1. Introduction
The following sections will discuss recent reports concerning the application of new organic dyes used individually as sensitizers for indoor DSSCs. Despite the growing number of studies published in the last few years, it must be pointed out that research in this area was mostly carried out by a few research groups focusing only on the development of specific compound classes, and that a systematic comparison of performances across a broad range of structurally diverse compounds is still lacking. As a result, it is currently difficult to identify general development trends or the emergence of “privileged” structures, if not within the body of work published by a single research group.
For this reason, compounds will be simply classified based on their central conjugated units, namely (1) fluorene-based dyes, (2) anthracene-based dyes, (3) benzopyrazine- and thienopyrazine-based dyes, followed by a section describing miscellaneous dyes not belonging to those categories.
2. Fluorene-Based Dyes
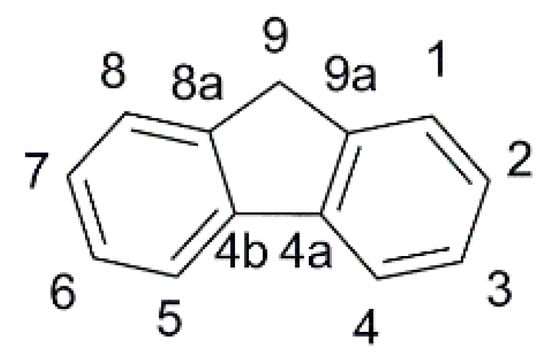
In the field of indoor DSSC, the fluorene scaffold (
Figure 1) and its derivatives were explored by Chow, Chang, and co-workers. They reported several dyes where such motif was used as an internal π-spacer
[1] or derivatized into internal acceptor moieties
[2], to obtain a library of fluorene-based dyes, featuring aromatic and aliphatic tertiary amines or phenothiazine donor groups, characterized by a fine-tuning of the frontier orbitals’ energies. Such systematic study of the interaction of fluorene with phenyl or thiophene rings directly connected as π spacers supported the rationale for the effect of torsion angles on the optical and electrochemical properties of this class of dyes.
Figure 1. The fluorene scaffold with the numbering of the ring positions.
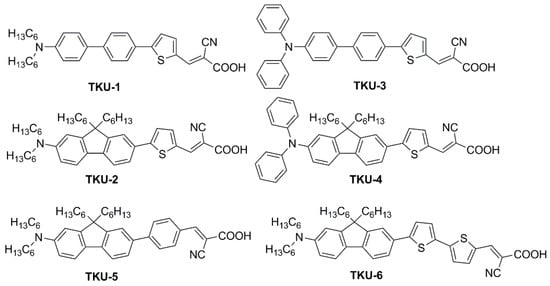
In the first report of indoor application of fluorene-containing dyes
[1], a series of D-π-A structures was described, where the fluorene moiety was introduced to replace a biphenyl spacer (present in model dyes
TKU-1 and
TKU-3,
Figure 2) aiming to increase the rigidity of the conjugated bridge.
Figure 2. Dyes containing a fluorene moiety as a replacement for a biphenyl spacer
[3].
All compounds presented a localized π-π∗ transition band below 400 nm, accompanied by an intramolecular charge transfer (ICT) transition band located in the visible region between 430 and 500 nm. Interestingly, the absorption features were influenced by the nature of the π-bridge section. Thus, varying the π-scaffold from biphenyl (TKU-1 and TKU-3) to fluorene (TKU-2 and TKU-4), the absorption spectra in solution were red-shifted thanks to a superior light-harvesting ability, in turn due to a more planar structure. The same effect was observed going from a structure where fluorene was directly connected to a phenyl ring (TKU-5) to one where it was linked to thiophene (TKU-2), and even more to a bithiophene moiety (TKU-6), thanks to a reduction of the dihedral angle between the aromatic planes. On the other hand, UV-Vis absorption spectra of the dyes adsorbed on TiO2 were measured in the hope of demonstrating the anti-aggregating effect of the hexyl chains placed on the tridimensional sp3 C9 site of the fluorene moiety. However, this proved not to be the case, as the absorption bands were strongly red-shifted and broadened compared to those of the samples in the THF solution, meaning that the alkyl chains were not bulky enough to prevent aggregation.
DSSCs built with biphenyl dyes (TKU-1 and TKU-3) were found to have longer lifetimes of the injected electrons than those fabricated with the fluorene-based analogs (TKU-2 and TKU-4). The same was observed for π-spacers directly connected to a phenyl ring (TKU-5) instead of thiophene (TKU-2), correlating this behavior to their more twisted molecular structure. The authors suggest that such twisted moieties can form a blocking layer that shields the TiO2 surface from the electrolyte, therefore reducing electron recombination. The above assumptions were found in agreement with the photovoltaic properties of the devices tested under AM 1.5 G irradiation conditions: dyes with planar moieties produce larger JSC (TKU-2 > TKU-1, TKU-4 > TKU-3, TKU-6 > TKU-2 > TKU-5) while twisted moieties produced higher VOC (reverse order).
All six TKU dyes were also exploited for the fabrication of indoor devices using an electrolyte with a low content of iodine so as not to hamper the light absorption of the dye. When comparing the six dyes, a different trend of the VOC was observed depending on the illumination conditions: low-intensity illumination (600 lux LED, or 600 lux T5 lamp) or simulated sunlight (AM 1.5 G condition) (Table 1).
Table 1. VOC values (in volts) registered for DSSCs built with dyes
TKU-1-6 under different illumination conditions
[1].
Interestingly, the authors were able to correlate it with the different bulkiness of the dyes: under low light intensity, TKU-1 and TKU-3, featuring only a few bulky substituents, display the largest drop in VOC with respect to the simulated sunlight irradiation. This suggests that charge recombination phenomena play a significant role for the DSSCs under low lighting intensity, more than under simulated sunlight conditions. This observation led the author to conclude that the best-performing dyes under (simulated) sunlight are not necessarily also the ideal ones under indoor light conditions and that their behavior should not be related only to the spectral overlap between the light source emission and the dye absorption, but also to the efficacy in shielding the TiO2 surface from the electrolyte.
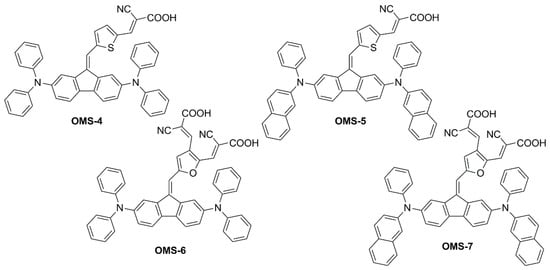
In a subsequent report
[2], the same authors explored the design of four multi-branched dyes featuring a dibenzofulvene unit connected with thienyl or furanyl as a conjugation bridge, T-shaped diarylamines (diphenylamine or phenyl-1-naphthylamine) as donors and different numbers of cyanoacrylic acid moieties as anchors (
OMS-4–
7,
Figure 3).
