Ocular drug administration encompasses a range of routes, each with its own advantages and limitations. The available methods include systemic delivery (such as oral, intravenous, and subcutaneous routes) as well as local delivery options (including topical eye drops, periocular or intravitreal injections, and intravitreal implants). While these approaches can be effective in delivering medications to the eye, they also have inherent drawbacks, which will be explored in greater detail in this entry. Notably, understanding the strengths and limitations of these ocular drug administration routes is crucial for optimizing therapy and achieving the desired therapeutic outcomes while minimizing potential adverse effects.
- Ocular drug administration
- Systemic delivery
- Topical eye drops
- Periocular injections
- Intravitreal injections
- Therapeutic goals
- Ophthalmology
1. Route of Administration
Various routes exist for ocular medication administration, each with distinct pros and cons. Systemic delivery (oral, intravenous, subcutaneous) and local methods (topical drops, periocular/IV injections, IV implants) are commonly used [1]. However, these methods have limitations. Table 1 and Figure 1 provide an overview of the advantages and disadvantages of different ocular drug administration approaches [2][3].
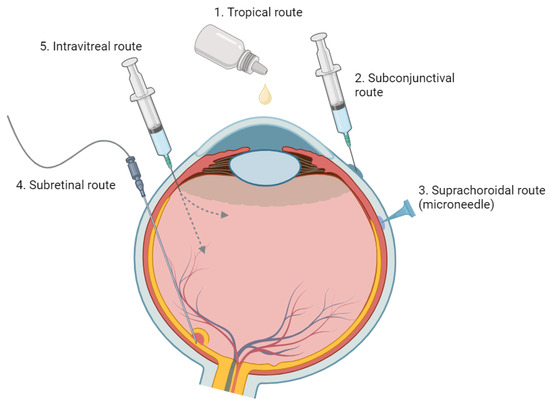
Figure 1. An Overview of Various Ophthalmic Medication Delivery Routes. This figure illustrates the range of administration methods used in ophthalmic medicine, including topical, subconjunctival, intravitreal, suprachoroidal, and subretinal techniques.
| Injection Method | Advantages | Disadvantages |
|---|---|---|
| Topical Eye Drops [4] | Prevalent, well-known method | Low bioavailability to posterior segment tissues |
| Non-invasive method for ocular drug delivery | Short duration of action, requiring frequent administration | |
| Relies on patient’s compliance | ||
| Local complications (ocular surface irritation, cataracts, ocular hypertension, periocular aesthetic issues) | ||
| Systemic Drug Administration | Noninvasive and potentially patient-preferred | High doses often required due to reduced accessibility to targeted ocular tissues |
| Usable as standalone or in combination with topical delivery | Potential systemic side effects due to high dosage, necessitating safety and toxicity considerations | |
| Effective bioavailability is challenging due to blood–ocular barriers | ||
| Intravitreal Injection [5] | Office-based, outpatient procedure | Requires frequent in-office visits |
| High bioavailability (bypass corneal and blood–retinal barriers) | Potential for severe complications (Endophthalmitis, retinal detachment, vitreous hemorrhage) | |
| Fewer systemic side effects compared to oral or IV administration | Local complications (increased IOP, cataract formation) | |
| Rapid therapeutic onset | Possible post-injection floaters | |
| Systemic absorption and side effects can still occur | ||
| Subretinal Injection [6] | Targeted treatment for the RPE and outer retina | Invasive procedure, requires vitrectomy |
| Reduced immune reactions for gene therapy using viral vectors (due to injection in an immune-privileged site) |
Limited distribution of injectate within subretinal space; effects confined to injection site |
2. Topical Administration
Topical administration, primarily through eye drops, is a commonly used non-invasive approach for ocular drug delivery. However, it presents challenges due to the eye's anatomy and physiology.
The concentration gradient from the tear reservoir to the cornea or conjunctiva drives passive absorption, but only a small fraction of the administered drop (approximately 20%) is retained in the eye [7]. Within a few minutes, about half of the medication leaves the eye, with a rapid turnover rate of around 15% per minute. Factors like reflex tearing, consecutive dosing, and the small cul-de-sac of the eye contribute to fast tear turnover, accelerating drug clearance and hindering effective absorption [2][3].
