Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Lyotropic liquid crystals (LLCs) are liquids that have crystalline structures. LLCs as drug delivery systems that can deliver hydrophobic, hydrophilic, and amphiphilic agents. Due to their unique phases and structures, LLCs can protect both small molecules and biologics from the gastrointestinal tract’s harsh environment, thus making LLCs attractive as carriers for oral drug delivery.
- lyotropic liquid crystals
- cubosomes
- hexosomes
- M cells
1. Introduction
Peroral is the most common delivery route for drug administration [1][2][3]. Oral dosing is the most preferred by patients and convenient to handle for healthcare providers because taking medicines by mouth is non-invasive and requires no sterility constraints compared to injectables [2][3][4]. However, an orally administered drug formulation must survive the gastrointestinal tract’s harsh environment, gastric acidic conditions (pH 1.2), the presence of digestive enzymes, the mucus and mucosal barriers, the enteric epithelia whose tissue morphology inhibits drug absorption, and the hepatic metabolism for local and/or systemic therapy (Figure 1) [1][2][3][4][5]. Notably, hepatic first-pass metabolism, also known as drug pre-systemic exposure, heavily reduces the pharmacokinetic effects of drugs [2][4][5]. Thus, the oral bioavailability of many small molecule active pharmaceutical ingredients (API) (of biopharmaceutical classification system (BCS) III (high solubilitypoor permeability) and IV (poor solubilitypoor permeability) drug classes) is often very poor, and the oral delivery of biologics remains a great challenge [2][5].
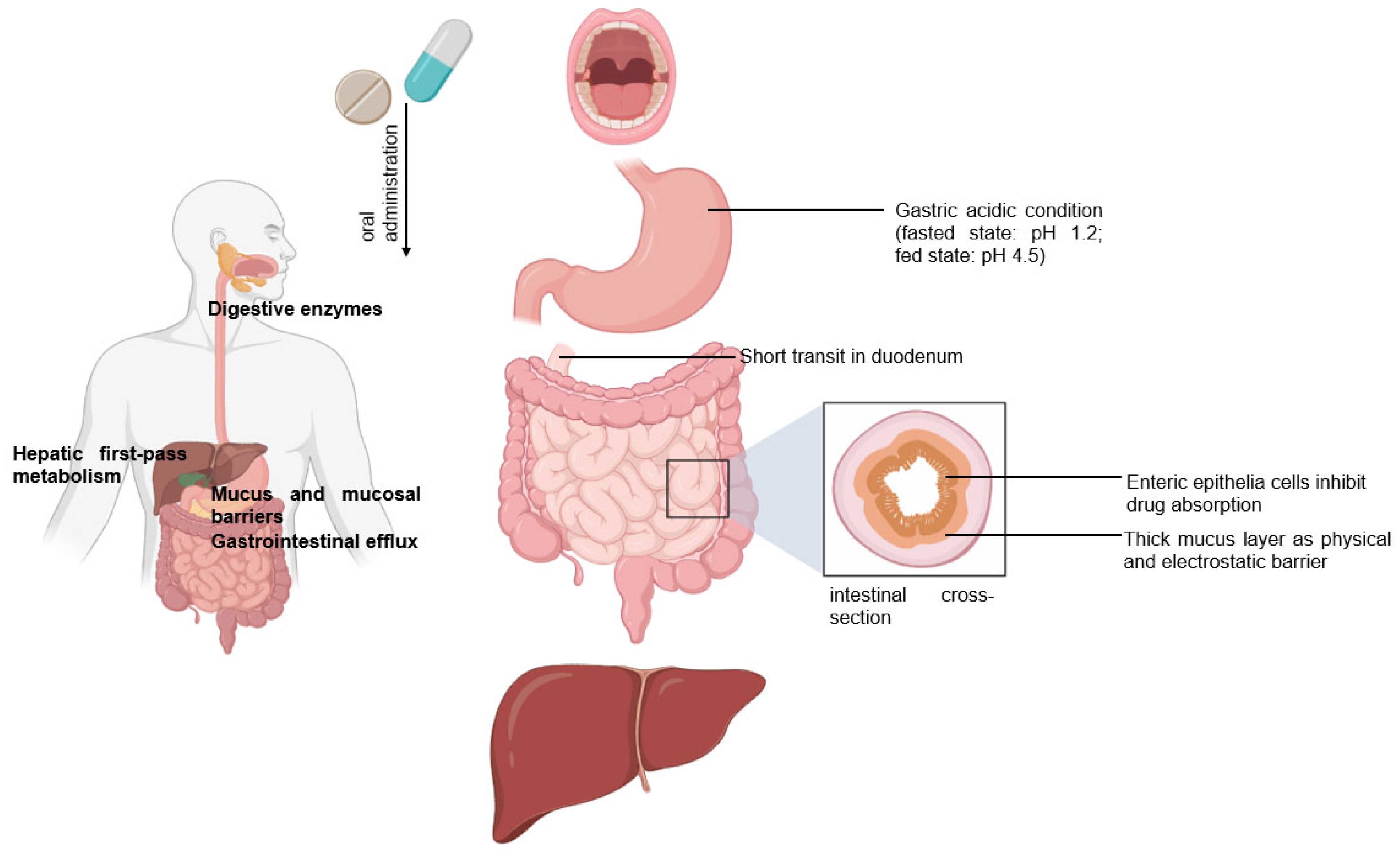
Figure 1. Following the oral administration of a therapeutic, the drug travels from the mouth to its site of action. The drug enters the digestive tract where it faces challenges.