Figure 3. Structures of multi-branched dibenzofulvene-based organic dyes
[2].
This was exploited to allow an electronic connection between the donor moieties located on the aromatic core and the acceptor/anchoring function on the sp2-hybridized C9 position. Indeed, all compounds exhibited UV-Vis absorption spectra with a significant component in the visible region which was red-shifted for the dyes having a double acceptor group and a phenyl-1-naphthylamine donor. The authors report that emissions were very weak for the entire series of dyes, and thus band gaps were estimated from the onset of the absorption spectra.
Other than improving optical properties, the dye structure was expected to be bulky enough to efficiently suppress intermolecular interactions and charge recombination phenomena in the cells. However, the lack of electron lifetime measurements and device efficiency measurements in the absence of the disaggregating additive chenodeoxycholic acid (CDCA) did not allow for proving this assumption. On the other hand, it was shown that the excessively planar structure was detrimental to device VOC. Compared to thiophene, when furan was directly connected with dibenzofulvene (dihedral angle with furan: 9°; with thiophene: 30°) high rates of recombination were observed for the corresponding cells under simulated sunlight, causing the VOC of the devices to drop (VOC = 0.63 and 0.55 V for OMS-4 and OMS-6, respectively; VOC = 0.61 and 0.55 V for OMS-5 and OMS-7, respectively).
Unfortunately, in this work, only the best-performing dye under AM 1.5 G simulated sunlight (
OMS-4) was tested under indoor lighting, preventing a comparison of the efficiency trends under different conditions, as performed in the previous study
[1]. Despite the dye absorption spectrum having a larger overlap with the emission of a simulated diffuse illumination standard (D65) than with that of a fluorescent lamp (CWF),
OMS-4-based devices showed the highest efficiency under the latter (PCE = 2.48% and 7.26% under 1000 lux D65 and 1000 lux CWF, respectively), highlighting once again that the efficiency of indoor devices is the result of several competing factors and is not only related to the spectral overlap between the light source emission and the dye absorption.
Finally, even though a DFT analysis highlighted the expected ICT upon photoexcitation, the authors noticed that the JSC of all devices was relatively low and addressed such behavior to the unfavorable 2,7-positions of the donor moiety in the benzofulvene system, preventing proper conjugation with the rest of the unsaturated scaffold of the dyes.
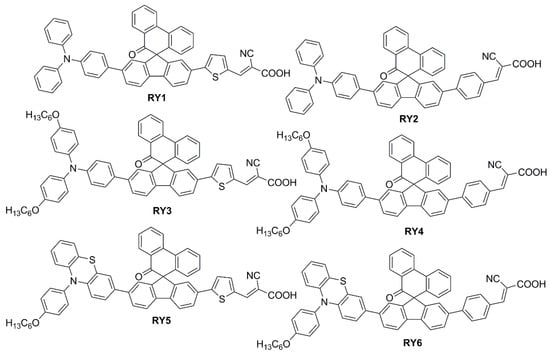
To modify the electronic and steric properties of the π spacer, the same research group reported also a small collection of dyes (
Figure 4) containing a spiro-phenanthrenone core
[3], which was supposed to act as an auxiliary acceptor moiety that, at the same time, could efficiently suppress intermolecular aggregation and prevent the electrolyte from reaching the TiO
2 surface, thanks to its bulky three-dimensional structure.
Figure 4. Structures of spiro-phenanthrenone-based organic dyes
RY1-6 [4].
The UV-Vis absorption spectra of the dyes in a MeCN/
tBuOH 1:1 solution are reported in Ref.
[4], demonstrating the red-shifting effect of the thiophene unit (
RY1,
3,
5) in comparison to the phenyl ring (
RY2,
4,
6) connected to the anchor, with the maxima of the ICT bands located around 450 nm. When adsorbed on TiO
2, the ICT transitions exhibited a slight blue-shift compared to those in solutions, which was ascribed to the deprotonation of the cyanoacrylic acid, but the difference between the two series of dyes remained clear.
As expected from the above design considerations, DSSCs built with such spiro-fluorene dyes and tested under AM 1.5 G conditions were found to lose efficiency when CDCA was used in the staining procedure, which was mainly due to a drop in photocurrents not compensated by the little gain in VOC. This was interpreted as a piece of evidence for the spiro-fluorene dyes to be able to promote vertical alignment and good dye coverage on TiO2 by themselves. Then, DSSCs were prepared with all the dyes of the series and tested under TL84 European fluorescent light and, in some cases, CWF cool white light as well. The different efficiencies recorded (see Table 2) were correlated to the different overlaps of the absorption spectra of the dyes and the emission profiles of the lamps.
Table 2. Efficiency of three
RY-series dyes under 2500 lux irradiation by different indoor lighting sources
[3].
Stability tests were also conducted on cells sensitized with dye RY3, either alone or co-sensitized with Ru-based dye N719. Both devices proved sufficiently stable, retaining 90% and 93% of their initial efficiency over 192 h indoor aging test, respectively.
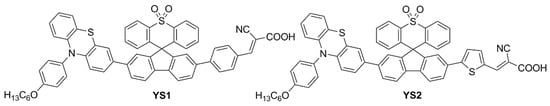
In a more recent paper, the same group also explored new electron-poor polycyclic moieties to functionalize the fluorene at the C9 site
[4]. Thioxanthenedioxide spiro fluorene was used as the auxiliary acceptor moiety in two dyes (
YS1 and
YS2,
Figure 5), in the hope of exploiting the interaction of the sulfone group with the Li
+ ions present in the liquid electrolyte to trap them and block the surface of TiO2. This was expected to increase the Fermi level of the semiconductor and thus enhance cell
VOC [5]. At the same time, the bulkiness of the central unit was anticipated to trap I
3− ions and retard charge recombination.
Figure 5. Structures of thioxanthenedioxide spiro fluorene-based organic dyes
YS1,
2 [4].
The weakening of the S=O double bond due to the interaction with Li+ ions was observed by the disappearance of the corresponding stretching peak in the FT-IR spectra. In indoor DSSCs, the new dyes were only used as co-sensitizers of N719, reaching PCE values of 20.82 ± 0.49% and 23.76 ± 0.96% for YS1 and YS2, respectively, under 2500 lux TL84 fluorescent light.
In summary, the above studies indicate that fluorene is a planar spacer with large conjugation that can foster broadband absorptions and allow good electron connections between donor and acceptor moieties in DSSC sensitizers, but only if the latter are located in positions 2 and 7 of the fluorene ring system, respectively. Position 9 is instead appropriate for steric and electronic modifications, especially spiro/derivatization with bulky polycyclic substituents. On the other hand, the ICT is not so effective between position 9 and positions 2 and 7. A common finding is that a thienyl π-spacer directly linked to fluorene can further broaden the absorption spectrum, while phenyl rings are less effective. The lower dihedral angle between the planes of fluorene and the adjacent thiophene reduces the HOMO-LUMO energy gap, resulting in red-shifted light absorptions and larger photocurrents. However, the thiophene insertion is usually accompanied by a lower photovoltage that can be ascribed to a detrimental interaction of sulfur atoms with iodine-based electrolytes and faster recombination rates.