Furthermore, drugs must traverse the hydrophobic tight junctions formed by the epithelium and endothelium, as well as the hydrophilic stroma layer of the cornea [8]. The low permeability of the cornea and sclera limits drug penetration, reducing the bioavailability of topically administered drugs.
Due to the barriers posed by the cornea and high tear turnover rates, frequent and high-dose applications are often required for topical administration. This can lead to local and systemic side effects, potentially compromising patient compliance [9]. Studies have shown that medication non-compliance rates in the general population are approximately 80% [10], and these challenges are even more pronounced in certain populations, such as the elderly and individuals with physical disabilities.
Additionally, topical drugs may affect unaffected tissue, resulting in side effects. For example, chronic use of topical steroids can lead to complications like cataracts and ocular hypertension [4]. Similarly, topical prostaglandins can cause undesirable periocular aesthetic concerns [11].
Although topical application is a primary method of ocular drug delivery, these complexities underscore the need for advancements in drug delivery methods to overcome the limitations associated with topical administration.
3. Systemic Administration
Oral delivery has been investigated as a potential route for ocular drug administration, either alone or in combination with topical delivery [12][13][14][15]. While it offers a non-invasive option for managing chronic retinal diseases, oral administration has limitations. It has reduced accessibility to targeted ocular tissues, requiring high doses for therapeutic effectiveness. However, high dosages can lead to systemic side effects, necessitating careful consideration of safety and toxicity [16][17].
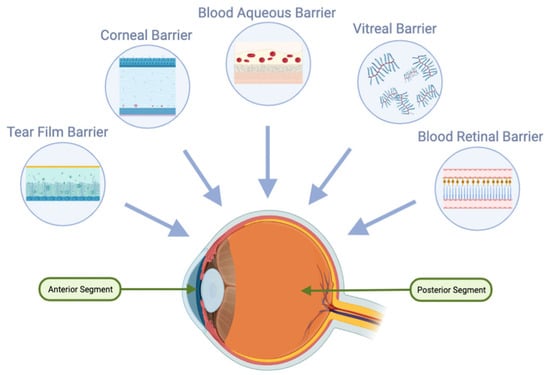
For oral delivery to be effective in ocular applications, achieving high oral bioavailability is crucial. Additionally, after oral absorption, molecules must traverse systemic circulation and overcome the blood-ocular barriers, including the blood-aqueous and blood-retinal barriers (Figure 2). The blood-retinal barrier consists of an inner barrier, protected by the fenestrated endothelium of retinal vasculature, and an outer barrier, maintained by tight junctions within the retinal pigment epithelium (RPE). These protective ocular structures present significant challenges for systemic drug administration [16][17].
Systemic medications, including steroidal and nonsteroidal anti-inflammatory drugs, as well as immunomodulatory agents (both biologic and nonbiologic), are effective for treating uveitic macular edema (UME). However, they are typically reserved for bilateral disease or cases unresponsive to local therapy due to potential adverse effects (AEs) such as infections and gastrointestinal (GI) disturbances [1][18][19]. Additionally, the use of nonsteroidal anti-inflammatory drugs and systemic immunomodulatory agents, either alone or in combination with steroids, may further increase the risk of GI disturbances [1][18][19].
4. Periocular Injection
Periocular drug administration offers an alternative to topical and systemic dosing, which struggle to achieve therapeutic drug concentrations in the posterior segment [2]. Compared to intravenous administration, periocular routes (subconjunctival, subtenon, retrobulbar, and peribulbar) are less invasive [2].
Subconjunctival injections can enhance absorption of water-soluble drugs by bypassing the conjunctival epithelial barrier. However, access to the posterior eye segment remains limited due to various barriers, including dynamic factors such as conjunctival blood and lymphatic circulation [20][21][22]. These dynamic barriers contribute to rapid drug elimination, reducing ocular bioavailability and vitreous drug levels after administration [20][21][22]. Although some molecules can reach the neural retina and photoreceptor cells through the permeable sclera [1][23], the high blood flow in the choroid can remove a significant portion of the drug before it reaches its target. Moreover, the presence of tight junctions within the retinal pigment epithelium forms blood-retinal barriers that restrict drug availability to the photoreceptor cells.
