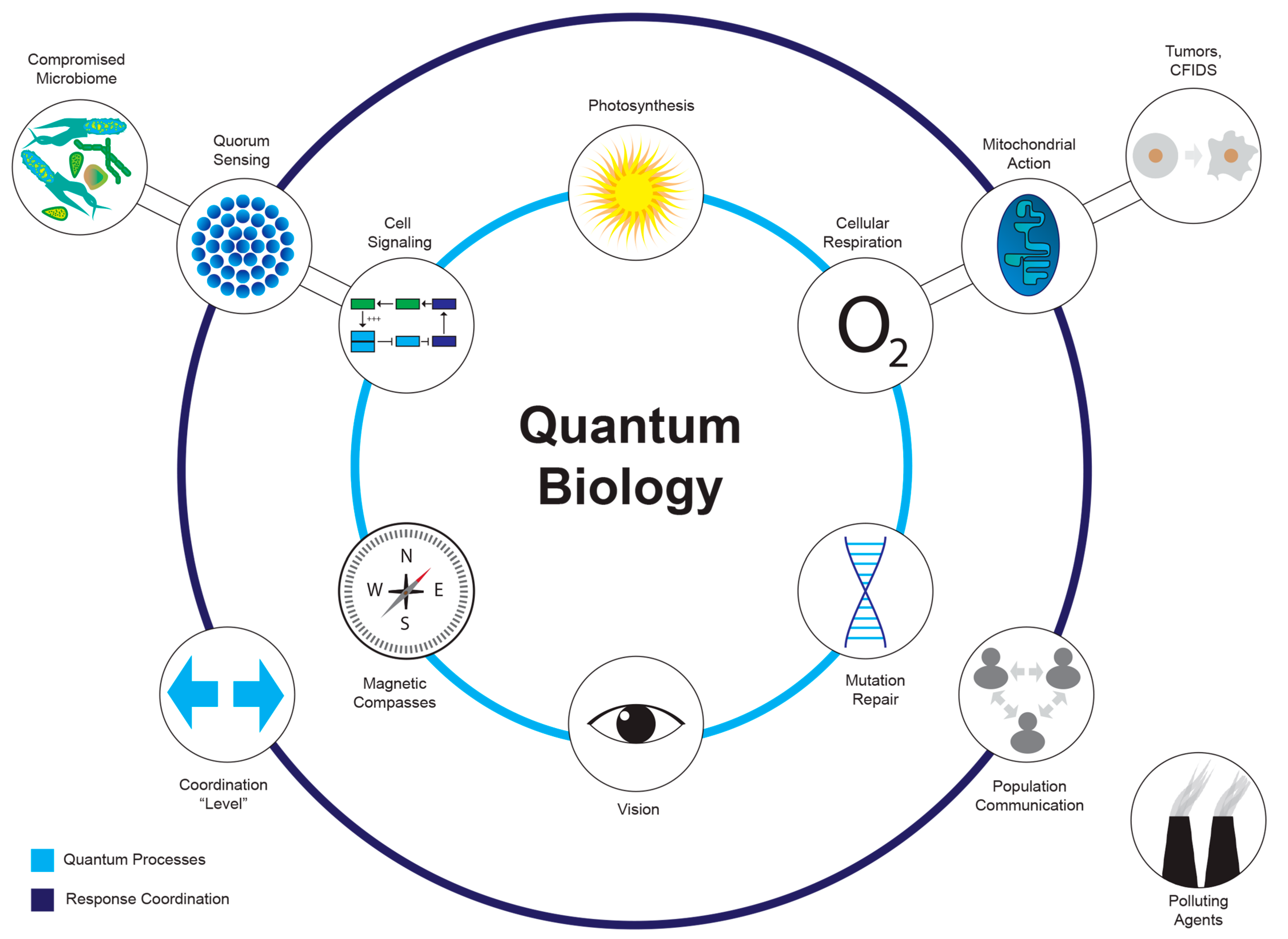
Figure 2. Quantum effects in biological processes. Exploring quantum influences on DNA replication and repair; cellular energy production (metabolism); cell signaling and communication; photosynthesis; enzyme catalysis; cellular communication; protein folding; and conformation.
1. Quantum Effects on Morphogenetic and Bioelectric Fields
In the late 18th century, Voltaire and Galvani’s early insights into the electrical nature of life formed the basis of modern research in quantum biology [151]. Their “electric fluid” concept can be seen as a precursor to the notion of quantum bioelectric fields, thought to comprise photons and other quantum particles, influencing various biological processes, such as morphogenesis, signal transduction, and nerve conduction [30]. Galvani’s experiments, demonstrating biological systems’ capacity to generate and respond to electrical signals [151], hinted at the involvement of quantum phenomena, such as entanglement, coherence, superposition, and non-locality, in bioelectric field organization and function.
Galvani’s groundbreaking experiments revealed the generation and transmission of bioelectric fields over substantial distances [67], exhibiting remarkable coherency [67], existing as both positive and negative entities simultaneously, and their susceptiblity to external stimuli like light and sound [31]. These findings imply that the particles involved in the organization and functioning of bioelectric fields may exist in an entangled state, maintain coherence, or even exist in superpositions of states, ultimately interacting non-locally with one another [44]. They also hint at the involvement of quantum phenomena in bioelectric field organization and function due to the overall complexity of the coherence of bioelectric fields, which are able to coordinate the activity of billions of cells in the body. They do this in a way that is highly efficient and precise, and this suggests that the particles involved in bioelectric field organization and function are interacting with each other in a quantum way [44].
Morphogenetic and bioelectric fields are pivotal in understanding the potential influence of quantum effects on various biological processes [152]. Morphogenetic fields are believed to guide the development of biological forms by coordinating cellular movement and tissue development. They are thought to organize the bodies of plants and animals through vibratory patterns and underlie their abilities to regenerate and heal after damage. Quantum entanglement and coherence could play a role in shaping morphogenetic fields [67], which guide biological form development through cellular coordination by influencing their organizational properties, influencing the behavior of biological molecules, or influencing the communication between cells (e.g., entangled photons could be used to control the movement of proteins and other molecules within cells) [153], and could lead to changes in cellular organization and tissue development. Furthermore, entangled particles could be used to transmit information between cells at a much faster speed than traditional chemical signaling [154], which could allow cells to coordinate their behavior more effectively and develop complex biological structures (e.g., entangled microtubules, transcription factors, immune cells, and mental information could coordinate cellular movement, regulate growth and differentiation, promote wound healing, and mediate consciousness effects on biology) [155]. Bioelectric fields are involved in various cellular processes [156][157], such as signal transduction, muscle contraction, and nerve conduction and the quantum effects such as superposition and non-locality could enhance its efficiency. Superposition might allow multiple bioelectric pathways, fostering faster nerve conduction [156]. In this scenario, a neuron could exist in a superposition of states—simultaneously firing and not firing. Such a state would empower the neuron to transmit signals to multiple targets simultaneously, allowing a significant acceleration in information processing within the brain. The bioelectric fields in different parts of the body could be correlated with each other [67], and could allow for the more efficient coordination of cellular activities. In the heart and lungs, these bioelectric fields could be correlated with each other to ensure that the two organs synchronize together efficiently to circulate blood and oxygen. The interaction of biophotons with molecules like DNA might mediate an effect called mitogenic [158], and quantum entanglement may facilitate long-distance cell communication through entangled biophotons, enabling coordinated cellular development [159].
- 2.
-
Quantum Effects on Mitogenic Radiation
In the 1920s, Alexander Gurwitsch conducted groundbreaking research on photonic communication in biology [160], discovering what he termed “mitogenic radiation” [161] determined to be in the visible spectrum. Gurwitsch’s pioneering experiments involved exposing onion root tips to ultraviolet (UV) light, resulting in the emission of radiation that stimulated the growth of other onion root tips [162]. It is now widely accepted that cells can communicate using photons. Mitogenic radiation involves the emission of biophotons, which can stimulate cell growth and division [163]. These biophotons may interact with molecules like DNA, potentially influencing gene expression related to cell growth and division. Recent research on mitogenic radiation has revealed its diverse applications in medicine, spanning from cancer treatment, regenerative healing, and tissue repair to addressing neurodegenerative diseases, cardiovascular health, and skin conditions. It may stimulate normal cell growth within the tumor microenvironment [164], potentially impeding the spread of cancer cells and enhancing their sensitivity to chemotherapy and radiation therapy. Additionally, quantum biology has intriguing connections to it. Biophotons are a central element in this link, as they are involved in various cellular processes, and it is possible that the photons emitted during this radiation interact with DNA, influencing gene expression or signaling pathways (e.g., to increase the expression of genes involved in activating signaling pathways in cell growth and division). Moreover, quantum entanglement might be at play, allowing cells to communicate over long distances through the entangled emission of biophotons [159]. If one cell emits a biophoton, the other cell will also emit a biophoton, even if they are separated by a large distance. The two biophotons will be entangled, meaning that they will share the same fate. If one biophoton interacts with a molecule in the cell, the other biophoton will also interact with the same molecule in the other cell, which could allow the two cells to communicate with each other over long distances.