3. Anthracene-Based Dyes
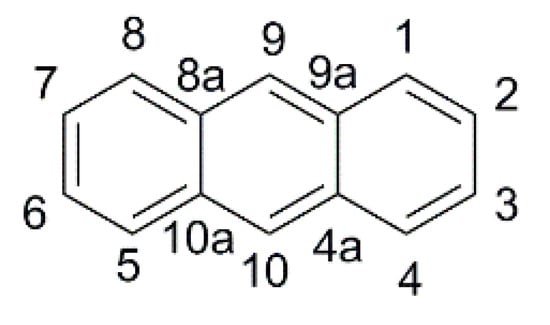
The thermal stability of the anthracene core and the ease of derivatization in 9- and 10- positions (
Figure 6) led to its application as a π-spacer in photosensitizers for DSSCs since 2009
[6], but only in 2015 the properties of anthracene-based dyes have been investigated under dim-light irradiation.
Figure 6. Anthracene scaffold with numbering of the ring positions.
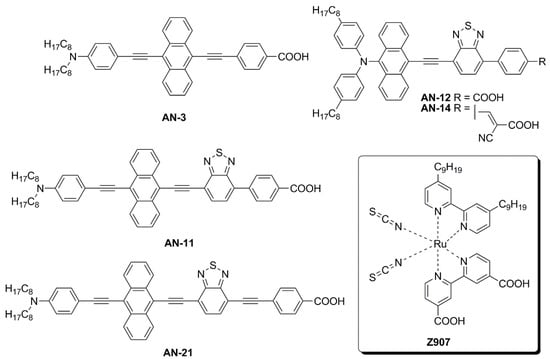
The first study in this field was carried out by Lin and co-workers, who reported the synthesis and characterization of five new anthracene-based dyes
[7]. After a first screening of the photovoltaic properties of the corresponding DSSCs under simulated AM 1.5 G irradiation (100 mW/cm
2), the
AN-3 dye (
Figure 7) was selected as the most promising one to be investigated under indoor conditions.
AN-3 was synthesized in a very straightforward way through a series of consecutive Sonogashira cross-coupling reactions, resulting in an estimated gram-scale production cost of around 65 USD/g, and exhibited a quite intense light absorption spectrum, mostly centered in the visible region, between 400 and 550 nm.
AN-3-containing DSSC modules (15 cells each) with an active area of 36 cm
2 were fabricated using an iodide-based gel as the electrolyte and the corresponding photovoltaic properties were investigated under irradiation by three different artificial light sources (T5, T8, and LED) at 200, 600, and 1000 lux, and compared with analogous DSSC modules containing an organometallic dye (
Z907—
Figure 7)
[8] as the reference. The two dyes showed comparable photovoltaic performances under indoor conditions at every illuminance (PCE = 2–3% at 200 lux; 3–5% at 600 lux, 4–6% at 1000 lux): interestingly, under T5 fluorescent light at 200 and 600 lux,
AN-3 even surpassed the efficiency of
Z907, probably because of the good overlapping between the light absorption spectrum of the dye and the T5 emission spectrum, which in this case proved to be a decisive factor.
Figure 7. Structures of anthracene-based dyes used under indoor lighting and of dye
Z907 [6][8][9].
Later, Lin’s group modified the structure of
AN-3 to enhance the push-pull properties of the dye, introducing an additional benzothiadiazole (BTDA) group as an electron-withdrawing motif (
AN-11—
Figure 11) and/or an
N,
N-diarylamino-anthryl group as the donor, with a carboxylic (
AN-12) or a cyanoacrylic acid (
AN-14) as the anchor
[9]. The combined effect of both the elongation of the π-structure and the introduction of an additional acceptor group in
AN-11 was evident in the comparison of the light absorption spectra of
AN-3 and
AN-11, since the dyes showed similar molar attenuation coefficients (ε) and comparable absorption bands, with a red-shift of around 30 nm for
AN-11 compared to
AN-3. On the other hand, the simultaneous modification of both the electron-donating and electron-withdrawing groups in
AN-12 and
AN-14 led to completely different absorption spectra for these two dyes, showing two well-resolved, but less intense absorption bands. The emission bands follow the same wavelength trend observed for the lowest energy absorption peaks, that is
AN-11 >
AN-14 >
AN-12 >
AN-3, but with different intensities, which could be related to different degrees of molecular aggregation of the dyes. Among this dye series,
AN-11 emerged as the sensitizer with the best light-harvesting properties (the most red-shifted
λmax, the highest
ε, the broadest light absorption spectrum—
Figure 8), both in solution and after adsorption on TiO
2, which affected the photovoltaic performance of the corresponding small-scale DSSCs (active area = 0.25 cm
2) under both T5 and LED fluorescence light at 1000 lux. Accordingly,
AN-11 gave rise to PCEs of approximately 10%, while
AN-12 and
AN-14 showed poorer efficiencies around 5–6%.
For this reason, a scale-up from small DSSCs to rigid (active area = 26.80 cm2) and flexible modules (active area = 19.80 cm2) containing the AN-11 dye was realized: both under T5 and LED illumination, the rigid AN-11-containing DSSC modules exhibited PCE values among 9 and 12%, always surpassing the performance of the corresponding Z907-containing modules, especially thanks to higher photocurrents, which in turn can be justified by the much greater molar attenuation coefficient and the broader light absorption spectrum of AN-11 compared to Z907. The best PCE value (11.94%) was recorded by AN-11 under T5 illumination at 1000 lux. Similar behavior was proven also for the flexible modules, even if the average PCE values were a bit lower, between 8–10%. Finally, both rigid and flexible AN-11-containing modules were proven to retain 87–90% of their initial PCE values after 600 h of T5 irradiation at 200 lux.
Compared to AN-3 and AN-11, the elongation of the π-structure in AN-21 caused a further red-shift in the light absorption band, which was centered at 544 nm in THF solution and was well-matched with the emission spectrum of a common light source such as T5. The DSSC module efficiencies were further increased moving from AN-11 to AN-21 and PCE values among 12–14% and 9–13% were recorded under T5 and LED irradiation respectively (best PCE: 13.48% under T5 illumination at 1000 lux). Finally, the authors demonstrated that the AN-21-containing module had the ability to power indoor applications by introducing it inside a remote controller and showing a signal of response upon command after being put under 500 lux of T5 light for 10 min.