5. Intravitreal Injection
IV administration is widely used as a first-line therapy for various ocular conditions, including neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), and macular edema secondary to retinal vein occlusion (RVO) [24][25][26]. It offers advantages such as direct drug delivery to the retina and vitreous, bypassing corneal and scleral barriers, and circumventing the blood-retinal barrier. These benefits ensure high bioavailability, rapid therapeutic effects, and improved patient compliance compared to topical eyedrops [27].
However, IV injections have drawbacks and potential complications. Severe complications include the risk of endophthalmitis, retinal detachment, and vitreous hemorrhage. IV steroids specifically can cause increased intraocular pressure and cataract development [25]. Minor side effects, such as floaters, and the potential for systemic absorption and associated side effects can also impact patient satisfaction and treatment adherence [25].
Another challenge with IV administration is the need for frequent injections due to the short half-life of drugs [28][29][30]. After injection, drugs are eliminated either anteriorly or posteriorly. Anterior elimination involves diffusion through the vitreous, aqueous turnover, and uveal blood flow. Posterior elimination requires permeation through the blood-retinal barrier, which can be passive or actively mediated. Hydrophilic drugs with larger molecular weights have longer half-lives, while hydrophobic drugs with smaller molecular weights have shorter half-lives, necessitating more frequent injections. The burden of regular in-office visits and patient-specific factors like age and prior vitrectomy can further complicate the treatment regimen and decrease compliance [30][31].
To overcome the limitations of short treatment duration and frequent in-office visits, intraocular implants have been developed. The Multicenter Uveitis Steroid Treatment (MUST) trial assessed a fluocinolone acetonide (FA) intraocular implant that releases the drug over approximately 30 months [32]. In the short term, the FA implant demonstrated superior efficacy in controlling uveitic inflammation and reducing macular edema compared to systemic corticosteroids, but the differences diminished after 24 months. However, the FA implant was associated with a fourfold increase in the risk of elevated intraocular pressure (IOP) requiring intervention. Extended follow-up after seven years revealed that patients receiving systemic therapy had better visual acuity than those with IV FA implants [33].
In the realm of gene therapy, intravitreal injection of an anti-VEGF transgene product (Adverum Biotechnologies) has shown promise but raises concerns about significant inflammatory responses [34][35][36][37]. The vitreous presents challenges for retinal gene delivery due to components like hyaluronan, which can lead to aggregation and immobilization of DNA/liposome complexes [38][39]. The inner limiting membrane (ILM) and the retinal pigment epithelium (RPE) also act as barriers to retinal gene delivery and drug transport to the choroid, respectively [40][41].
Emerging alternatives like subretinal and subconjunctival (SC) drug delivery hold potential for longer-lasting effects, reducing injection frequency, and minimizing gene therapy-induced inflammation [42].
6. Subretinal Injection
Subretinal delivery is a promising approach for retinal gene therapy, particularly for retinal degeneration and vascular diseases. This method involves directly introducing viral vectors into the immune-privileged subretinal space, allowing targeted treatment for the retinal pigment epithelium (RPE) and outer retina while minimizing immune reactions [6].
The FDA-approved gene therapy Voretigene neparvovec-rzyl (Luxturna) for RPE65-associated inherited retinal dystrophy has shown promising outcomes [43][44]. Gene therapy also holds potential for conditions like diabetic retinopathy (DR) and age-related macular degeneration (AMD), suggesting the possibility of a single-dose treatment for these chronic diseases. Early studies using subretinal adenoviral vector anti-VEGF gene therapy have demonstrated a significant reduction in treatment burden and a favorable safety profile for neovascular AMD [45].
However, it's important to acknowledge that subretinal delivery presents its own challenges. It requires an invasive vitrectomy procedure for administration, and the localized distribution of the injected material may limit therapeutic effects to the vicinity of the injection site [42].
References
- Ghate, D.; Edelhauser, H.F. Ocular Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287.
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373.
- Carnahan, M.C.; Goldstein, D.A. Ocular Complications of Topical, Peri-Ocular, and Systemic Corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483.
- Tyagi, P.; Kadam, R.S.; Kompella, U.B. Comparison of Suprachoroidal Drug Delivery with Subconjunctival and Intravitreal Routes Using Noninvasive Fluorophotometry. PLoS ONE 2012, 7, e48188.