-
Photosynthesis, vision, and magnetic compasses
Light harvesting is a process whereby pigments such as chlorophyll or rhodopsin trap sunlight and convert the radiant energy into electrical chemical energy. However, the exact quantum nature of light harvesting remains a topic of debate [61]. Cryptochromes, a class of photoreceptor proteins found in both plants and animals, contribute to these processes by generating triplet states through the absorption of photons [165][166]. These triplet states are critical in understanding the quantum aspects of photosynthesis and magnetoreception, which allows organisms to detect the Earth’s magnetic field. Experimental evidence supports the role of cryptochromes in magnetic field detection, especially in birds, which rely on these proteins for navigation. When cryptochromes are disrupted, it can lead to navigation difficulties when using the Earth’s magnetic field. Flavin adenine dinucleotide (FAD), a pivotal cofactor, plays essential roles in various biological processes [167]. It exists in two redox states, oxidized (FAD) and reduced (FADH2), and takes part in electron transfer reactions within enzymes, contributing to ATP generation through the electron transport chain. The quantum properties of FAD are closely tied to its biological functions; for instance, its ability to exist in two redox states is crucial for electron transfer reactions. Moreove, the flavin ring in FAD is associated with quantum tunneling, a process that contributes to the generation of triplet states in cryptochromes. The quantum effects responsible for energy transfer between chromophores in photosynthesis, are instrumental in energy dissipation within these biological systems. This exciton transfer play a pivotal role in safeguarding cells from damage, particularly by dissipating excess energy in the form of heat [44]. Quantum tunneling and entanglement are among the quantum phenomena that facilitate this energy dissipation in biological systems [168][169]. Recent evidence suggests that stable states lasting several femtoseconds are sufficient for the intermediates to survive long enough to influence biological processes [170], and light-harvesting chlorophyll pigments can enable mammalian mitochondria to capture photonic energy and produce ATP [171]. It is interesting to note in this context that the ionizing radiation-induced bystander effect (RIBE) seems to be triggered by light emission during the return to the ground state of excited molecules, resulting in certain situations in a block of complex 1 in the mitochondrial electron transport chain (ETC) [172]. In the retina, rhodopsin captures light, and it is thought that the energy requirements for vision involve quantum tunneling in complex 1 of the mitochondrial ETC. In this context, the light harvesting is also suspected to underlie the so-called “magnetic compass” allowing bird and insect migrations to be so accurate.
-
Cellular Respiration
Outside of the light-harvesting functions, respiration in mitochondria is thought to involve non-trivial quantum effects including entanglement, superposition, and tunneling [44]. Tunneling has recently been demonstrated to occur in complex 1, as noted above, but is likely to occur also in other complexes involved in electron transfer [120]. These processes lead to very efficient electron transfer, and it has been speculated that aging or certain degenerative diseases involving chronic fatigue and related loss of energy may involve defects at the quantum level [173]. Furthermore, the role of infrared (IR), at 700 nanometers to 1 mm, and its manipulation in influencing quantum behavior, especially within contexts such as the electron transport chain (ETC) [174] or its direct effects on water [175][176], emphasizes the significance of IR in modulating quantum processes within cellular environments. It is emitted by objects above absolute zero and is absorbed by water molecules, and it exerts a range of biological effects, including its influence on cellular respiration, gene expression, and cell signaling. Quantum effects may significantly mediate the biological impacts of IR (e.g., quantum entanglement can enable long-distance IR-mediated signaling, overcoming physical barriers between cells). Quantum tunneling also plays a role in the transfer of IR-mediated energy, allowing effects to propagate through barriers [177][178].
-
Mutation/repair in DNA
One of the major areas of research in quantum biology concerns DNA replication, mutation, and repair. The key idea here is that, for replication to occur with minimal effort, the energy required to break a hydrogen bond between AT or CG base pairs can be reduced if the base pairs enter a superposition tautomeric state during which they tunnel [179][180]. However, when in the tautomeric state, they may be more prone to mutations. The Löwdin theory of mutation, published in 1963 and formalized in 1965, provides a convincing, if theoretical, discussion of how mismatch and missense mutations as well as deletions could be accounted for by this theory [181][182]. Quantum events (mainly entanglement) are also postulated to explain the ability of restriction endonucleases to coordinate breaks in the complementary strands of DNA to enable repair processes to occur.
-
Cell signaling after energy capture/deposition
During the last thirty years or so, it has become increasingly apparent to radiobiologists that irradiated cells communicate with unirradiated cells and transmit a “memory” of the irradiation to their progeny [183]. While the occurrence of these events is not now disputed, no fully acceptable mechanism has emerged. This is particularly true of attempts to explain how non-clonal mutations emerge in the distant progeny of irradiated cells, or how cells or even organisms that are not targeted by radiation and show no ionizing radiation energy deposited in them can display all the signs of having been exposed, including chromosomal mutations, the upregulation of their repair capacity, mitochondrial changes, and cell transformation [183]. Theories involving quantum biology have not yet been advanced in this field, but many of the processes involved are thought to involve quantum behaviors. These include signaling, which involves ion gradients, and mitochondrial the ETC, the emission of UVA photons within which triggers release of exosomes that are captured by bystander cells and somehow lead to the ETC complex 1 block, as mentioned above [184][185]. The ETC collects electrons from NADH or FADH2 and transfers them through a series of electron carriers within multiprotein respiratory complexes (complex I to IV) to oxygen, generating an electrochemical gradient [186]. Their involvement extends to various diseases, including cancer, Alzheimer’s disease, and Parkinson’s disease [187][188]. As mentioned above, infrared radiation influences the electron transport chain (ETC) [189] in various ways (e.g., exciting electrons within the ETC complexes [190], leading to increased electron transport and ATP production, or enhancing the fluidity of the mitochondrial membrane, optimizing the efficiency of electron transport). IR radiation directly impacts water molecules within biological systems [191], leading to increased rotational and vibrational energy. IR can also break hydrogen bonds between water molecules, resulting in changes to water structure and dynamics.
Many of these processes may incorporate non-trivial quantum phenomena, including tunneling, superposition, and entanglement [32]. Notably, neurotransmitters and their inhibitors, such as serotonin, L-deprenyl, nicotine, ondansetron, or reserpine, possess the capacity to modulate or interfere with these forms of communication [67]. Tunneling mechanisms enable ions to traverse polarized cell membranes, offering a far more efficient means of generating bioelectric fields compared to classical diffusion [157][192]. Moreover, the concept that bioelectric fields could exist in a superposition of states is intriguing, as it could enhance the transmission of information in a more efficient manner than classical fields [193], which might play a crucial role in information processing within the brain, facilitating the brain’s ability to concurrently process data from various sources [194]. Additionally, the possibility that bioelectric fields might be entangled opens up the potential for long-distance communication, which could be instrumental in how neurons interact with one another and with other cells in the body [195][196]. Tunneling may also be integral in the generation of action potentials in neurons, which are rapid changes in the electrical potential facilitating neuron-to-neuron communication [192].
2.3. Anywhere Response Is Coordinated
The second major suite of biological actions that may involve quantum-level mechanisms is those where synchronization occurs at a level where classical mechanisms cannot explain the observations or where nonlinearity and thresholds predominate in the mechanism.
-
Mitochondrial action
Mitochondria, like chloroplasts, are microbial-like organelles that many biologists believe were “captured” by primitive eukaryotic cells and harnessed to perform specific functions [197]. Respiration and photosynthesis are prime processes thought to involve quantum biology, as discussed above, but other functions of mitochondria may also involve quantum processes. For example, water interfaces in the mitochondrial membrane capture red–near-infrared (R-NIR) energy, and this quantum-like process is suggested to be responsible for the ability of low-level light therapy (LLLT) to energize people [198][199]. The synchronization of the biological functions of mitochondria may involve entanglement, explaining how the proton pump function in complex 1 can be associated with the reduction of quinone by NADH, even though several nanometers may separate them [199][200]. Mitochondria, the powerhouses of the cell, are central to cellular respiration and many other cellular functions, responsible for energy production, contain their own DNA, and are believed to have evolved from bacteria [201][202].