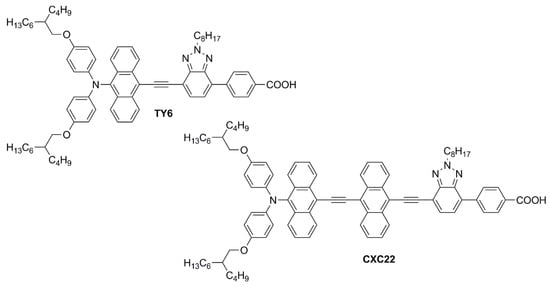
Simultaneously, Wei, Yeh et al. investigated anthryl-based dyes containing an
N,
N-
bis(
p-alkyloxy-phenyl)amine as the donor, a benzotriazole as the additional acceptor group, and benzoic acid as the anchoring moiety
[10][11]. The two photosensitizers (
TY6 and
CXC22—
Figure 8) differed in the elongation of the π-structure, with
CXC22 bearing an additional ethynyl-anthryl bridge in comparison to
TY6. Both molecules were prepared by assembling the proper building blocks through the most common cross-coupling reactions (Sonogashira, Buchwald–Hartwig, and Suzuki–Miyaura) and exhibited three main absorption maxima in the UV-Vis region, with
CXC22 showing red-shifted (λ
max = 535
vs. 516 nm for
CXC22 and
TY6, respectively) and more intense bands. Initially,
TY6 was employed as a DSSC photosensitizer in combination with an iodine-based electrolyte, either in the presence or in the absence of chenodeoxycholic acid (CDCA) as a co-adsorbent: the properties of the corresponding small-scale devices (active area = 0.25 cm
2) were investigated under dim-light irradiation
[10].
Figure 8. Structures of anthracene-based dyes with benzotriazole as the additional acceptor group
[10][11].
Using T5 as the light source, TY6-containing DSSCs exhibited remarkable PCE values ranging from 18.76 to 26.19% at variable illuminance levels (300–6000 lux) without CDCA, while the efficiencies rose to 21.40–28.56% in the presence of the co-adsorbent, especially thanks to a significant increase in terms of Voc. Under the same light source, DSSCs containing N719 as the reference dye showed efficiencies varying between 12.00 and 18.91% (300–6000 lux). Once again, the improved performances registered with TY6 compared to N719 were attributed to the better matching between its light absorption profile and the emission spectrum of T5. Using a LED light source, the spectral matching with TY6 was poorer and the PCE values were accordingly lower, but still higher than those obtained with N719 (14.0–20.7% vs. 12.1–17.6%, respectively; illuminance: 300–6000 lux).
[Cu(dmp)
2]
2+/+(TFSI)
2/1 redox couple (
E0 = +0.88 V vs. NHE) was then employed in both
TY6- and
CXC22-containing DSSCs
[11]. Although the chosen light source was again T5, efficiencies of
TY6-containing devices fell in the range of 14.56–21.34% (illuminance: 300–6000 lux), especially because of a significant loss in terms of
Jsc. However, under the same conditions,
CXC22 generated 71% higher photocurrents, which led to impressive PCE values ranging from 21.60% (300 lux) to 37.07% (6000 lux). Again, the increase in the photovoltaic performance was ascribed to the superior optical properties of
CXC22 in comparison to
TY6 and, consequently, to its better ability to harvest indoor light. At the same time, it should be pointed out that the very high PCEs achieved in this study were also due to excellent
Voc values, which were reached thanks to the use of a copper-based redox mediator, having a much more positive redox potential compared to the traditional iodine-based one: a similar observation was made also in some studies dealing with the co-sensitization approach.
The long-term stability of dye CXC22 was demonstrated by irradiating test cells containing I−/I3− as the redox couple and 3-methoxypropionitrile (MPN) as the electrolyte solvent under one-sun illumination at 60 °C for 500 h: under these conditions, 96% of the initial PCE value was retained. Unfortunately, the stability test carried out on the CXC22-based DSSCs containing the [Cu(dmp)2]2+/+(TFSI)2/1 redox couple was ruinous, with only a 66% initial PCE value retained, even if the experimental conditions were milder (in the dark and at room temperature). Since the stability of the dye was already demonstrated, the reason for the failed test was ascribed to a possible reaction of the additive 4-tert-butylpyridine (4-TBP) with the copper complexes.
In conclusion, the anthracene core appears to be a smart choice for the elongation of the π-scaffold of organic D-π-A or D-A-π-A photosensitizers for indoor applications, thanks to its high thermal stability, good intramolecular charge transfer ability, and the possibility of obtaining high-responsive molecules in the entire visible region. Such advantageous optical properties can especially be achieved when the connection of the anthracenes with the other sections of the dyes occurs through C-C triple bonds, which keep the π-structure planar and increase the conjugation level. Finally, it must be highlighted that molecular diversity can be introduced quite easily on the 9- and 10-positions of the anthracene core thanks to the exploitation of the most common metal-catalyzed cross-coupling reactions and the commercial availability of dihalo-derivatives, such as 9,10-dibromoanthracene, which make the synthesis of anthryl-derivatives particularly affordable.
4. Benzopyrazine and Thienopyrazine-Based Dyes
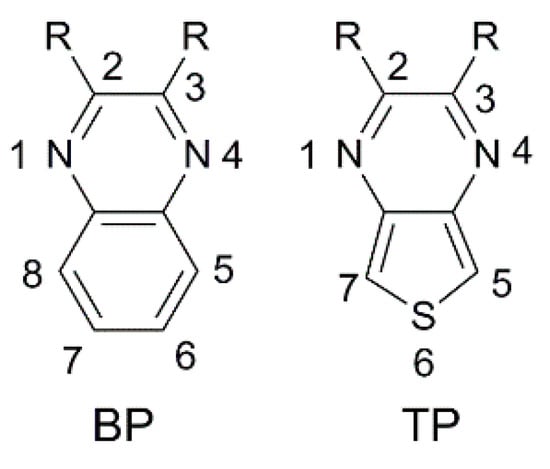
Benzo [3,4-
b]pyrazine (
BP) is an electron-poor heterocyclic moiety (
Figure 9), popularly used as the auxiliary acceptor in small band-gap polymeric materials with a D-A configuration
[12]. For comparison, together with
BP, a more electron-deficient entity, the thieno [3,4-
b]pyrazine (
TP), is often investigated.
Figure 9. Benzopyrazine (BP) and thienopyrazine (TP) scaffolds with numbering of the ring positions.
The optical properties and solubility of the
BP- and
TP-molecules can be finely tuned introducing proper alkyl or aryl substituents on the 2,3-positions of the core. In this field, Diau and co-workers reported the synthesis and characterization of four new pyrazine-based dyes (
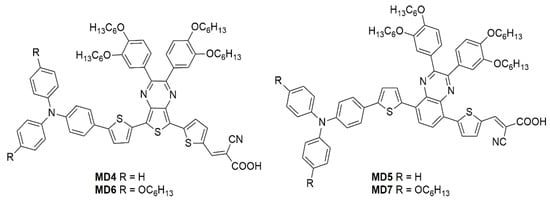
MD4-7—
Figure 10), which have been used in combination with I
−/I
3− as the redox mediator to fabricate DSSCs and explore their photovoltaic properties both under 1 Sun irradiation and T5 fluorescent light at different illuminance levels, from 300 to 6000 lux
[13].