The intestinal lymphatic pathway can be considered as the ultimate solution for drug transport to avoid first-pass metabolism [1][2][5][6][7]. The intestine absorbs fat both through passive diffusion and protein-facilitated transfer [2][5][6][7][8]. The exogenous lipoprotein pathway utilizing chylomicrons-the fat carriers assembled in the intestinal epithelium, is responsible for transferring the food-derived lipids and fat-soluble vitamins to the cells [5][6][7][8]. Therefore, by targeting the chylomicrons in enterocytes, lipid-based drug formulations can be absorbed from the intestine via lymphatic transport through the chylomicron pathway, and they enter the bloodstream through the thoracic lymph duct (Figure 2a). Another promising lymphatic pathway for oral drug delivery is the microfold cells (M cells) channel [5][7][9]. The epithelium covering mucosa-associated lymphoid tissues is where M cells are typically found. In the ileum, M cells mediate the interface between the lumen and the lymphatic system by capturing and transporting pathogen-like particles. To initiate an immune response, M cells actively deliver luminal particles and antigens to intestinal dendritic cell subsets lying under lymphoid follicles (Figure 2b). The lymphatic vessels are closely associated with blood vessels, and they are well-distributed throughout the intestinal wall and lined with endothelial cells that are highly permeable to lipophilic molecules.
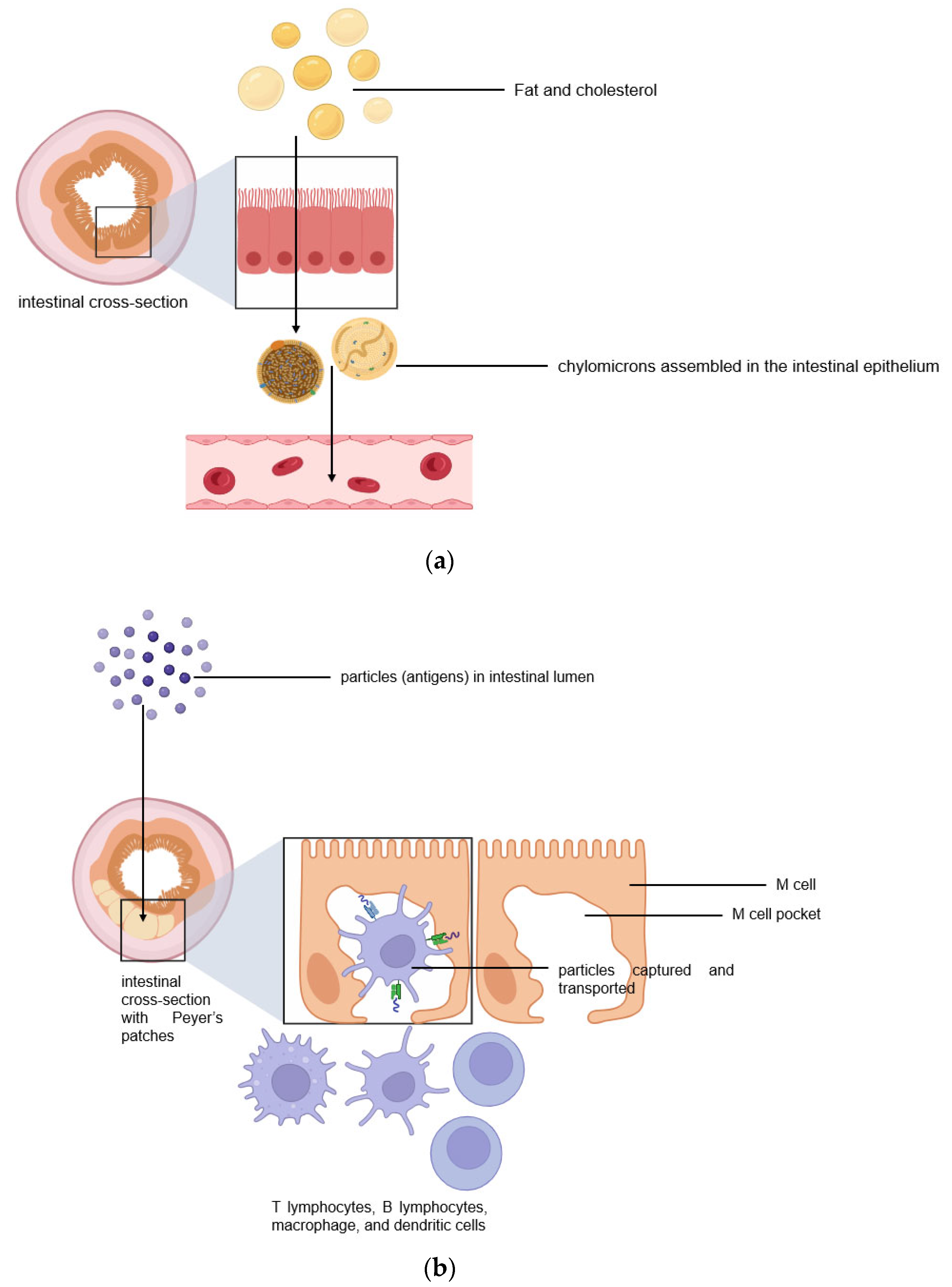
Figure 2. Two pathways for efficient intestinal lymphatic transport: (a) the chylomicron pathway and (b) the M cell pathway.