Quantum biology considerations extend to the role of cryptochromes within mitochondria, which may influence processes such as energy capture and electron transfer [203][204]. It is worth noting that cryptochromes are involved in the responses to blue and ultraviolet-A (UVA) light, playing a role in synchronizing biological functions. The generation and manipulation of triplet states in cryptochromes, initiated by photon absorption, are of particular interest in understanding the quantum mechanisms driving mitochondrial action [205]. Emerging evidence suggests that mitochondria communicate with each other through photons, thereby influencing various cellular processes [68]. This inter-mitochondrial photon communication contributes to the coordination of mitochondrial function, ultimately impacting overall cellular health. Chromophoric networks, comprised of molecules capable of absorbing and transferring light energy, play a pivotal role in these quantum processes. Notable examples include tryptophan and the aromatic networks found in biological systems [206][207]. One fascinating quantum effect within chromophoric networks is tryptophan in microtubules (e.g., super-radiance), where molecules collectively emit light, resulting in brighter emission compared to individual molecules [208]. This super-radiance phenomenon has the potential to enhance the efficiency of energy transfer processes in mitochondria [209][210]. How does super-radiance play a role in boosting the mitochondria’s energy capture and electron transfer processes? In addition to this, Herbert Fröhlich proposed that quantum effects influence biological systems through coherent vibrations, where all chromophores in a network vibrate in phase [210]. These coherent vibrations might extend to water molecules within mitochondria and could play a role in energy transfer and signal transduction [209], including plant leaves and nerve cells, which implies that these coherent vibrations may contribute to the energy transfer processes within mitochondria [211][212][213][214], further bridging the gap between established biology and the quantum world. The participation of cryptochromes, known for their sensitivity to blue and ultraviolet-A light, in processes related to energy capture and electron transfer within these organelles is important. Cryptochromes have the potential to synchronize and influence biological functions, which is crucial in understanding the quantum mechanisms that drive mitochondrial action [203][204].
-
Tissue/organ/organism-level response
Related areas to cellular signaling discussed earlier include emergent responses at the level of the tissue and organ and tissue coordination via the nervous system. These can be reduced to functions of fundamental processes such as electromagnetic activity or electron transport involved in enzyme activity, DNA replication, ion-gated membrane channels, and, of course, energy production (photosynthesis and respiration), which have all been shown to involve quantum chemistry [215]. Interesting questions concern decision making at the tissue or organ level. For example, in irradiated cells, are there critical dose thresholds where apoptosis, which is a low-dose response to eliminate damaged cells, might be prevented so that organ function can be maintained even if compromised? Alternatively, are there damage-sensing mechanisms that inform cells on the best course of action to preserve tissue function? Are consciousness and psychopathological behavior emergent properties of the quantum brain? While such ideas are largely speculative, they are actively being discussed in quantum biology and quantum neurobiology [216][217]. The influence of coherent vibrations in water molecules, particularly influenced by Fröhlich’s ideas, could potentially play a role in various biological processes at this scale, which might include the coordination of tissues and organs through electromagnetic activity, electron transport involved in enzyme activity, DNA replication, and even consciousness. The quantum aspects of decision making within cells and the coordination of tissues become fascinating topics.
-
Quorum sensing
Quorum sensing is a phenomenon first described in bacteria that refers to the ability to detect and respond to changes in population density by regulating gene expression [218]. Cryptochromes are sensitive to blue light and have the ability to generate and manipulate radical pairs upon photon absorption, potentially contributing to synchronizing the functions of social organisms, including both bacteria and animals [166][219]. Beyond their involvement in population-level communication, alongside chemical signaling, electrical signaling plays a vital role in the quorum sensing process [220][221]. In bacteria, a certain threshold number of individuals are required to trigger a gene expression change. It was thought that signals could only be chemical molecules, but, recently, evidence has emerged for electrical signaling [218]. This led Majumdar and Pal in 2017 to question whether quantum mechanics could explain the long-distance synchronization of functions in, for example, biofilms [222]. The function of potassium ion-gated channels, which are important in density-related signaling in a wide range of species, is also suggested to involve two entangled K ions. A further area where quorum sensing is suspected to involve quantum biology is in the aggregation of slime molds. These exist as single-celled amoeba-like organisms, but, when food is short, they respond to a variety of chemical or electrochemical signals and aggregate into a multicellular, differentiated form to produce fruiting bodies, the spores of which remain dormant until food is again plentiful.
-
Population-level communication (bacteria/social animals)
Quorum sensing is a specific subtype of communication and signaling within and between cells and organisms. The field has been reviewed recently by Matarèse et al., 2020 [12][223], looking at chemical and physical signaling across the plant, microbial, and animal kingdoms and reviewing the evidence for acoustic signaling across the kingdoms of living organisms. Direct experimental evidence for quantum biological effects in social organisms as opposed to subcellular processes is scarce, but the whole organism-level communication mechanisms involving ion-gated channels and electrical or electrochemical gradients can be explained more easily by postulating the involvement of quantum processes [32]. One experiment that may involve quantum entanglement but could also be explained as a post-conditioning effect is described in Mothersill et al., 2018 [224]. The authors and others demonstrated that if irradiated fish swam with unirradiated fish, the unirradiated group upregulated a suite of proteins that were protective against radiation damage [225]. However, in this series of experiments, the fish met before one group was irradiated and did not meet again. Both groups of bystander fish (those meeting before and those meeting after their partners were irradiated) induced protective responses and bystander signaling.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242216464
References
- Mothersill, C.; Rusin, A.; Seymour, C. The Development of Bio-Assays Based on Non-Targeted Effects of Radiation; a Potential Worm-Hole into Ecosystem Level Biomarkers. In NATO Science for Peace and Security Series A: Chemistry and Biology; Springer: Berlin/Heidelberg, Germany, 2022.
- Seiler, A.; Fagundes, C.P.; Christian, L.M. The Impact of Everyday Stressors on the Immune System and Health. In Stress Challenges and Immunity in Space: From Mechanisms to Monitoring and Preventive Strategies; Springer: Berlin/Heidelberg, Germany, 2019.
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127.
- Kadhim, M.A.; Hill, M.A. Non-Targeted Effects of Radiation Exposure: Recent Advances and Implications. Radiat. Prot. Dosimetry 2015, 166, 118–124.
- Mothersill, C.; Rusin, A.; Seymour, C. Relevance of Non-Targeted Effects for Radiotherapy and Diagnostic Radiology; A Historical and Conceptual Analysis of Key Players. Cancers 2019, 11, 1236.
- Han, X.; Chen, Y.; Zhang, N.; Huang, C.; He, G.; Li, T.; Wei, M.; Song, Q.; Mo, S.; Lv, Y. Single-Cell Mechanistic Studies of Radiation-Mediated Bystander Effects. Front. Immunol. 2022, 13, 849341.
- Tang, H.; Cai, L.; He, X.; Niu, Z.; Huang, H.; Hu, W.; Bian, H.; Huang, H. Radiation-Induced Bystander Effect and Its Clinical Implications. Front. Oncol. 2023, 13, 1124412.
- Calabrese, E.J.; Kozumbo, W.J. The Hormetic Dose-Response Mechanism: Nrf2 Activation. Pharmacol. Res. 2021, 167, 105526.
- Hauptmann, M.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; Lubin, J.H.; Preston, D.L.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. J. Natl. Cancer Inst. Monogr. 2020, 2020, 188–200.
- Hall, W.A.; Paulson, E.; Li, X.A.; Erickson, B.; Schultz, C.; Tree, A.; Awan, M.; Low, D.A.; McDonald, B.A.; Salzillo, T.; et al. Magnetic Resonance Linear Accelerator Technology and Adaptive Radiation Therapy: An Overview for Clinicians. CA Cancer J. Clin. 2022, 72, 34–56.
- Connell, P.P.; Hellman, S. Advances in Radiotherapy and Implications for the next Century: A Historical Perspective. Cancer Res. 2009, 69, 383–392.
- Matarèse, B.F.E.; Lad, J.; Seymour, C.; Schofield, P.N.; Mothersill, C. Bio-Acoustic Signaling; Exploring the Potential of Sound as a Mediator of Low-Dose Radiation and Stress Responses in the Environment. Int. J. Radiat. Biol. 2020, 98, 1083–1097.
- Kadhim, M.; Salomaa, S.; Wright, E.; Hildebrandt, G.; Belyakov, O.V.; Prise, K.M.; Little, M.P. Non-Targeted Effects of Ionising Radiation-Implications for Low Dose Risk. Mutat. Res. Rev. Mutat. Res. 2013, 752, 84–98.