Figure 10. Benzopyrazine and thienopyrazine-based dyes
[13].
The synthetic pathway for MD dyes includes the functionalization of the corresponding fused-pyrazine cores through a Vilsmeier–Haack reaction, introducing an aldehyde moiety as the precursor of the final cyanoacrylic anchoring group, and a Stille–Migita cross-coupling to insert the two different donor groups. The thieno [3,4-b]pyrazine-based dyes, MD4 and MD6, showed absorption maxima (λmax) both in the blue and red region of the visible spectrum: the lowest energy absorption peaks were located at 633 nm and 642 nm for MD4 and MD6, respectively, with λonset > 750 nm and molar attenuation coefficients still exceeding 104 M−1 cm−1 at 700 nm. On the other hand, the absorption maxima of MD5 and MD7 were located at 513 and 532 nm, respectively, with a blue-shift of around 110–120 nm compared to the TP-containing species: this behavior is coherent with the more electron-deficient nature of the thieno [3,4-b]pyrazine-core. As expected, increasing the donor strength of the electron-rich moiety by employing a p-alkyloxy-substituted triarylamine led to a red-shift of the absorption spectra of MD6 and MD7 in comparison to MD4 and MD5. Solubility tests in THF solution did not indicate a relevant dye aggregation up to a concentration of 3 × 10−4 M, but a slight dye aggregation was evident once adsorbed on 4 µm-thick TiO2 films. Indeed, in all the spectra, the usual behavior of H-aggregates of the dyes adsorbed on titania, that is a red-shift of the λonset and a blue-shift of the λmax, was recognizable for all the sensitizers, even though MD4 and MD6 seemed to have a higher tendency to aggregate than their BP-congeners.
For these reasons, CDCA (1 mM) was used as the co-adsorbent and added during dye soaking to minimize the aggregation of the dyes and to passivate the TiO2 surface to suppress the dark current.
Thanks to their almost panchromatic absorption in the visible region, the MD-dyes demonstrated a good spectral matching with the emission spectrum of the T5 fluorescent lamp. Both BP-based dyes (MD5 and MD7) showed PCE values higher than 15% even under only 300 lux illumination, and MD7 exhibited the highest efficiency among all the tested sensitizers, reaching 27.17% PCE under 6000 lux irradiance, which is comparable with the efficiency of the N719-based cells fabricated and measured under similar conditions (27.64%).
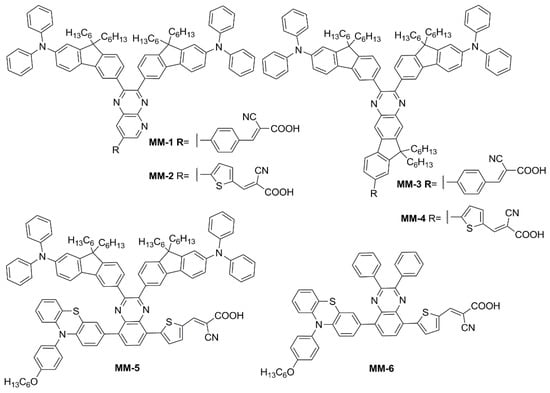
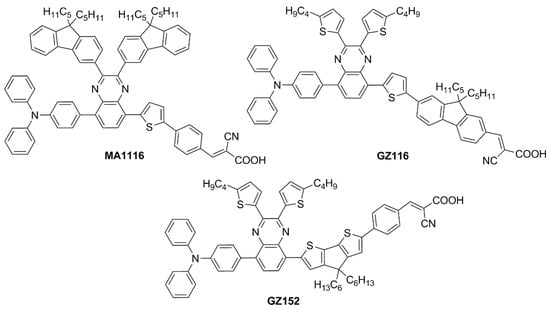
Subsequently, in 2019 Chang et al. proposed a series of Y-shaped D-A-π-A dyes containing
BP and
BP-like moieties. Six metal-free organic dyes (
MM-1–
6,
Figure 11) were synthesized through stepwise modifications on the
BP structure by introducing electron-donating substituents at different positions of the
BP moiety
[14].
Figure 11. Electron-rich benzopyrazine-based dyes
MM1–
6 [14].
The synthesis of compounds MM-1–6 involved an initial condensation reaction between a diketone precursor and the respective diamine to form the BP unit; subsequently, the other building blocks were assembled via Sonogashira, Stille–Migita, and Suzuki cross-couplings. All the MM-dyes exhibited two broad and intense light absorption peaks in the 359–484 nm range. Compared with the phenylene π-spacers in MM-1 and MM-3, the introduction of a thiophene ring in MM-2 and MM-4 induced a higher planarity of the system, extending its π-conjugation and, therefore, causing a red-shift of the absorption maxima by approximately 18–22 nm. Even more red-shifted peaks were observed for the dyes MM-5 and MM-6, thanks to the introduction of a phenothiazine-based donor group into their structures. Spectra of the dyes adsorbed on a mesoporous TiO2 film appeared quite similar to those in solutions. A slight red-shift in light absorption was observed for dyes MM-1 and MM-2, which was ascribed to a possible J-aggregation of the molecules in the solid state, while H-aggregation and/or deprotonation of the cyanoacrylic moiety could justify the blue-shift of the λmax for MM-4 and MM-6.
DSSC prepared with the MM-dyes and I−/I3− as the redox mediator showed promising power conversion efficiencies (PCEs) under 1 Sun illumination: in particular, the dyes carrying a donor group (a phenothiazine) at 5 or 8-position of BP (MM-5 and MM-6) displayed higher photocurrents and PCEs than those containing the donors only at 2,3-positions (MM-1–4). The authors also explored the possibility of using these dyes under the illumination of a TL84 fluorescent lamp in the intensity range of 600–2500 lux. The devices containing the sensitizers MM-1–4 showed unremarkable PCE values up to 13% even under a stronger illumination of 2500 lux, possibly due to their narrower absorption bandwidths; on the other hand, the two dyes MM-5 and MM-6 displayed PCE values higher than 18% under 1000 lux. MM-6 showed the best performance among all the sensitizers, reaching a PCE of 28.95% (2500 lux) when using deoxycholic acid as a co-absorbent. The authors further looked into the effect of co-adsorption, mixing MM-3 and MM-6 at different ratios, to get photoanodes with broader light absorption and produce higher currents. MM-3 and MM-6 were subsequently deposited on TiO2, finding that the optimized deposition times for MM-3 and MM-6 were 4 and 8 h, respectively. The best PCE was 30.45% under TL84 fluorescent indoor light source at 2500 lux. A stability test of the devices was carried out at 25 °C under both TL84 indoor light and AM 1.5 G in ambient conditions. The device containing both MM-3 and MM-6 retained 80–82% of the initial efficiency at every illuminance (600–2500 lux) after 168 h, indicating good stability in these conditions.