- Yiu, G.; Chung, S.H.; Mollhoff, I.N.; Nguyen, U.T.; Thomasy, S.M.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2020, 16, 179–191.
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A Comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754.
- Loftsson, T.; Stefánsson, E. Aqueous Eye Drops Containing Drug/Cyclodextrin Nanoparticles Deliver Therapeutic Drug Concentrations to Both Anterior and Posterior Segment. Acta Ophthalmol. 2022, 100, 7–25.
- Uchino, M.; Yokoi, N.; Shimazaki, J.; Hori, Y.; Tsubota, K.; on behalf of the Japan Dry Eye Society. Adherence to Eye Drops Usage in Dry Eye Patients and Reasons for Non-Compliance: A Web-Based Survey. J. Clin. Med. 2022, 11, 367.
- Foley, L.; Larkin, J.; Lombard-Vance, R.; Murphy, A.W.; Hynes, L.; Galvin, E.; Molloy, G.J. Prevalence and Predictors of Medication Non-Adherence among People Living with Multimorbidity: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e044987.
- Holló, G. The Side Effects of the Prostaglandin Analogues. Expert Opin. Drug Saf. 2007, 6, 45–52.
- Santulli, R.J.; Kinney, W.A.; Ghosh, S.; Decorte, B.L.; Liu, L.; Tuman, R.W.A.; Zhou, Z.; Huebert, N.; Bursell, S.E.; Clermont, A.C.; et al. Studies with an Orally Bioavailable Alpha V Integrin Antagonist in Animal Models of Ocular Vasculopathy: Retinal Neovascularization in Mice and Retinal Vascular Permeability in Diabetic Rats. J. Pharmacol. Exp. Ther. 2008, 324, 894–901.
- Shirasaki, Y.; Miyashita, H.; Yamaguchi, M. Exploration of Orally Available Calpain Inhibitors. Part 3: Dipeptidyl Alpha-Ketoamide Derivatives Containing Pyridine Moiety. Bioorg. Med. Chem. 2006, 14, 5691–5698.
- Kampougeris, G.; Antoniadou, A.; Kavouklis, E.; Chryssouli, Z.; Giamarellou, H. Penetration of Moxifloxacin into the Human Aqueous Humour after Oral Administration. Br. J. Ophthalmol. 2005, 89, 628–631.
- Sakamoto, H.; Sakamoto, M.; Hata, Y.; Kubota, T.; Ishibashi, T. Aqueous and Vitreous Penetration of Levofloxacin after Topical and/or Oral Administration. Eur. J. Ophthalmol. 2007, 17, 372–376.
- hirasaki, Y. Molecular Design for Enhancement of Ocular Penetration. J. Pharm. Sci. 2008, 97, 2462–2496.
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future Perspective in Topical Glaucoma Therapeutics. Int. J. Pharm. 2002, 248, 1–14.
- Gipson, I.K.; Argüeso, P. Role of Mucins in the Function of the Corneal and Conjunctival Epithelia. Int. Rev. Cytol. 2003, 231, 1–49.
- Geroski, D.H.; Edelhauser, H.F. Transscleral Drug Delivery for Posterior Segment Disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48.
- Hosseini, K.; Matsushima, D.; Johnson, J.; Widera, G.; Nyam, K.; Kim, L.; Xu, Y.; Yao, Y.; Cormier, M. Pharmacokinetic Study of Dexamethasone Disodium Phosphate Using Intravitreal, Subconjunctival, and Intravenous Delivery Routes in Rabbits. J. Ocul. Pharmacol. Ther. 2008, 24, 301–308.
- Weijtens, O.; Feron, E.J.; Schoemaker, R.C.; Cohen, A.F.; Lentjes, E.G.; Romijn, F.P.; van Meurs, J.C. High Concentration of Dexamethasone in Aqueous and Vitreous after Subconjunctival Injection. Am. J. Ophthalmol. 1999, 128, 192–197.
- Kim, S.H.; Csaky, K.G.; Wang, N.S.; Lutz, R.J. Drug Elimination Kinetics Following Subconjunctival Injection Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Pharm. Res. 2008, 25, 512–520.