Lyotropic liquid crystals (LLCs) result when amphiphiles (amphiphilic mesogens) dissolve into suitable solvent-forming solutions that flow like liquids but still have ordered crystalline structures of solid crystals [10][11]. The well-defined, thermodynamically stable structures of LLCs such as lamellar, hexagonal, and cubic (Figure 3 and Figure 4) are formed by the self-assembly of amphiphilic lipids due to hydrophobic forces. By dispersing these mesophase structures into water in the presence of stabilizers, they can be transformed into liposomes, hexosomes, and cubosomes, collectively known as LLC drug delivery systems [11]. In recent years, due to their unique structures offering matrices for prolonged drug release as well as their amphipathic nature for the encapsulation of hydrophobic, amphiphilic, and hydrophilic molecules of various molecular weights, LLC drug delivery systems have drawn significant attention as one of the advanced systems in the field of colloidal dispersions [11][12][13]. Figure 4 shows how a hydrophobic drug, a hydrophilic drug, and an amphiphilic drug are encapsulated in cubosomes. Many lipids that form LLCs are biocompatible and biodegradable and are applicable to the oral route of administration [12][14]. Intestinal lymphatic absorption is a pathway that an LLC drug delivery system may follow to gain access to the systemic circulation after its oral administration. A lipid-based LLC drug delivery system targeting the intestine can enhance intestinal lipid fluidity.
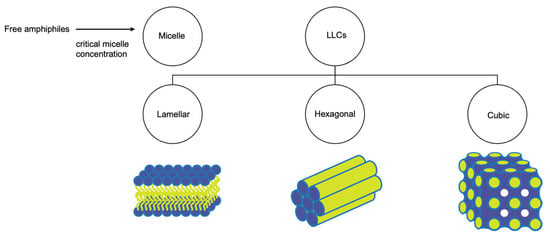
Figure 3. Structural classification of LLCs.
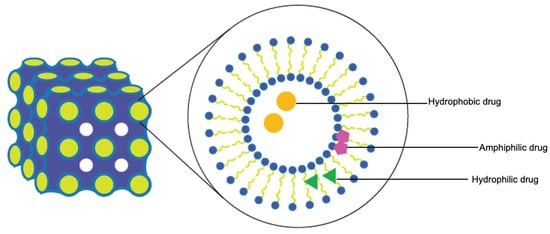
Figure 4. Cubosomes encapsulate hydrophobic, hydrophilic, and amphiphilic model drugs.