Sun et al. in 2021 described a series of new organic
BP-based dyes with a D-A-π-A scaffold integrated with fluorenyl units, which were used to extend the conjugation of the π-spacer or for the decoration of the 2 and 3 positions of the
BP scaffold (
Figure 12)
[15]. The synthesis of the dyes (
GZ and
MA series) was carried out through a series of Miyaura borylation and Suzuki–Miyaura coupling procedures and concluded with a Knoevenagel condensation. Among all the reported structures, the broader absorption bands in the visible region of
GZ116 and
MA1116 (
Figure 12) compared to other dyes made these two molecules promising candidates for indoor applications. In
N,
N-DMF solution, indeed, these dyes exhibited two or more main absorption peaks in the range of 335–600 nm: furthermore, both molecules showed almost panchromatic light-harvesting properties, with the absorption edges approaching 600 nm.
Figure 12. Structures of benzopyrazine-based dyes with extended conjugation
[15][16].
Thus, solar cells containing GZ116 and MA116 as the photosensitizers and I–/I3– as the redox mediator were investigated under indoor conditions using a T5 fluorescent lamp with different light intensities, between 300 and 6000 lux. The devices fabricated with MA116 displayed a high PCE of 26.81% (Jsc = 0.93 mA cm–2, Voc = 0.68 V, and FF = 0.76), while GZ116 displayed a PCE of 25.38% (Jsc = 0.88 mA cm–2, Voc = 0.69 V, and FF = 0.76) at 6000 lux illumination. Both dyes exhibited a comparable performance to the standard organometallic photosensitizer N719, whose cells were fabricated and measured under the same conditions. These studies demonstrated that placing a fluorenyl unit on the electron-poor quinoxaline scaffold could be a promising approach for boosting both the photocurrent and the photovoltage of DSSC devices for outdoor and indoor applications.
Later, the same group studied a new dye named
GZ152 (
Figure 12) to assess the effect of a longer π-conjugation in sensitizers containing a 2,3-
bis(5-butylthiophen-2-yl)quinoxaline as an auxiliary acceptor unit
[16]. The cyclopentadithiophene (CPDT) unit was introduced as an extended π-conjugated section to further modulate the optical properties of
GZ152, which was tested in DSSCs using an I
−/I
3−-based electrolyte under simulated AM 1.5 G light irradiation, giving a maximum PCE of 8.61%. Once adsorbed on TiO
2, the dye displayed a broad, almost panchromatic light absorption in the visible region, which was well-matched with the emission of the T5 fluorescent lamp. Thus, the performance of
GZ152-containing DSSCs was further evaluated under T5 illumination with variable light intensity from 500 to 6000 lux. As expected, the PCE values increased with the illumination intensity, and the best performance was achieved at 6000 lux, with a PCE of 28.31% (
Jsc = 1.15 mA cm
−2,
Voc = 0.62 V, and
FF = 0.77).
In summary, BP and TP units can be used as auxiliary acceptors in the conjugated spacer of D-π-A or D-A-π-A dyes for indoor applications thanks to a beneficial red-shift of the absorption spectra, especially ascribed to a deepening of the LUMO energy level, and an improved photostability. The functionalization of these heterocyclic cores can be easily tuned by following robust synthetic strategies based on the most common cross-coupling reactions allowing for the introduction of the various different building blocks.
5. Miscellaneous Dyes
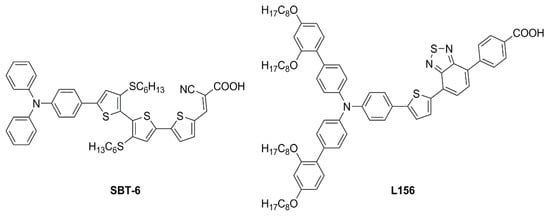
In the race toward increasing performances, several other scaffolds have been the subject of individual studies, leading to the characterization of a variety of photochemical properties and enriching the dye library for low-light environment conditions. In 2020, Ho’s group focused on some dithioalkylbithiophene (SBT)-based chromophores (
Figure 12) to investigate the effect of extending π-bridge conjugation and introducing different S-alkyl chains onto the molecules’ backbones
[17]. The strong S(Thio)-S(R) intramolecular interaction appeared to increase the planarity of the molecules, broadening their absorption spectra (
λonset > 600 nm) and leading to high
JSC values in full Sun photovoltaic characterization (AM 1.5 G, 100 mW cm
−2). Due to the obvious proneness to aggregation of such compounds, the authors also performed an extensive study on the addition of the nonconductive co-adsorbent CDCA in combination with the sensitizers. Thanks to the addition of CDCA (10 mM) the best-performing
SBT-6-based device exhibited a remarkably improved efficiency of up to 9.47% with the I
−/I
3− couple as the electrolyte under 1 Sun illumination. The latter configuration was then tested under a T5 light source and compared with the standard
N719 dye under increasing light intensity (from 900 to 6000 lux). A good PCE of 23.57% was recorded under 6000 lux illumination, higher than the performance reported for
N719.
Figure 13. Structures of dithioalkylbithiophene dyes
SBT-6 and
L156 [17][18].
In the same year, Ferdowsi and co-authors presented a work describing the novel dye
L156 (
Figure 13), containing a triphenylamine (TPA) segment and 4-(benzo[
c][1,2,5]thiadiazol-4-yl)benzoic acid as electron-rich and -deficient moieties, respectively, connected by a thiophene π bridge
[18]. Following an extensive optimization of the DSSC devices setup under simulated AM 1.5 G sunlight, the sensitizer was eventually tested under indoor-light conditions both at 200 lux and 1000 lux. Samples were prepared by using 30NR-D (dispersive titania paste) with 30 nm average particle size, and different concentrations of [Cu(tmby)
2]
2+/1+ (tmby, 4,4′,6,6′-tetramethyl-2,2′-bipyridine; 0.06, 0.03, 0.01 M Cu
2+) as the redox mediator. While the performances reported at 200 lux were generally low, the best efficiencies were achieved for devices featuring the lowest Cu
2+ concentration (0.01 M) under 1000 lux of illumination, reaching the interesting result of 21.9% PCE and an output power density (
Pout) of 67.3 µW cm
−2.
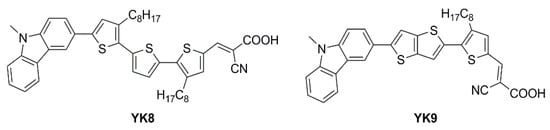
An extensive study was presented in 2021 by Haridas and co-workers reporting two new molecularly engineered, cost-effective, metal-free, carbazole-based D–π–A sensitizers (
YK8 and
YK9—
Figure 14) containing two different π-spacers, suitable for indoor photovoltaic applications
[19].