- Yahyapour, R.; Salajegheh, A.; Safari, A.; Amini, P.; Rezaey-An, A.; Amraee, A.; Najafi, M. Radiation-Induced Non-Targeted Effect and Carcinogenesis; Implications in Clinical Radiotherapy. J. Biomed. Phys. Eng. 2018, 8, 435–446.
- Laurier, D.; Rühm, W.; Paquet, F.; Applegate, K.; Cool, D.; Clement, C. Areas of Research to Support the System of Radiological Protection. Radiat. Environ. Biophys. 2021, 60, 519–530.
- Morgan, W.F.; Sowa, M.B. Non-Targeted Effects Induced by Ionizing Radiation: Mechanisms and Potential Impact on Radiation Induced Health Effects. Cancer Lett. 2015, 356, 17–21.
- Morgan, W.F. Non-Targeted and Delayed Effects of Exposure to Ionizing Radiation: I. Radiation-Induced Genomic Instability and Bystander Effects In Vitro. Radiat. Res. 2012, 178, AV223–AV236.
- Morgan, W.F. Non-Targeted and Delayed Effects of Exposure to Ionizing Radiation: II. Radiation-Induced Genomic Instability and Bystander Effects in Vivo, Clastogenic Factors and Transgenerational Effects. Radiat. Res. 2003, 159, 581–596.
- Liu, Y.J.; Wang, C. A Review of the Regulatory Mechanisms of Extracellular Vesicles-Mediated Intercellular Communication. Cell Commun. Signal. 2023, 21, 1–12.
- Cagatay, S.T.; Mayah, A.; Mancuso, M.; Giardullo, P.; Pazzaglia, S.; Saran, A.; Daniel, A.; Traynor, D.; Meade, A.D.; Lyng, F.; et al. Phenotypic and Functional Characteristics of Exosomes Derived from Irradiated Mouse Organs and Their Role in the Mechanisms Driving Non-Targeted Effects. Int. J. Mol. Sci. 2020, 21, 8389.
- López-Díaz FJ Cross-Talk between TGF-β and P53 Regulates the Stress Response. Cancer Discov. 2013, 3, 715.
- Averbeck, D. Low-Dose Non-Targeted Effects and Mitochondrial Control. Int. J. Mol. Sci. 2023, 24, 11460.
- Rouleau, N.; Karbowski, L.M.; Persinger, M.A. Experimental Evidence of Classical Conditioning and Microscopic Engrams in an Electroconductive Material. PLoS ONE 2016, 11, e0165269.
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7, 100.
- Rohli, R.V.; Li, C. Energy Transfer and Electromagnetic Radiation. In Meteorology for Coastal Scientists; Springer: Berlin/Heidelberg, Germany, 2021.
- Chelkowski, S.; Bandrauk, A.D. Control of Molecular Vibrational Excitation and Dissociation by Chirped Intense Infrared Laser Pulses. Rotational Effects. J. Chem. Phys. 1993, 99, 4279–4287.
- Phelps, A.V. Rotational and Vibrational Excitation of Molecules by Low-Energy Electrons. Rev. Mod. Phys. 1968, 40, 399–410.
- Krisko, A.; Radman, M. Phenotypic and Genetic Consequences of Protein Damage. PLoS Genet. 2013, 9, e1003810.
- Reynaud, E. Protein Misfolding and Degenerative Diseases. Nat. Educ. 2010, 3, 28.
- Harris, M.P. Bioelectric Signaling as a Unique Regulator of Development and Regeneration. Development 2021, 148, dev180794.
- Funk, R.H.W. Endogenous Electric Fields as Guiding Cue for Cell Migration. Front Physiol. 2015, 6, 143.
- Kim, Y.; Bertagna, F.; D’souza, E.M.; Heyes, D.J.; Johannissen, L.O.; Nery, E.T.; Pantelias, A.; Jimenez, A.S.P.; Slocombe, L.; Spencer, M.G.; et al. Quantum Biology: An Update and Perspective. Quantum Rep. 2021, 3, 80–126.
- Popp, F.-A. Biophotons—Background, Experimental Results, Theoretical Approach and Applications. In Integrative Biophysics; Springer: Berlin/Heidelberg, Germany, 2003.
- Bischof, M. A Tribute to Fritz-Albert Popp on the Occasion of His 70th Birthday. Indian J. Exp. Biol. 2008, 46, 267–272.
- Popp, F.A.; Li, K.H.; Gu, Q. Recent Advances in Biophoton Research and Its Applications; World Scientific Publishing Co. Pte. Ltd.: London, UK, 1992.
- Popp, F.-A.; Gu, Q.; Li, K.-H. Biophoton Emission: Experimental Background and Theoretical Approaches. Mod. Phys. Lett. B 1994, 8, 1269–1296.
- Popp, F.A.; Chang, J.J.; Herzog, A.; Yan, Z.; Yan, Y. Evidence of Non-Classical (Squeezed) Light in Biological Systems. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2002, 293, 98–102.
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front Plant Sci. 2021, 12, 619987.
- Marais, A.; Adams, B.; Ringsmuth, A.K.; Ferretti, M.; Gruber, J.M.; Hendrikx, R.; Schuld, M.; Smith, S.L.; Sinayskiy, I.; Krüger, T.P.J.; et al. The Future of Quantum Biology. J. R. Soc. Interface 2018, 15, 20180640.
- Vepsäläinen, A.P.; Karamlou, A.H.; Orrell, J.L.; Dogra, A.S.; Loer, B.; Vasconcelos, F.; Kim, D.K.; Melville, A.J.; Niedzielski, B.M.; Yoder, J.L.; et al. Impact of Ionizing Radiation on Superconducting Qubit Coherence. Nature 2020, 584, 551–556.
- Lambert, N.; Chen, Y.-N.; Cheng, Y.-C.; Li, C.-M.; Chen, G.-Y.; Nori, F. Quantum Biology. Nat. Phys. 2013, 9, 10–18.
- Cao, J.; Cogdell, R.J.; Coker, D.F.; Duan, H.G.; Hauer, J.; Kleinekathöfer, U.; Jansen, T.L.C.; Mančal, T.; Dwayne Miller, R.J.; Ogilvie, J.P.; et al. Quantum Biology Revisited. Sci. Adv. 2020, 6, eaaz4888.
- Streltsov, A.; Singh, U.; Dhar, H.S.; Bera, M.N.; Adesso, G. Measuring Quantum Coherence with Entanglement. Phys. Rev. Lett. 2015, 115, 020403.
- Mazzoccoli, G. Chronobiology Meets Quantum Biology: A New Paradigm Overlooking the Horizon? Front Physiol. 2022, 13, 892582.
- Manousakis, E. Quantum mechanics and path integrals. In Practical Quantum Mechanics; Springer: Berlin/Heidelberg, Germany, 2015.
- Styer, D.F. The Strange World of Quantum Mechanics; Cambridge University Press: Cambridge, UK, 2014.
- Zettili, N.; Zahed, I. Quantum Mechanics: Concepts and Applications. Am. J. Phys. 2003, 71, 93.
- Klein, M.J. Max Planck and the Beginnings of the Quantum Theory. Arch. Hist. Exact. Sci. 1975, 1, 459–479.
- Feit, M.D.; Fleck, J.A.; Steiger, A. Solution of the Schrödinger Equation by a Spectral Method. J. Comput. Phys. 1982, 47, 412–433.
- Shirley, J.H. Solution of the Schrödinger Equation with a Hamiltonian Periodic in Time. Phys. Rev. 1965, 138, B979–B987.
- Ettlinger, H.J.; Dirac, P.A.B. The Principles of Quantum Mechanics. Am. Math. Mon. 1931, 38, 524.
- Ananthaswamy, A. Particle, Wave, Both or Neither? The Experiment That Challenges All We Know about Reality. Nature 2023, 618, 454–456.
- Duffy, J.; Loch-Temzelides, T. A Double-Slit Experiment with Human Subjects. PLoS ONE 2021, 16, e0246526.
- Parker, S. A Single-Photon Double-Slit Interference Experiment. Am. J. Phys. 1971, 39, 420–424.
- Fleming, G.R.; Scholes, G.D. Quantum Biology: Introduction. In Quantum Effects in Biology; Cambridge University Press: Cambridge, UK, 2014.
- Sarovar, M.; Ishizaki, A.; Fleming, G.R.; Whaley, K.B. Quantum Entanglement in Photosynthetic Light-Harvesting Complexes. Nat. Phys. 2010, 6, 462–467.
- Bothma, J.P.; Gilmore, J.B.; McKenzie, R.H. The Role of Quantum Effects in Proton Transfer Reactions in Enzymes: Quantum Tunneling in a Noisy Environment? New J. Phys. 2010, 12, 055002.