2. LLC Drug Delivery Systems for Efficient Intestinal Lymphatic Targeting
Preparation and Characterization of Oral LLC Drug Delivery Systems
An LLC system includes water (aqueous phase), amphiphilic mesogen (lipid phase), and surfactant. The mesophase is often determined by the hydrophobicity and hydrophilicity of the system. When the concentration of micelles dispersed in water is above the critical aggregation concentration (CMC), micelles are forced to pack into a structure (micellar cubic phase—discontinuous cubic phase) [12][15]. The lamellar phase consists of lipid bilayers with hydrophilic heads (outer layer) and hydrophobic tails (inner layer). In water, these lipid bilayers are arranged linearly with water channels. Small changes in temperature and concentration can cause transitions between mesophases (Figure 4). Bicontinuous cubic and hexagonal phases are made of a 3D network of bicontinuous lipid bilayers arranged in a pattern of infinite periodic minimal surfaces (bicontinuous cubic phase) and in cylinders (hexagonal phase). These 3D networks have two distinct, continuous, non-intersecting, hydrophilic sections, and hydrophobic components are placed within. The cubic and hexagonal phase dispersions are especially advantageous for oral delivery because their structure could protect the drug in the gastrointestinal tract’s harsh environment [16]. Moreover, LLCs with a hydrophilic surface can easily traverse the water layer and contact the endothelial cell layer. Cubosomes were reported to have the ability to penetrate through the endothelial cell membrane [17][18].
Cubosomes and hexosomes are typically prepared for drug delivery by forming dispersions using energy input methods. Glyceryl monooleate and phytantriol are the two most used lipids to form cubic and hexagonal phases. Surfactants and stabilizers are often incorporated into the preparation to maintain its colloidal stability. The “gold standard” steric stabilizer for the preparation of LLC is poloxamer 407 (Pluronic® F127 (BASF, Ludwigshafen, Germany), polyethylene-polypropylene glycol, hydrophilic–lipophilic balance (HLB) = 18) [19][20]. Poloxamer 407 kinetically stabilizes the formation of LLC by working on the interfacial tension, because it is composed of polyethylene oxide (PEO)-polypropylene oxide-PEO block copolymer that contains both hydrophilic and hydrophobic parts. The hydrophobic part interacts with the lipid bilayer, while the hydrophilic part faces the aqueous portion [21]. Bile salts, amphiphilic proteins, block polymers, other non-ionic surfactants, and other types of stabilizers (PEGylated lipids and customized lipid–copolymer (lipidic polymers)) including polysorbate 80, Cremophor®, and Labrasol® (BASF, Ludwigshafen, Germany), were investigated for their ability to stabilize LLCs [19][22]. The choice of surfactants for oral LLC systems was based on their required ability to protect APIs from premature metabolism by liver enzymes. Their high HLB value indicates their tendency to solubilize lipid (low HLB) in water, thereby forming the desired structure required to encapsulate a drug. For surfactants with HLB values > 6, cholesterol addition is recommended to form bilayered vesicles in the case of lamellar LLCs [23]. A chosen stabilizer is also required to have the bioactivity to inhibit the function of P-glycoprotein (P-gp) in the intestine, thereby increasing intestinal absorption [24]. Moreover, it was suggested that the PEO blocks of Poloxamer 407, covering the outer surfaces of formed cubosomes and/or hexosomes drain relatively faster into the lymphatic system, avoiding detection by lymph node macrophages, thus reaching the blood circulation and remaining there for a long period of time [25].
LLC drug delivery systems are characterized by their mesophase identification, drug release patterns, stability, and drug entrapment efficiency (EE) that is calculated as follows:
For LLC formulations, EE and in vitro release are the most important characteristics. Both cubosomes and hexosomes can encapsulate the drug within the LLC system, and depending on the drug solubility, a water-insoluble drug can be found in the hydrophobic sections (surfactant hydrophobic parts and hydrophobic tails) and a water-soluble drug can be found in the hydrophilic sections (water channels) of the network. A general procedure to produce an LLC dispersion is to dissolve hydrophobic drugs in the oil phase with surfactants and then homogenize the oil phase mixture and water. Barauskas et al. compared different nanoparticle dispersions of self-assembled lipid mesophases and suggested that the release profile of hydrophobic drug-loaded cubosomes was significantly improved [26]. This can be explained by the fact that it is more difficult for hydrophobic drugs to escape from the LLC system compared to hydrophilic ones. The affinity of hydrophobic drugs with hydrophobic cores of reversed cubic phase is strong. Meanwhile, hydrophilic drugs trapped inside the LLC systems can flow out to the outer water through the water channels. Interestingly, amphiphilic drugs can be trapped in both hydrophilic and hydrophobic sections and along the interface of lipid and water (Figure 3 and Figure 4). The drug release from an LLC drug delivery system is based on the principle of drug diffusion following the Higuchi diffusion kinetic model [11][12][27][28]. Studies have shown that the LLC structure and the nature of lipids could be utilized to control the drug release rate, but an initial burst release before a sustained drug release seems unavoidable [11][12][29]. Initial burst release phenomenon and drug release kinetics are independent and are not related to EE. An LLC product with a high drug loading may cause local or systemic toxicity due to an initial burst release [29].