Figure 14. Structures of carbazole-based D–π–A dyes
YK8 and
YK9 [19].
The YK8 sensitizer was endowed with bis-octyl chains on a terthiophene core to reduce aggregation and prevent charge carrier recombination, potentially yielding a better electron lifetime, whereas the YK9 dye was designed and synthesized using a more planar thienothiophene linker for better donor–acceptor interactions, facilitating rapid electron migration to the TiO2 layer. The J/V measurements of the corresponding DSSCs were conducted under Osram 14 W T2 cool daylight fluorescent tube illumination at various intensities (700, 1000, 1500, and 2000 lux). A conventional iodide/triiodide electrolyte was exploited for all the experiments. As commonly reported, the authors observed an increase of JSC and VOC for both YK8 and YK9 dyes as the light intensity increased, reaching an impressive maximum power conversion efficiency of 30.24% ± 1.23% for YK8 at 1500 lux, while the DSSCs employing YK9 showed a maximum PCE of 20.11% ± 0.96% at 2000 lux. As expected, compared to YK9, the lower aggregation on the surface of TiO2 and the reduction of charge recombination phenomena due to the larger number of alkyl chains allowed YK8-containing cells to achieve an improved electron lifetime, leading to a better open-circuit voltage. In addition, the extended π linker led to a better electron diffusion length contributing to superior charge collection efficiency. Further, the red-shifted absorption in the 550–700 nm range for the YK8 sensitizer matches the spectra of the cool daylight fluorescent tube used for illumination, leading to better light harvesting, as shown by the corresponding EQE spectra. Finally, YK8-based cells presented a higher shunt resistance at 1500 lux, which may have also contributed to its better PCE at this particular light intensity. Interestingly, Haridas’ group showed a custom-designed self-powered temperature sensor with an LCD that drew power from the indoor solar cells containing the YK8 sensitizer. Four solar cells, each having an active area of 0.31 cm2 were serially interconnected (net active area of 1.24 cm2) to successfully power the temperature sensor under an illumination of 500 lux.
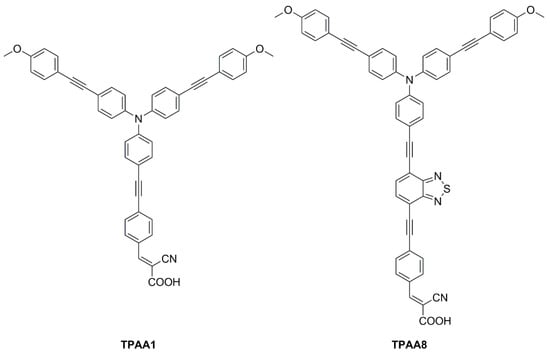
In 2021, Yoosuf et al. expanded their previous work on D-π-A propeller-shaped dyes (
TPAA1—
Figure 15)
[20] by synthesizing and testing a new molecularly engineered D-A-π-A triphenylamine dye featuring conjugated triple bonds and a benzothiadiazole unit as auxiliary acceptor (
TPAA8—
Figure 15)
[21]. Photovoltaic performances of cells built with
TPAA1 and
TPAA8 sensitizers were measured under 1000 lux illumination intensity using a warm white LED light, employing both I
–/I
3– and [Co(bpy)
3]
2+/3+ as redox couples. The best performance under indoor light was showcased by
TPAA8, delivering a maximum power conversion efficiency of 11.03% along with a [Co(bpy)
3]
2+/3+ redox mediator, while 8.74% was achieved when using an I
−/I
3− redox mediator. For comparison,
TPAA1 exhibited very low power conversion efficiencies of 0.30% and 2.50% along with [Co(bpy)
3]
2+/3+ and the I
−/I
3− electrolyte, respectively.
Figure 15. Structures of D-π-A propeller-shaped dyes
TPAA1 and
TPAA8 [20][21].
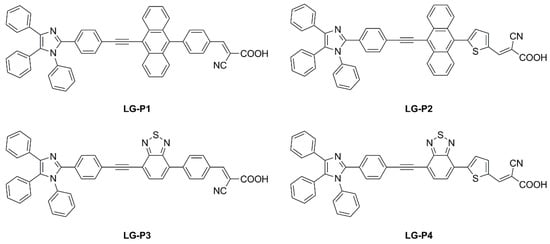
Giribabu et al. recently published three articles detailing their extensive work on triphenylimidazole-based metal-free organic dyes. In these works, four sensitizers were synthesized through a series of optimized Sonogashira cross-coupling reactions and then tested in combination with copper-based electrolytes
[22][23], as well as with the standard I
−/I
3− redox couple
[24]. Firstly, different structures of D-D-π-A (
LG-P1—
Figure 16) and D-A-π-A (
LG-P3—
Figure 16) dyes were investigated, bearing anthracene and benzothiadiazole as the auxiliary donor and acceptor units, respectively
[22]. The dyes were designed to have a relatively positive ground state oxidation potential to make them compatible with copper electrolyte [Cu(dmp)
2]
2+/1+. Nonetheless, efficiencies under 1000 lux daylight LED illumination were low, mainly limited by strong recombination processes. In particular, such processes were more intense on the
LG-P1 sensitizer, leading to PCE values of 0.13%, while
LG-P3-based devices showed a 9.14% efficiency.
Figure 16. Structures of triphenylimidazole-based dyes
LG-P1–
4 [22].
Charge extraction (CE), open-circuit voltage decay (OCVD), and electrochemical impedance spectroscopy (EIS) experiments confirmed that the more planar geometry and better conjugation of the D–A–π–A
LG-P3 dye contributed to its better photon absorption, injection, and charge collection efficiencies, resulting in an improved IPCE profile and
Jsc values. Interestingly, a more recent work from the same group expanded the study to dyes
LG-P2 and
LG-P4 (
Figure 16). The latter were similar to sensitizers
LG-P1 and
LG-P3, respectively, but bore a thiophene group as a π linker instead of benzene, which was supposed to guarantee better orbital overlap between the adjacent donor/acceptor groups and the π spacer thanks to their lower dihedral angles
[23]. In contrast to the previous report, the device employing the
LG-P2 dye having anthracene as the auxiliary donor in a D-D-π-A architecture displayed a better PCE of 7.46%, while
LG-P4, featuring the benzothiadiazole unit, led to an efficiency of 0.97%. Again, strong recombination processes are linked to the lower injection driving force, hampering the performance of
LG-P4 dye.
More recently, in 2022 Giribabu et al. expanded their studies by employing the aforementioned molecules in I
−/I
3− electrolyte-based DSSC devices
[24]. Under the very same indoor illumination conditions used in the previous papers (1000 lux daylight LED), sensitizer
LG-P1 resulted in the best-performing compound with a PCE of 10.53%. Interestingly, in tests performed using a fluorescent lamp (1000 lux daylight CFL), dye
LG-P2 showed the top efficiency among the novel sensitizers, reaching a PCE of 9.19%.