- Tuszynski, J.A. From Quantum Chemistry to Quantum Biology: A Path toward Consciousness. J. Integr. Neurosci. 2020, 19, 687–700.
- Cheng, H.P.; Deumens, E.; Freericks, J.K.; Li, C.; Sanders, B.A. Application of Quantum Computing to Biochemical Systems: A Look to the Future. Front Chem. 2020, 8, 587143.
- Holland, R.A. True Navigation in Birds: From Quantum Physics to Global Migration. J. Zool. 2014, 293, 1–15.
- Frederiksen, A.; Teusch, T.; Solov’yov, I.A. Quantum Effects in Biological Systems. In Dynamics of Systems on the Nanoscale; Springer International Publishing: Cham, Switzerland, 2022.
- Calvillo, L.; Redaelli, V.; Ludwig, N.; Qaswal, A.B.; Ghidoni, A.; Faini, A.; Rosa, D.; Lombardi, C.; Pengo, M.; Bossolasco, P.; et al. Quantum Biology Research Meets Pathophysiology and Therapeutic Mechanisms: A Biomedical Perspective. Quantum Rep. 2022, 4, 148–172.
- Nishiura, N.; Kaneko, K. Evolution of Phenotypic Fluctuation under Host-Parasite Interactions. PLoS Comput. Biol. 2021, 17, e1008694.
- Aristarhov, S. Heisenberg’s Uncertainty Principle and Particle Trajectories. Found Phys. 2023, 53, 1–12.
- Busch, P.; Heinonen, T.; Lahti, P. Heisenberg’s Uncertainty Principle. Phys. Rep. 2007, 452, 155–176.
- Cai, J. Quantum Biology: Explore Quantum Dynamics in Biological Systems. Sci. China Inf. Sci. 2016, 59, 1–7.
- Hammerschlag, R.; Levin, M.; McCraty, R.; Bat, N.; Ives, J.A.; Lutgendorf, S.K.; Oschman, J.L. Biofield Physiology: A Framework for an Emerging Discipline. Glob. Adv. Health Med. 2015, 4, 35–41.
- Fels, D. Cellular Communication through Light. PLoS ONE 2009, 4, e5086.
- Takeda, M.; Kobayashi, M.; Takayama, M.; Suzuki, S.; Ishida, T.; Ohnuki, K.; Moriya, T.; Ohuchi, N. Biophoton Detection as a Novel Technique for Cancer Imaging. Cancer Sci. 2004, 95, 656–661.
- Sjostedt, S.; Bezak, E. Non-Targeted Effects of Ionising Radiation and Radiotherapy. Australas Phys. Eng. Sci. Med. 2010, 33, 219–231.
- Zhou, H.; Ivanov, V.N.; Gillespie, J.; Geard, C.R.; Amundson, S.A.; Brenner, D.J.; Yu, Z.; Lieberman, H.B.; Hei, T.K. Mechanism of Radiation-Induced Bystander Effect: Role of the Cyclooxygenase-2 Signaling Pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 14641–14646.
- Benfatto, M.; Pace, E.; Curceanu, C.; Scordo, A.; Clozza, A.; Davoli, I.; Lucci, M.; Francini, R.; De Matteis, F.; Grandi, M.; et al. Biophotons and Emergence of Quantum Coherence—A Diffusion Entropy Analysis. Entropy 2021, 23, 554.
- Wang, S.; Qiu, L.; Liu, X.; Xu, G.; Siegert, M.; Lu, Q.; Juneau, P.; Yu, L.; Liang, D.; He, Z.; et al. Electron Transport Chains in Organohalide-Respiring Bacteria and Bioremediation Implications. Biotechnol. Adv. 2018, 36, 1194–1206.
- Peters, J.W.; Fisher, K.; Newton, W.E.; Dean, D.R. Involvement of the P Cluster in Intramolecular Electron Transfer within the Nitrogenase MoFe Protein. J. Biol. Chem. 1995, 270, 27007–27013.
- Gray, H.B.; Winkler, J.R. Electron Flow through Metalloproteins. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1563–1572.
- Zhang, P.; Yuly, J.L.; Lubner, C.E.; Mulder, D.W.; King, P.W.; Peters, J.W.; Beratan, D.N. Electron Bifurcation: Thermodynamics and Kinetics of Two-Electron Brokering in Biological Redox Chemistry. Acc. Chem. Res. 2017, 50, 2410–2417.
- Reece, S.Y.; Nocera, D.G. Proton-Coupled Electron Transfer in Biology: Results from Synergistic Studies in Natural and Model Systems. Annu. Rev. Biochem. 2009, 78, 673–699.
- Huynh, M.H.V.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2007, 107, 5004–5064.
- Paganetti, H. Range Uncertainties in Proton Therapy and the Role of Monte Carlo Simulations. Phys. Med. Biol. 2012, 57, R99–R117.
- Mohan, R. A Review of Proton Therapy–Current Status and Future Directions. Precis. Radiat. Oncol. 2022, 6, 164–176.
- Ooms, M.D.; Dinh, C.T.; Sargent, E.H.; Sinton, D. Photon Management for Augmented Photosynthesis. Nat. Commun. 2016, 7, 12699.
- Applebury, M.L. Dynamic Processes of Visual Transduction. Vision Res. 1984, 24, 1445–1454.
- Pathania, N.; Qureshi, T. Quantifying Entanglement with Coherence. Int. J. Theor. Phys. 2022, 61.
- Schrödinger, E. Die Gegenwärtige Situation in Der Quantenmechanik. Naturwissenschaften 1935, 23, 807–812.
- Li, T.; Yin, Z.Q. Quantum Superposition, Entanglement, and State Teleportation of a Microorganism on an Electromechanical Oscillator. Sci. Bull. 2016, 61, 163–171.
- Friedman, J.R.; Patel, V.; Chen, W.; Tolpygo, S.K.; Lukens, J.E. Quantum Superposition of Distinct Macroscopic States. Nature 2000, 406, 43–46.
- Fein, Y.Y.; Geyer, P.; Zwick, P.; Kiałka, F.; Pedalino, S.; Mayor, M.; Gerlich, S.; Arndt, M. Quantum Superposition of Molecules beyond 25 KDa. Nat. Phys. 2019, 15, 1242–1245.
- Romero, E.; Augulis, R.; Novoderezhkin, V.I.; Ferretti, M.; Thieme, J.; Zigmantas, D.; Van Grondelle, R. Quantum Coherence in Photosynthesis for Efficient Solar-Energy Conversion. Nat. Phys. 2014, 10, 676–682.
- Yuan, J.; Hazarika, A.; Zhao, Q.; Ling, X.; Moot, T.; Ma, W.; Luther, J.M. Metal Halide Perovskites in Quantum Dot Solar Cells: Progress and Prospects. Joule 2020, 4, 1160–1185.
- Sharma, D.; Jha, R.; Kumar, S. Quantum Dot Sensitized Solar Cell: Recent Advances and Future Perspectives in Photoanode. Sol. Energy Mater. Sol. Cells 2016, 155, 294–322.
- Ollivier, H.; Zurek, W.H. Quantum Discord: A Measure of the Quantumness of Correlations. Phys. Rev. Lett. 2002, 88, 017901.
- Garrison, J.; Chiao, R. Quantum Optics; Oxford University Press: Oxford, UK, 2008; ISBN 9780198508861.
- Lvovsky, A.I.; Raymer, M.G. Continuous-Variable Optical Quantum-State Tomography. Rev. Mod. Phys. 2009, 81, 299–332.
- Bae, J.; Kwek, L.C. Quantum State Discrimination and Its Applications. J. Phys. A Math Theor. 2015, 48, 083001.
- Niaz, M.; Fernández, R. Understanding Quantum Numbers in General Chemistry Textbooks. Int. J. Sci. Educ. 2008, 30, 869–901.
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; Von Molnár, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A Spin-Based Electronics Vision for the Future. Science 2001, 294, 1488–1495.
- Žutić, I.; Fabian, J.; Sarma, S. Das Spintronics: Fundamentals and Applications. Rev. Mod. Phys. 2004, 76, 1–9.
- Chilton, N.F. Molecular Magnetism. Annu. Rev. Mater Res. 2022, 52, 79–101.
- Pauli, W. The Connection between Spin and Statistics. Phys. Rev. 1940, 58, 716–722.
- Born, M. Statistical Interpretation of Quantum Mechanics. Science 1955, 122, 675–679.
- Dirac, P. The Quantum Theory of the Electron. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character 1928, 117, 610–624.