An LLC’s mesophase behavior can be affected by the choice of surfactant and mesogen and by several other factors. On the other hand, LLC mesophase behavior affects the physiochemical characteristics, EE (cubosomes were reported to be better than hexosomes in most cases), and drug release patterns (cubic phase often released drug faster than hexagonal phase) of the LLC system [30][31][32]. It is important to identify the LLC phase, especially when the phase determines whether the drug has been incorporated into it. Structural details of an LLC can be observed using transmission electron microscopy (TEM), but small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) can visualize the structure as well as the phase behavior of LLCs using lattice parameters of the phase. The morphology of an LLC can be determined using TEM, in which samples are drop-casted on a mesh grid. Traditional TEM, in which materials are dried and stained on carbon grids before being observed using a microscope, is not recommended because of dehydration. Cryogenic electron microscopy (Cryo-EM) has been used to obtain the high-resolution images of LLCs, enabling the direct visualization of the internal phase structure and size assessment without fixating or staining [33]. To analyze LLC mesophases, experiments that are used are complex, and more than one technique can be used. Experiments are often run at various concentrations of mesogens. As an LLC phase transition occurs, fluctuations in heat capacities and enthalpy can be measured using differential scanning calorimetry (DSC). Particle size and zeta potential values measured using dynamic light scattering are often determined for the stability tests and quality control of LLCs.
The physical properties, stability, and physiological behavior of LLCs are highly critical characteristics. The nanostructured LLCs were considered as the template for nanostructured biodegradable hydrogel networks [34]. The choice of materials and the concentration of mesogens dramatically affected the network diffusivity and permeability, thus leading to changes in the degradation rate of the lamellar phase. For nanostructured LLCs, being nanosized may facilitate the release of drug from the carriers, and no significant relation between the mean particle size value and the release rate as well as the degradation rate was observed.
This entry is adapted from the peer-reviewed paper 10.3390/liquids3040029
References
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524.
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411.
- Viswanathan, P.; Muralidaran, Y.; Ragavan, G. Chapter 7—Challenges in oral drug delivery: A nano-based strategy to overcome. In Micro and Nano Technologies, Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–201.
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129.
- Zhang, Z.; Lu, Y.; Qi, J.; Wu, W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm. Sin. B 2021, 11, 2449–2468.
- Yáñez, J.A.; Wang, S.W.; Knemeyer, I.W.; Wirth, M.A.; Alton, K.B. Intestinal lymphatic transport for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 923–942.
- Trevaskis, N.L.; Kaminskas, L.M.; Porter, C.J. From sewer to saviour—Targeting the lymphatic system to promote drug exposure and activity. Nat. Rev. Drug Discov. 2015, 14, 781–803.
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194.
- Fievez, V.; Plapied, L.; des Rieux, A.; Pourcelle, V.; Freichels, H.; Wascotte, V.; Vanderhaeghen, M.L.; Jerôme, C.; Vanderplasschen, A.; Marchand-Brynaert, J.; et al. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur. J. Pharm. Biopharm. 2009, 73, 16–24.
- Hansen, J.-P.; McDonald, I.R. Chapter 12—Applications to Soft Matter. In Theory of Simple Liquids, 4th ed.; Hansen, J.-P., McDonald, I.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 511–584.
- Huang, Y.; Gui, S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RCS Adv. 2018, 8, 6978–6987.
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic liquid crystal systems in drug delivery. Drug Discov. Today 2010, 15, 1032–1040.
- Kim, D.H.; Jahn, A.; Cho, S.-J.; Kim, J.S.; Ki, M.-H.; Kim, D.-D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2015, 45, 1–11.
- Ganem-Quintanar, A.; Quintanar-Guerrero, D.; Buri, P. Monoolein: A review of the pharmaceutical applications. Drug Dev. Ind. Pharm. 2000, 26, 809–820.