- Prausnitz, M.R.; Noonan, J.S. Permeability of Cornea, Sclera, and Conjunctiva: A Literature Analysis for Drug Delivery to the Eye. J. Pharm. Sci. 1998, 87, 1479–1488.
- Modi, Y.S.; Tanchon, C.; Ehlers, J.P. Comparative Safety and Tolerability of Anti-VEGF Therapy in Age-Related Macular Degeneration. Drug Saf. 2015, 38, 279–293.
- Massa, H.; Nagar, A.M.; Vergados, A.; Dadoukis, P.; Patra, S.; Panos, G.D. Intravitreal Fluocinolone Acetonide Implant (ILUVIEN®) for Diabetic Macular Oedema: A Literature Review. J. Int. Med. Res. 2019, 47, 31–43.
- Adelman, R.A.; Parnes, A.J.; Bopp, S.; Saad Othman, I.; Ducournau, D. Strategy for the Management of Macular Edema in Retinal Vein Occlusion: The European VitreoRetinal Society Macular Edema Study. BioMed Res. Int. 2015, 2015, 870987.
- Gao, L.; Zhao, X.; Jiao, L.; Tang, L. Intravitreal Corticosteroids for Diabetic Macular Edema: A Network Meta-Analysis of Randomized Controlled Trials. Eye Vis. 2021, 8, 35.
- Hussain, R.M.; Hariprasad, S.M.; Ciulla, T.A. Treatment Burden in Neovascular AMD:Visual Acuity Outcomes Are Associated with Anti-VEGF Injection Frequency. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 780–784.
- Ciulla, T.A.; Bracha, P.; Pollack, J.; Williams, D.F. Real-World Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema in the United States. Ophthalmol. Retina 2018, 2, 1179–1187.
- Ciulla, T.; Pollack, J.S.; Williams, D.F. Visual Acuity Outcomes and Anti-VEGF Therapy Intensity in Macular Oedema Due to Retinal Vein Occlusion: A Real-World Analysis of 15 613 Patient Eyes. Br. J. Ophthalmol. 2021, 105, 1696–1704.
- Chin, H.-S.; Park, T.-S.; Moon, Y.-S.; Oh, J.-H. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005, 25, 556–560.
- Multicenter Uveitis Steroid Treatment Trial Research Group. The Multicenter Uveitis Steroid Treatment (MUST) Trial: Rationale, Design and Baseline Characteristics. Am. J. Ophthalmol. 2010, 149, 550–561.e10.
- Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group. Association between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs. Systemic Anti-Inflammatory Therapy and Visual Acuity at 7 Years Among Patients with Intermediate, Posterior, or Panuveitis. JAMA 2017, 317, 1993–2005.
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307.
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, Durability, and Safety of Intravitreal Faricimab up to Every 16 Weeks for Neovascular Age-Related Macular Degeneration (TENAYA and LUCERNE): Two Randomised, Double-Masked, Phase 3, Non-Inferiority Trials. Lancet 2022, 399, 729–740.
- Genentech: Press Releases|Friday, 28 January 2022. Available online: https://www.gene.com/media/press-releases/14943/2022-01-28/fda-approves-genentechs-vabysmo-the-firs (accessed on 6 July 2023).
- Genentech: Press Releases|Friday, 22 October 2021. Available online: https://www.gene.com/media/press-releases/14935/2021-10-22/fda-approves-genentechs-susvimo-a-first- (accessed on 6 July 2023).
- Pitkänen, L.; Ruponen, M.; Nieminen, J.; Urtti, A. Vitreous Is a Barrier in Nonviral Gene Transfer by Cationic Lipids and Polymers. Pharm. Res. 2003, 20, 576–583.
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van de Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A Barrier to Nonviral Ocular Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3553–3561.
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to AAV-Mediated Retinal Transduction from the Vitreous. Mol. Ther. J. Am. Soc. Gene Ther. 2009, 17, 2096–2102.
- Kansara, V.; Muya, L.; Wan, C.-R.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392.
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-HRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860.
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology 2021, 128, 1460–1468.
- REGENXBIO Announces Additional Positive Interim Phase I/IIa and Long-Term Follow-Up Data of RGX-314 for the Treatment of Wet AMD|Regenxbio Inc. Available online: https://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-additional-positive-interim-phase-iiia-and/ (accessed on 6 July 2023).