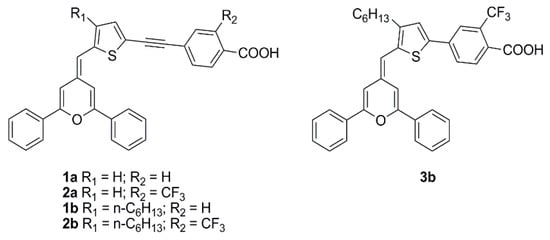
The pyranylidene scaffold has been used by Orduna and coworkers as donor moiety for a series of small sensitizers in which insertion of triple bond and/or trifluoromethyl groups in the π-bridge was studied (
Figure 17)
[25]. Test cells sensitized with CH
2Cl
2 solutions containing 0.1 mM conc. of the dye and 0.3 mM of anti-aggregating additive CDCA were first analyzed under standard AM 1.5 G illumination using I
−/I
3− redox couple as the electrolyte. Compound
2b achieved the best performances within the series, demonstrating a positive influence of the acetylenic bond in the molecule backbone due to the increased electron transfer between the donor and acceptor units. Moreover, the presence of alkyl chains limited the aggregation (compounds
1b and
2b), while the incorporation of a CF
3 group in the benzene ring (
2a-b) increased the
VOC values, improving the efficiencies. EIS experiments seemed to confirm that the alkyl substituent and the CF
3 group reduce the recombination processes. Tests carried out in lower light conditions (77 mW cm
−2) or with a fluorescent lamp (OSRAM 930/18W) at 2000 lux (0.708 mW cm
−2) and 1000 lux (0.350 mW cm
−2) showed a similar trend. The highest values were obtained at 1000 lux for compound
2b reaching a η of 5.64%, with
JSC of 59.7 µA cm
−2,
VOC of 445 mV, and
FF of 0.74.
Figure 17. Structures of DSSC dyes containing the pyranylidene scaffold
[25].
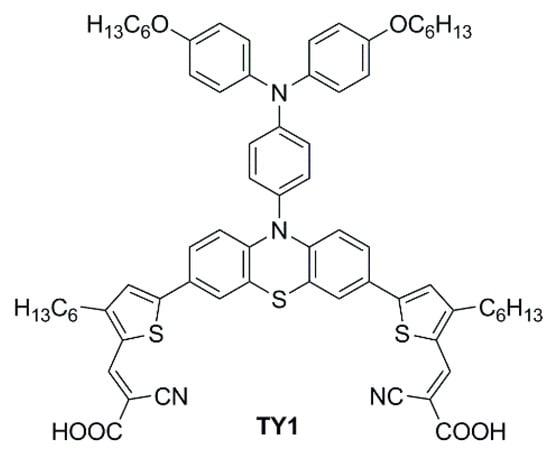
In 2023, Li et al. reported a series of sensitizers bearing a double-anchored phenothiazine scaffold, arranged in a D(-π-A)
2 skeleton
[26]. A preliminary photovoltaic study was conducted under simulated AM 1.5 G illumination using I
−/I
3− as a redox mediator. The best-performing dye was
TY1 (
Figure 18), whose cells showed a good PCE of 8.33%, with an open-circuit voltage (
VOC) of 0.72 V, a short-circuit current density (
JSC) of 16.19 mA cm
−2, and a fill factor (
FF) of 0.71. The authors also performed an extensive study on the addition of the nonconductive co-adsorbent CDCA in combination with
TY1, finding that a high concentration of CDCA (CDCA/
TY1 ≈ 33/1) is necessary to successfully occupy the TiO
2 surface not covered by the dye, decreasing the aggregation between dye molecules and leading to enhanced
JSC.
Figure 18. Structure of the double-anchored phenothiazine dye
TY1 [26].
Finally, TY1 was tested in indoor photovoltaic conditions using a halogen lamp (Philips TL5 lamp, 14 W, 6500 K), under different light intensities. The optimal TY1/CDCA-based cell reached good efficiencies of 21.16% at 1000 lux (office environment), 19.53% at 600 lux (conference hall), and 16.75% at 300 lux (living room). Moreover, a durability test was also performed exhibiting a decent long-term stability within 20 days.
6. Novel Organic Sensitizers for Indoor DSSCs: Conclusions
Despite the large number of dyes recently reported for indoor DSSC applications, a clear development path for organic structures specially designed for dim-light harvesting appears difficult to identify, except when a number of consecutive studies were conducted on specific dye classes by the same research groups. Nevertheless, the recurrence of some building blocks, such as fluorene, anthracene, and benzopyrazine, allows for drafting of some potential guidelines to be followed for the design of such kinds of sensitizers:
-
D-A-π-A structure: despite some exotic configurations that were tested over the years, the most reliable design for DSSC dyes is still the D-A-π-A structure, regardless of whether the energy source is the Sun or artificial light (e.g., dyes
CXC22 [11] and
GZ152 [16]). This configuration usually ensures intense and broad light absorption bands, well-aligned HOMO/LUMO energy levels, and long-term stability.
-
Matching with the light source: in most cases, the DSSCs that show the best PCE values under indoor conditions are those that contain sensitizers featuring either a light absorption spectrum well-matched to the emission spectrum of the light source or a panchromatic absorption in the visible region (e.g.,
AN21 [27] and
YK8 [19]). A careful design of the structure of the dye is mandatory to obtain the desired dye optical properties.
-
Bulkiness: the introduction of bulky alkyl or (hetero)aryl groups into the dye structures (e.g.,
RY3 [3] and
MD5,7 [13]) was revealed to be beneficial for reducing undesired aggregation on the semiconductor and shielding the TiO
2 layer from the electrolyte, thus limiting recombination.
From the synthetic point of view, the best-performing dyes are usually prepared by exploiting the most common metal-catalyzed cross-coupling processes (e.g., Suzuki–Miyaura and Stille–Migita reactions), which are consolidated organic transformations, potentially scalable for an industrial application. Furthermore, in the perspective of increasing sustainability and limiting the costs of potential scaled-up synthesis, more attention should be paid to the design of greener processes, for example by avoiding the use of toxic and flammable solvents and/or reducing the employment of organometallic species through the exploitation of procedures based on C-H activation reactions
[28][29].
To conclude, an enormous number of new individual dye structures are theoretically still available for DSSC indoor applications. To limit the chemical space to cover, however, future research in this field should focus on finding the perfect match between the dye light absorption properties and the emission spectrum of the light source used in each specific application, differently from the outdoor conditions where the only light source is the Sun. Furthermore, greater efforts should be made to design dyes having the right energy level alignment to work with organometallic redox mediators, like Co- and Cu-based molecules, since they have not been thoroughly explored so far in this field.
This entry is adapted from the peer-reviewed paper 10.3390/ma16237338