- Varshalovich, D.A.; Moskalev, A.N.; Khersonskii, V.K. Quantum Theory of Angular Momentum; World Scientific Publishing Co. Pte. Ltd.: London, UK, 1988.
- Luo, S. Quantum Discord for Two-Qubit Systems. Phys. Rev. A 2008, 77.
- Plenio, M.B.; Virmani, S. An Introduction to Entanglement Measures. Quantum Inf. Comput. 2007, 7, 1–51.
- Horodecki, R.; Horodecki, P.; Horodecki, M.; Horodecki, K. Quantum Entanglement. Rev. Mod. Phys. 2009, 81, 865–942.
- Griffiths, D.J.; Schroeter, D.F. Introduction to Quantum Mechanics, 1st ed.; Cambridge University Press: Cambridge, UK, 2018; ISBN 1108100341.
- Einstein, A.; Podolsky, B.; Rosen, N. Can Quantum-Mechanical Description of Physical Reality Be Considered Complete? Phys. Rev. 1935, 47, 777.
- Karimi, E.; Boyd, R.W. Classical Entanglement? Science 2015, 350, 1172–1173.
- Galanis, C.; Vlachos, A. Hebbian and Homeostatic Synaptic Plasticity—Do Alterations of One Reflect Enhancement of the Other? Front Cell Neurosci. 2020, 14, 50.
- Cleve, R.; Buhrman, H. Substituting Quantum Entanglement for Communication. Phys. Rev. A 1997, 56, 1201.
- Zeilinger, A. Experiment and the Foundations of Quantum Physics. Rev. Mod. Phys. 1999, 71, S288–S297.
- Ursin, R.; Tiefenbacher, F.; Schmitt-Manderbach, T.; Weier, H.; Scheidl, T.; Lindenthal, M.; Blauensteiner, B.; Jennewein, T.; Perdigues, J.; Trojek, P. Entanglement-Based Quantum Communication over 144 Km. Nat. Phys. 2007, 3, 481–486.
- Yang, X.; Wei, K.; Ma, H.; Sun, S.; Liu, H.; Yin, Z.; Li, Z.; Lian, S.; Du, Y.; Wu, L. Measurement-Device-Independent Entanglement-Based Quantum Key Distribution. Phys. Rev. A 2016, 93, 052303.
- Rochlin, G.I. Tunneling Phenomena in Solids. J. Franklin Inst. 1971, 292, 66.
- Dimitrova, T.L.; Weis, A. The Wave-Particle Duality of Light: A Demonstration Experiment. Am. J. Phys. 2008, 76, 137–142.
- Kunitski, M.; Eicke, N.; Huber, P.; Köhler, J.; Zeller, S.; Voigtsberger, J.; Schlott, N.; Henrichs, K.; Sann, H.; Trinter, F.; et al. Double-Slit Photoelectron Interference in Strong-Field Ionization of the Neon Dimer. Nat. Commun. 2019, 10, 1.
- Bach, R.; Pope, D.; Liou, S.H.; Batelaan, H. Controlled Double-Slit Electron Diffraction. New J. Phys. 2013, 15, 033018.
- Wemer, H. The Physical Principles of the Quantum Theory; Dover Publication: Mineola, NY, USA, 1949; ISBN 0486601137.
- Kennard, E.H. On the Quantum Mechanics of a System of Particles. Phys. Rev. 1928, 31, 876–890.
- Klinman, J.P.; Kohen, A. Hydrogen Tunneling Links Protein Dynamics to Enzyme Catalysis. Annu. Rev. Biochem. 2013, 82, 471–496.
- Truhlar, D.G.; Gao, J.; Alhambra, C.; Garcia-Viloca, M.; Corchado, J.; Sánchez, M.L.; Villà, J. The Incorporation of Quantum Effects in Enzyme Kinetics Modeling. Acc. Chem. Res. 2002, 35, 341–349.
- Nagel, Z.D.; Klinman, J.P. Tunneling and Dynamics in Enzymatic Hydride Transfer. Chem. Rev. 2006, 106, 3095–3118.
- Kržan, M.; Vianello, R.; Maršavelski, A.; Repič, M.; Zakšek, M.; Kotnik, K.; Fijan, E.; Mavri, J. The Quantum Nature of Drug-Receptor Interactions: Deuteration Changes Binding Affinities for Histamine Receptor Ligands. PLoS ONE 2016, 11, e0154002.
- Jaeger, G. Entanglement, Information, and the Interpretation of Quantum Mechanics. Choice Rev. Online 2010, 47, 3870.
- Andersen, M.L.; Stobbe, S.; Sørensen, A.S.; Lodahl, P. Strongly Modified Plasmon-Matter Interaction with Mesoscopic Quantum Emitters. Nat. Phys. 2011, 7, 215–218.
- Wolf, F.A. Mind into Matter: A New Alchemy of Science and Spirit; Red Wheel/Weiser: Newburyport, MA, USA, 2001.
- Eibenberger, S.; Gerlich, S.; Arndt, M.; Mayor, M.; Tüxen, J. Matter–Wave Interference of Particles Selected from a Molecular Library with Masses Exceeding 10000 Amu. Phys. Chem. Chem. Phys. 2013, 15, 14696–14700.
- Roger, P. The Road to Reality: A Complete Guide to the Laws of the Universe, 1st ed.; Jonathan Cape: London, UK, 2004.
- Jozsa, R.; Linden, N. On the Role of Entanglement in Quantum-Computational Speed-Up. Proc. R. Soc. A Math. Phys. Eng. Sci. 2003, 459, 2011–2032.
- Gröblacher, S.; Paterek, T.; Kaltenbaek, R.; Brukner, Č.; Zukowski, M.; Aspelmeyer, M.; Zeilinger, A. An Experimental Test of Non-Local Realism. Nature 2007, 446, 871–875.
- Fan, Y.; Guo, X.; Yang, X. Quantifying Coherence of Quantum Channels via Trace Distance. Quantum Inf. Process 2022, 21, 1–16.
- Li, C.M.; Lambert, N.; Chen, Y.N.; Chen, G.Y.; Nori, F. Witnessing Quantum Coherence: From Solid-State to Biological Systems. Sci. Rep. 2012, 2, srep00885.
- Roztocki, N.; Soja, P.; Weistroffer, H.R. The Role of Information and Communication Technologies in Socioeconomic Development: Towards a Multi-Dimensional Framework. Inf. Technol. Dev. 2019, 25, 171–183.
- Flöther, F.F.; Griffin, P.F. How Can Quantum Technologies Be Applied in Healthcare, Medicine and the Life Sciences? Res. Dir. Quantum Technol. 2023, 1.
- Shimazoe, K.; Tomita, H.; Watts, D.; Moskal, P.; Kagawa, A.; Thirolf, P.G.; Budker, D.; Levin, C.S. Quantum Sensing for Biomedical Applications. In Proceedings of the 2021 IEEE Nuclear Science Symposium and Medical Imaging Conference Record, NSS/MIC 2021, Yokohama, Japan, 16–23 October 2021.
- Um, M.; Ro, D.; Chang, I.J.; Lee, H.M. A Radiation-Hardened Readout Integrated Circuits for Sensor Systems. In Proceedings of the 2020 IEEE International Conference on Consumer Electronics-Asia, ICCE-Asia 2020, Seoul, Republic of Korea, 1–3 November 2020.
- Jezzard, P.; Clare, S. Principles of nuclear magnetic resonance and MRI. In Functional Magnetic Resonance Imaging: An Introduction to Methods; Oxford Academic: Oxford, UK, 2012.
- Li, M.; Jiang, F.; Xue, L.; Peng, C.; Shi, Z.; Zhang, Z.; Li, J.; Pan, Y.; Wang, X.; Feng, C.; et al. Recent Progress in Biosensors for Detection of Tumor Biomarkers. Molecules 2022, 27, 7327.
- Casacio, C.A.; Madsen, L.S.; Terrasson, A.; Waleed, M.; Barnscheidt, K.; Hage, B.; Taylor, M.A.; Bowen, W.P. Quantum-Enhanced Nonlinear Microscopy. Nature 2021, 594, 201–206.
- Datta, A. Quantum-Enhanced Stimulated Emission Microscopy. In Proceedings of the Emerging Imaging and Sensing Technologies for Security and Defence V; and Advanced Manufacturing Technologies for Micro- and Nanosystems in Security and Defence III, Online Only, UK, 21–25 September 2020; SPIE: Bellingham, WA, USA, 2020.
- Wendin, G. Quantum Information Processing with Superconducting Circuits: A Review. Rep. Prog. Phys. 2017, 80, 106001.