- Ruckenstein, E.; Nagarajan, R. Critical micelle concentration and the transition point for micellar size distribution. J. Phys. Chem. 1981, 85, 3010–3014.
- Chen, Y.; Ma, P.; Gui, S. Cubic and hexagonal liquid crystals as drug delivery systems. Biomed. Res. Int. 2014, 2014, 815981.
- Yang, Z.; Tan, Y.; Chen, M.; Dian, L.; Shan, Z.; Peng, X.; Wu, C. Development of amphotericin B-loaded cubosomes through the SolEmuls technology for enhancing the oral bioavailability. AAPS PharmSciTech 2012, 13, 1483–1491.
- Nazaruk, E.; Majkowska-Pilip, A.; Bilewicz, R. Lipidic Cubic-Phase Nanoparticles-Cubosomes for Efficient Drug Delivery to Cancer Cells. ChemPlusChem 2017, 82, 570–575.
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473.
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In vitro and in vivo evaluation of cubosomes containing 5-fluorouracil for liver targeting. Acta Pharm. Sin. B 2015, 5, 79–88.
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11.
- Wei, Y.; Zhang, J.; Zheng, Y.; Gong, Y.; Fu, M.; Liu, C.; Xu, L.; Sun, C.C.; Gao, Y.; Qian, S. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019, 9, 6287–6298.
- Paecharoenchai, O.; Teng, L.; Yung, B.C.; Teng, L.; Opanasopit, P.; Lee, R.J. Nonionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine 2013, 8, 1865–1873.
- Nguyen, T.T.; Duong, V.A.; Maeng, H.J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103.
- Helvig, S.Y.; Andersen, H.; Antopolsky, M.; Airaksinen, A.J.; Urtti, A.; Yaghmur, A.; Moghimi, S.M. Hexosome engineering for targeting of regional lymph nodes. Materialia 2020, 11, 100705.
- Barauskas, J.; Cervin, C.; Jankunec, M.; Spandyreva, M.; Ribokaite, K.; Tiberg, F.; Johnsson, M. Interactions of lipid-based liquid crystalline nanoparticles with model and cell membranes. Int. J. Pharm. 2010, 391, 284–291.
- Zewail, M.; Gaafar, P.M.E.; Ali, M.M.; Abbas, H. Lipidic cubic-phase leflunomide nanoparticles (cubosomes) as a potential tool for breast cancer management. Drug Deliv. 2022, 29, 1663–1674.
- Paolino, D.; Tudose, A.; Celia, C.; Di Marzio, L.; Cilurzo, F.; Mircioiu, C. Mathematical Models as Tools to Predict the Release Kinetic of Fluorescein from Lyotropic Colloidal Liquid Crystals. Materials 2019, 12, 693.
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control Release 2001, 73, 121–136.
- Nazaruk, E.; Miszta, P.; Filipek, S.; Górecka, E.; Landau, E.M.; Bilewicz, R. Lyotropic Cubic Phases for Drug Delivery: Diffusion and Sustained Release from the Mesophase Evaluated by Electrochemical Methods. Langmuir 2015, 31, 12753–12761.
- Mohammad, Y.; Prentice, R.N.; Boyd, B.J.; Rizwan, S.B. Comparison of cubosomes and hexosomes for the delivery of phenytoin to the brain. J. Colloid. Interface Sci. 2022, 605, 146–154.
- Tran, N.; Mulet, X.; Hawley, A.M.; Hinton, T.M.; Mudie, S.T.; Muir, B.W.; Giakoumatos, E.C.; Waddington, L.J.; Kirbyb, N.M.; Drummond, C.J. Nanostructure and cytotoxicity of self-assembled monoolein–capric acid lyotropic liquid crystalline nanoparticles. R. Soc. Chem. Adv. 2015, 5, 26785–26795.
- Gao, M.; Kim, Y.K.; Zhang, C.; Borshch, V.; Zhou, S.; Park, H.S.; Jákli, A.; Lavrentovich, O.D.; Tamba, M.G.; Kohlmeier, A.; et al. Direct observation of liquid crystals using cryo-TEM: Specimen preparation and low-dose imaging. Microsc. Res. Techn. 2014, 77, 754–772.
- Clapper, J.D.; Iverson, S.L.; Guymon, C.A. Nanostructured Biodegradable Polymer Networks Using Lyotropic Liquid Crystalline Templates. Biomacromolecules 2007, 8, 2104–2111.
This entry is offline, you can click here to edit this entry!