- Weedbrook, C.; Pirandola, S.; García-Patrón, R.; Cerf, N.J.; Ralph, T.C.; Shapiro, J.H.; Lloyd, S. Gaussian Quantum Information. Rev. Mod. Phys. 2012, 84, 621–669.
- Braunstein, L.S.; Van Loock, P. Quantum Information with Continuous Variables. Rev. Mod. Phys. 2005, 77, 513–577.
- Pauls, J.A.; Zhang, Y.; Berman, G.P.; Kais, S. Quantum Coherence and Entanglement in the Avian Compass. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2013, 87, 062704.
- Sun, Y.; Wang, C.; Dai, J. Biophotons as Neural Communication Signals Demonstrated by in Situ Biophoton Autography. Photochem. Photobiol. Sci. 2010, 9, 315–322.
- Popp, F.A.; Nagl, W.; Li, K.H.; Scholz, W.; Weingärtner, O.; Wolf, R. Biophoton Emission-New Evidence for Coherence and DNA as Source. Cell Biophys. 1984, 6, 33–52.
- Sawant, S.G.; Randers-Pehrson, G.; Geard, C.R.; Brenner, D.J.; Hall, E.J. The Bystander Effect in Radiation Oncogenesis: I. Transformation in C3H 10T1/2 Cells in Vitro Can Be Initiated in the Unirradiated Neighbors of Irradiated Cells. Radiat. Res. 2001, 155, 397–401.
- Brenner, D.J.; Little, J.B.; Sachs, R.K. The Bystander Effect in Radiation Oncogenesis: II. A Quantitative Model. Radiat. Res. 2001, 155, 402–408.
- Giovannetti, V.; Lloyd, S.; Maccone, L. Quantum-Enhanced Measurements: Beating the Standard Quantum Limit. Science 2004, 306, 1330–1336.
- Joo, J.; Munro, W.J.; Spiller, T.P. Quantum Metrology with Entangled Coherent States. Phys. Rev. Lett. 2011, 107, 083601.
- Piccolino, M.; Bresadola, M.; Wade, N. The controversy between Galvani and Volta over animal electricity. In Shocking Frogs; Oxford University Press: Oxford, UK, 2014.
- Tyler, S.E.B. The Work Surfaces of Morphogenesis: The Role of the Morphogenetic Field. Biol. Theory 2014, 9, 194–208.
- Kim, H.J.; Lee, S. Relation between Quantum Coherence and Quantum Entanglement in Quantum Measurements. Phys. Rev. A 2022, 106, 022401.
- Ren, J.G.; Xu, P.; Yong, H.L.; Zhang, L.; Liao, S.K.; Yin, J.; Liu, W.Y.; Cai, W.Q.; Yang, M.; Li, L.; et al. Ground-to-Satellite Quantum Teleportation. Nature 2017, 549, 70–73.
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial-Mesenchymal Transition and Its Transcription Factors. Biosci. Rep. 2022, 42, BSR20211754.
- Bhavsar, M.B.; Leppik, L.; Costa Oliveira, K.M.; Barker, J.H. Role of Bioelectricity During Cell Proliferation in Different Cell Types. Front Bioeng. Biotechnol. 2020, 8, 603.
- George, L.F.; Bates, E.A. Mechanisms Underlying Influence of Bioelectricity in Development. Front. Cell Dev. Biol. 2022, 10, 772230.
- Camelo, L.G. Pathophysiology of Biophoton: Vibratory Impact Syndrome Leading to Physical Effects and Metabolic Changes—Part 1. Neurosci. Med. 2021, 12, 126–162.
- Zou, N. Quantum Entanglement and Its Application in Quantum Communication. Proc. J. Phys. Conf. Ser. 2021, 1827, 012120.
- Belousov, L.V. “Our Standpoint Different from Common...” (Scientific Heritage of Alexander Gurwitsch). Russ. J. Dev. Biol. 2008, 39, 307–315.
- Gurwitsch, A.A. A Historical Review of the Problem of Mitogenetic Radiation. Experientia 1988, 44, 545–550.
- Jung, S.K.; Lee, K.W.; Byun, S.; Lee, E.J.; Kim, J.E.; Bode, A.M.; Dong, Z.; Lee, H.J. Myricetin Inhibits UVB-Induced Angiogenesis by Regulating PI-3 Kinase in Vivo. Carcinogenesis 2009, 31, 911–917.
- Kaiser, S.; Scheuring, D. To Lead or to Follow: Contribution of the Plant Vacuole to Cell Growth. Front Plant Sci. 2020, 11, 553.
- Syljuåsen, R.G. Cell Cycle Effects in Radiation Oncology. In Radiation Oncology; Springer: Berlin/Heidelberg, Germany, 2019.
- Fraikin, G.Y.; Belenikina, N.S. Photochemistry and Signaling Activity of Plant Cryptochromes: A Review. Biol. Bull. 2023, 50, 266–275.
- Fraikin, G.Y. Photosensory and Signaling Properties of Cryptochromes. Moscow Univ. Biol. Sci. Bull. 2022, 77, 54–63.
- Chadwick, G.L.; Skennerton, C.T.; Laso-Pérez, R.; Leu, A.O.; Speth, D.R.; Yu, H.; Morgan-Lang, C.; Hatzenpichler, R.; Goudeau, D.; Malmstrom, R.; et al. Comparative Genomics Reveals Electron Transfer and Syntrophic Mechanisms Differentiating Methanotrophic and Methanogenic Archaea. PLoS Biol. 2022, 20, e3001508.
- Lanting, T.; Przybysz, A.J.; Smirnov, A.Y.; Spedalieri, F.M.; Amin, M.H.; Berkley, A.J.; Harris, R.; Altomare, F.; Boixo, S.; Bunyk, P.; et al. Entanglement in a Quantum Annealing Processor. Phys. Rev. X 2014, 4, 021041.
- Devault, D. Quantum Mechanical Tunnelling in Biological Systems. Q Rev. Biophys. 1980, 13, 387–564.
- Ball, P. Is Photosynthesis Quantum-Ish? Phys. World 2018, 31, 249–251.
- Xu, C.; Zhang, J.; Mihai, D.M.; Washington, I. Light-Harvesting Chlorophyll Pigments Enable Mammalian Mitochondria to Capture Photonic Energy and Produce ATP. J. Cell Sci. 2014, 127, 388–399.
- Yeles, C.; Vlachavas, E.I.; Papadodima, O.; Pilalis, E.; Vorgias, C.E.; Georgakilas, A.G.; Chatziioannou, A. Integrative Bioinformatic Analysis of Transcriptomic Data Identifies Conserved Molecular Pathways Underlying Ionizing Radiation-Induced Bystander Effects (RIBE). Cancers 2017, 9, 160.
- Sia, P.I.; Luiten, A.N.; Stace, T.M.; Wood, J.P.M.; Casson, R.J. Quantum Biology of the Retina. Clin. Exp. Ophthalmol. 2014, 42, 582–589.
- Sun, L.; Zhao, L.; Peng, R.Y. Research Progress in the Effects of Terahertz Waves on Biomacromolecules. Mil. Med. Res. 2021, 8, 1–8.
- Lindinger, M.I. Structured Water: Effects on Animals. J. Anim. Sci. 2021, 99, skab063.
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic Contamination of Drinking Water: A Systematic Review. PLoS ONE 2020, 15, e0236838.
- Pal, S.; Batra, P.; Krisnanda, T.; Paterek, T.; Mahesh, T.S. Experimental Localisation of Quantum Entanglement through Monitored Classical Mediator. Quantum 2021, 5, 478.
- Khrennikov, A. Quantum Versus Classical Entanglement: Eliminating the Issue of Quantum Nonlocality. Found Phys. 2020, 50, 1762–1780.
- Slocombe, L.; Al-Khalili, J.S.; Sacchi, M. Quantum and Classical Effects in DNA Point Mutations: Watson-Crick Tautomerism in at and GC Base Pairs. Phys. Chem. Chem. Phys. 2021, 23, 4141–4150.
- Slocombe, L.; Sacchi, M.; Al-Khalili, J. An Open Quantum Systems Approach to Proton Tunnelling in DNA. Commun. Phys. 2022, 5, 1–9.
- Löwdin, P.O. Quantum Genetics and the Aperiodic Solid. Some Aspects on the Biological Problems of Heredity, Mutations, Aging, and Tumors in View of the Quantum Theory of the DNA Molecule. In Advances in Quantum Chemistry; Academic Press: Cambridge, MA, USA, 1966; pp. 213–360.
- Guallar, V.; Douhal, A.; Moreno, M.; Lluch, J.M. DNA Mutations Induced by Proton and Charge Transfer in the Low-Lying Excited Singlet Electronic States of the DNA Base Pairs: A Theoretical Insight. J. Phys. Chem. A 1999, 103, 6251–6256.
- Yang, M. A Quantum Mechanical Approach to Understanding DNA Mutations. Berkeley Sci. J. 2019, 24, 37–40.
- Yin, M.; O’Neill, L.A.J. The Role of the Electron Transport Chain in Immunity. FASEB J. 2021, 35, e21974.
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z. Bin Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15.
- Cogliati, S.; Cabrera-Alarcón, J.L.; Enriquez, J.A. Regulation and Functional Role of the Electron Transport Chain Supercomplexes. Biochem. Soc. Trans. 2021, 49, 2655–2668.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Fedunov, R.G.; Ivanov, A.I. Effect of the Excitation Pulse Frequency on the Ultrafast Photoinduced Electron Transfer Dynamics. J. Chem. Phys. 2005, 122, 064501.
- Parlak, C.; Ramasami, P. Theoretical and Experimental Study of Infrared Spectral Data of 2-Bromo-4-Chlorobenzaldehyde. SN Appl. Sci. 2020, 2, 1–9.
- Vatansever, F.; Hamblin, M.R. Far Infrared Radiation (FIR): Its Biological Effects and Medical Applications. Photonics Lasers Med. 2012, 1, 255–266.
- Sengupta, B.; Stemmler, M.; Laughlin, S.B.; Niven, J.E. Action Potential Energy Efficiency Varies among Neuron Types in Vertebrates and Invertebrates. PLoS Comput. Biol. 2010, 6, e1000840.
- Aubrun, G.; Lami, L.; Palazuelos, C.; Plávala, M. Entanglement and Superposition Are Equivalent Concepts in Any Physical Theory. Phys. Rev. Lett. 2022, 128, 160402.
- Gidon, A.; Aru, J.; Larkum, M.E. Does Brain Activity Cause Consciousness? A Thought Experiment. PLoS Biol. 2022, 20, e3001651.
- Kim, J.H.; Chae, J.W.; Jeong, Y.C.; Kim, Y.H. Long-Range Distribution of High-Quality Time-Bin Entangled Photons for Quantum Communication. J. Korean Phys. Soc. 2022, 80, 203–213.
- Xu, G.; Xiao, K.; Li, Z.; Niu, X.X.; Ryan, M. Controlled Secure Direct Communication Protocol via the Three-Qubit Partially Entangled Set of States. Comput. Mater. Contin. 2019, 58, 809–827.
- Sommer, A.P.; Haddad, M.K.; Fecht, H.J. Light Effect on Water Viscosity: Implication for ATP Biosynthesis. Sci. Rep. 2015, 5, 12029.
- Nesterov, S.V.; Smirnova, E.G.; Yaguzhinsky, L.S. Mechanism of Energy Storage and Transformation in the Mitochondria at the Water–Membrane Interface. Biochemistry 2022, 87, 179–190.
- Kühlbrandt, W. Structure and Function of Mitochondrial Membrane Protein Complexes. BMC Biol. 2015, 13, 89.
- Dröse, S.; Krack, S.; Sokolova, L.; Zwicker, K.; Barth, H.D.; Morgner, N.; Heide, H.; Steger, M.; Nübel, E.; Zickermann, V.; et al. Functional Dissection of the Proton Pumping Modules of Mitochondrial Complex I. PLoS Biol. 2011, 9, e1001128.
- How Mitochondria Evolved from Bacteria. Nat. India 2022.
- Carvalho, D.S.; Andrade, R.F.S.; Pinho, S.T.R.; Góes-Neto, A.; Lobão, T.C.P.; Bomfim, G.C.; El-Hani, C.N. What Are the Evolutionary Origins of Mitochondria? A Complex Network Approach. PLoS ONE 2015, 10, e0134988.
- Damulewicz, M.; Mazzotta, G.M. One Actor, Multiple Roles: The Performances of Cryptochrome in Drosophila. Front Physiol. 2020, 11, 99.
- Ponnu, J.; Hoecker, U. Signaling Mechanisms by Arabidopsis Cryptochromes. Front Plant Sci. 2022, 13, 844714.
- Sullivan, C.M.; Nienhaus, L. Generating Spin-Triplet States at the Bulk Perovskite/Organic Interface for Photon Upconversion. Nanoscale 2022, 15, 998–1013.
- Nebgen, B.; Emmert, F.L.; Slipchenko, L.V. Vibronic Coupling in Asymmetric Bichromophores: Theory and Application to Diphenylmethane. J. Chem. Phys. 2012, 137, 084112.
- Jansen, T.L.C.; Saito, S.; Jeon, J.; Cho, M. Theory of Coherent Two-Dimensional Vibrational Spectroscopy. J. Chem. Phys. 2019, 150, 100901.
- Nishiyama, A.; Tanaka, S.; Tuszynski, J.A. Non-Equilibrium Quantum Brain Dynamics: Super-Radiance and Equilibration in 2 + 1 Dimensions. Entropy 2019, 21, 1066.
- Yan, W.; Diao, S.; Fan, Z. The Role and Mechanism of Mitochondrial Functions and Energy Metabolism in the Function Regulation of the Mesenchymal Stem Cells. Stem Cell Res. Ther. 2021, 12, 1–17.
- Schweber, S. Herbert Fröhlich: A Physicist Ahead of His Time. Phys. Today 2016, 69, 52.
- Fröhlich, H. Coherent Excitation in Active Biological Systems. In Modern Bioelectrochemistry; Springer: Berlin/Heidelberg, Germany, 1986.
- Frohlich, H. Coherent Electric Vibrations in Biological Systems and the Cancer Problem. IEEE Trans. Microw. Theory Tech. 1978, 26, 613–618.
- Pokorný, J. Coherent Vibration Interaction among Cells in Biological Systems. Czechoslov. J. Phys. 1980, 30, 1339–1342.
- Cho, K.H.; Rhee, Y.M. Computational Elucidations on the Role of Vibrations in Energy Transfer Processes of Photosynthetic Complexes. Phys. Chem. Chem. Phys. 2021, 23, 26623–26639.
- Daniel, C. Ultrafast Processes: Coordination Chemistry and Quantum Theory. Phys. Chem. Chem. Phys. 2021, 23, 43–58.
- Feinberg, T.E.; Mallatt, J. Phenomenal Consciousness and Emergence: Eliminating the Explanatory Gap. Front Psychol. 2020, 11, 1041.
- Hameroff, S. How Quantum Brain Biology Can Rescue Conscious Free Will. Front Integr. Neurosci. 2012, 6, 93.
- York, A. Biofilms: Shocking Biofilms. Nat. Rev. Microbiol. 2017, 15, 132–133.
- Lopez, L.; Fasano, C.; Perrella, G.; Facella, P. Cryptochromes and the Circadian Clock: The Story of a Very Complex Relationship in a Spinningworld. Genes 2021, 12, 672.
- Wu, L.; Luo, Y. Bacterial Quorum-Sensing Systems and Their Role in Intestinal Bacteria-Host Crosstalk. Front Microbiol. 2021, 12, 611413.
- Fleitas Martínez, O.; Rigueiras, P.O.; da Pires, Á.S.; Porto, W.F.; Silva, O.N.; de la Fuente-Nunez, C.; Franco, O.L. Interference with Quorum-Sensing Signal Biosynthesis as a Promising Therapeutic Strategy Against Multidrug-Resistant Pathogens. Front Cell Infect. Microbiol. 2019, 8, 444.
- Majumdar, S.; Pal, S. Cross-Species Communication in Bacterial World. J. Cell Commun. Signal 2017, 11, 187–190.
- Matarèse, B.F.E.; Rahmoune, H.; Vo, N.T.K.; Seymour, C.B.; Schofield, P.N.; Mothersill, C. X-Ray-Induced Bio-Acoustic Emissions from Cultured Cells. Int. J. Radiat. Biol 2022, 99, 1285–1290.
- Mothersill, C.; Smith, R.; Wang, J.; Rusin, A.; Fernandez-Palomo, C.; Fazzari, J.; Seymour, C. Biological Entanglement–Like Effect After Communication of Fish Prior to X-Ray Exposure. Dose-Response 2018, 16, 1559325817750067.
- Smith, R.W.; Moccia, R.D.; Seymour, C.B.; Mothersill, C.E. Irradiation of Rainbow Trout at Early Life Stages Results in a Proteomic Legacy in Adult Gills. Part A; Proteomic Responses in the Irradiated Fish and in Non-Irradiated Bystander Fish. Environ. Res. 2018, 163, 297–306.
This entry is offline, you can click here to edit this entry!
